Abstract
Two new lanostane triterpenes named ganoderic acid AW1 (1) and ganoderic acid AW2 (2) were isolated from the fungal fruiting bodies of the cultivated new isolate of the Egyptian Ganoderma sp in addition to the known compounds ganomycine A (3), pinellic acid (4), and ergosterol peroxide (5). Their structures were elucidated by detailed analysis of their 1D and 2D-NMR data, as well as high-resolution mass spectroscopy. Ganoderic acid AW1 (1) showed good antimalarial activity against the chloroquine sensitive strain of Plasmodium falciparum with an IC50 value of 257.8 nM with no cytotoxicity up to the concentration of 9 μM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that half of the world’s population are at risk of malarial infection especially infants, children under the age of five and pregnant women. The number of malarial infected cases increased in the year of 2016 and reached 216 million cases in 91 countries, while death toll as a consequence of malaria was near one million globally between 2015 and 2016 (https://www.who.int/news-room/fact-sheets/detail/malaria, accessed October 1st, 2019). Antimalarial drugs, although available, are suffering from the global spread of resistance especially against the old drugs, chloroquine, and sulfadoxine–pyrimethamine, and they are no longer as effective (White 2004). Due to drug resistance against artemisinin (Cui et al. 2015), the World Health Organization recommend the artemisinin-based combination therapy (Eastman and Fidock 2009). That being said, there is an urgent need for the discovery and development of new antimalarial drugs with different mechanisms of action to control malaria. Decreasing the death associated with malaria is listed in The United Nation’ Millennium development goals as a key target to be achieved.
The basidiomycete macrofungi, Ganoderma which belongs to the family Ganodermataceae, have been used for centuries as a medicinal remedy in China, Japan, and Korea for the treatment of several diseases including cancer and diabetes (Paterson 2006). Approximately 200 species have been identified that belong to Ganoderma genus and they are distributed globally (Cole and Schweikert 2003). Ganoderma lucidum is the most widely used in the medicinal applications. The genus is well known for producing highly oxygenated lanostanes triterpenes, as well as polysaccharides (Cole and Schweikert 2003). Recently, several new oxygenated lanostane with promising anticancer activities have been reported (Satria et al. 2018) (Isaka et al. 2018) (Peng et al. 2019) (Su et al. 2018). Antibacterial and antifungal activities, along with anticancer activity, are also very commonly associated with the lanostane secondary metabolites, which are isolated from either the fruiting bodies or the mycelium of the genus Ganoderma (Xia et al. 2014). However, there is very limited literature reporting the antimalarial activity for them. For example, three nortriterpenes, ganoboninketals (A–C), isolated from the fruiting bodies of Ganoderma boninense exhibited modest antimalarial activities with IC50 range of 1.7–7.9 μM (Ma et al. 2014). Furthermore, ganodermalactones F, schisanlactone B, and colossolactone E from the culture of Ganoderma sp. KM01 showed modest antimalarial activity with IC50 range of 6.00–10.0 μM (Lakornwong et al. 2014).
With our interest in exploring the Egyptian environment for drug leads, we developed a scale-up in house cultivation method for the Ganoderma sp EGDA mushroom strain, which was recorded for the first time at the North East Nile Delta, Egypt. It was identified as a novel strain based on classical and modern techniques including fruit bodies, spore structure and culture properties as a traditional taxonomic criteria; and DNA sequences analysis of the ribosomal 5.8S rRNA gene containing the flanking internal transcribed spacers as a molecular criteria (El-Fallal et al. 2015).
Materials and methods
General experimental procedure
Nuclear Magnetic Resonance spectra (1D and 2D) were recorded with a Bruker Avance IIITM spectrometer (Bruker Daltonics, Bremen, Germany) at 500 MHz for proton, and 125 MHz for carbon. The data obtained were processed using ACD-NMR processor software ver. 12.01. The solvent used was CD3OD. Chemical shifts data were reported in parts per million (ppm) on the δ scale; coupling constants (J) were expressed in Hz. Normal phase chromatography was carried out using silica gel G60–230 (Merck, Darmstadt, Germany) packed by the wet method in the stated solvent. Sephadex LH20 column chromatography was carried out using sephadex (SIGMA-ALDRICH, Missouri, USA). Reversed phase chromatography was performed using phase-bonded octadecylsilyl-silica gel (RP-C18, BAKERBOND® Octadecyl, C18) 40 µm. Pre-coated silica gel plates (TLC) F245(20 × 20 cm) were used for analytical separation, using ascending technique and suitable solvent systems with visualization using p-anisaldehyde/H2SO4 and vanillin solution.
Ganoderma cultivation
Mycelial growth
Pure Ganoderma sp EGDA mycelia were developed from the already preserved culture maintained on potato dextrose agar (PDA) slants. The pure mycelia were plated onto PDA medium supplied with traces of streptomycin sulfate and incubated for 7 days at 30 °C.
Spawn preparation
Hundred grams of sorghum gains were placed in glass jars and mixed with 3% of a 4:1 ratio of calcium sulfate and calcium carbonate mixture, respectively. Moistened with 50 mL water, then autoclaved for 30 min at 15 p.s.i. After cooling, the spawn medium was inoculated with five agar disks from fully grown PDA cultures. Incubation was carried out at 30 °C for 2 weeks.
Substrate preparation and culturing: the culturing substrate of Ganoderma sp EGDA was prepared by mixing 500 g of sawdust with 30 gm wheat bran, 5 g calcium sulfate, and 1 g magnesium sulfate. Polypropylene bags were filed two third with the previously prepared mixture to leave air space for ventilation. The free end of the bag was pulled up tightly through a plastic neck, the edge then covered with cotton plugs and autoclaved twice for 45 min at 15 p.s.i. After cooling, the bags were inoculated with 10% sorghum grain spawn by localized spawning technique (Chen 2004). The bags were then incubated at 30 °C for 2 weeks until the mycelia colonized the substrate. Then they are transferred to a mushroom house for maturation of the culture blocks at ambient temperature (27–30 °C) until dense and leathery yellowish white mycelia could be observed. The plugs necks were removed and the bags were placed on shelves and totally covered with a plastic sheet. After the primordia appeared, the water was sprayed daily. Water spraying was stopped when the color of the fruiting bodies caps were formed.
Extraction and isolation
The fresh cultivated mushroom’s fruiting bodies (30 g) were allowed to complete dryness in shade for about 4 days and then were extracted with methanol at 40 oC under reflux for 5 h. The methanolic extract was evaporated under vacuum to a reduced volume using rotary evaporator to produce a thick brownish residue (~7 g). This residue was suspended in 20 mL of H2O and then extracted with EtOAc (~50 mL × 3). Organic layers were combined and dried over Na2SO4 anhydrous and then allowed to complete dryness under reduced pressure to produce (~4 g) of brownish residue. The EtOAc extract was resuspended on methanol (50 mL) and kept in the fridge overnight, for the removal of fats, and was filtered and dried down to produce a brownish residue (2.5 g). The de-fated EtOAc extract was loaded on 2 g silica gel and left in open air until complete dryness. This extract was then fractionated on a silica gel column using 63 g of silica (1:~25 wt/wt ratio). Fractionation started with 100% petroleum ether with 10% increment of wa EtOAc (3% H2O and 1% HOAc EtOAc) (commercial ethyl acetate was mixed with 3% (v/v) water and 1% (v/v) acetic acid and shake well until clear solution was observed). Similar fractions were combined based on their similarities on thin layer chromatography (TLC, pet. ether: EtOAc 1:1) and then dried down by rotavap which yielded eight different pools
TLC optimization was completed first for choosing the best isocratic solvent that will better resolve each pool in a practical approach. The less polar pool, pool 1, was purified using a silica column with an isocratic solvent system (10 column volumes) of 90:10 (pet. ether: wa EtOAc) and then the polarity of the isocratic system was increased to 88:12 (pet. ether: wa EtOAc) for an additional four column volumes followed by a column wash with one column volume of wa EtOAc and one column volume of MeOH. Around 1 mL fractions were collected and analyzed by TLC. Fractions 78–90 were combined together based on their similarities on the TLC (88:12 pet.ether: wa EtOAc) and delivered the known compound, ergosterol peroxide (5) (5.7 mg) (Nowak et al. 2016). Pool 3 was purified by an isocratic solvent system (ten column volumes) of 70:30 (pet. ether: wa EtOAc) using silica column and then the polarity of the isocratic system was increased to 50:50 (pet. ether: wa EtOAc) for an additional four column volumes followed by a column wash with one column volume of wa EtOAc and one column volume of MeOH. Around 1 mL fractions were collected and analyzed by TLC. Fractions 13–17 showed one pure compound, pinellic acid (4) (3 mg) (Nagai et al. 2002). Fractions (145–180) were combined with the column wash and named G2. The TLC profile of G2 showed the presence of at least three major compounds with moderate to high polarity (TLC solvent: 100% wa EtOAc). The fraction was loaded on 500 mg of silica and after complete dryness was loaded on top of a glass column containing 5 g silica gel. Elution started first with an isocratic system of 60:40 (pet. ether: wa EtOAC). Five column volumes (~100 mL) were eluted with a flow rate of 1 mL/min and analyzed by TLC. Ganomycine 1 (3) (1.8 mg) was eluted first. A major spot was shown to be consistent in the TLC (Rf = 0.32, 100% wa EtOAc) during the elution of the last column volume eluted from the G2 column with some minor spots below this major spot. These fractions were combined and dried down with a rotavap which yielded a yellowish residue (~50 mg) and was given the code G2-W. Further purification of G2-W with a sephadex LH20 column with an isocratic solvent system composed of (DCM: MeOH, 1:1) delivered the new compound, named ganoderic acid AW1 (1) (3.1 mg). Right after the elution of compound 1, another compound was eluted from the sephadex LH20 column. This compound is confirmed to be a new related analog to compound 1 and named ganoderic acid AW2 (2) (2.7 mg).
Ganoderic acid AW1 (1)
Yellowish amorphous oil; HR-ESIMS m/z 551.26071 [M + Na]+ (calculated for C30H40O8Na; 551.26154); [α] +44.2 (MeOH, c 0.095); ECD (MeOH) λmax 210 (Δε −5.9), 223 (0), 242 (−1.1); NMR data: (Table 1).
Ganoderic acid AW2 (2)
White amorphous solid; HR-ESIMS m/z 553.27643 [M + Na]+ (calculated for C30H42O8Na; 553.27719); \([\alpha ]_D^{25}\) +30.2 (MeOH, c 0.205); ECD (MeOH) λmax 215 (Δε −4.1), 223 (0), 242 (−3.2); NMR data: (Table 1)
In vitro antimalarial and antimicrobial activities
The antimalarial activity was determined against D6 (chloroquine sensitive) and W2 (chloroquine resistant) strains of Plasmodium falciparum in an in vitro assay as described elsewhere (Bharate et al. 2007). Artemisinin and chloroquine were included as the drug controls, and IC50 values were computed from the dose–response curves using Microsoft Excel software (Bharate et al. 2007).
Results and discussions
Mushroom cultivation
Generally, Ganoderma sp EGDA required about 100 days to produce mature fruiting bodies (Basidiocarps) after inoculation with 10% spawn mixture. The stages of Ganoderma sp EGDA cultivation and fruit bodies’ development are summarized in Fig. S22 (Supplementary material).
Structure elucidation of isolated compounds
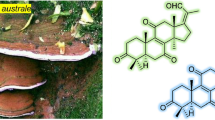
Ganoderic acid AW1 (1) was obtained as a yellowish oil. Its molecular formula of C30H40O8 was determined from its HR-ESIMS data, which was consistent with its 13C-NMR spectra (MeOH, d4, 125 MHz) that showed 30 carbon resonances. The 1H-NMR (MeOH, d4, 500 MHz) (Table 1) revealed the presence of five singlet methyl groups at δH 1.87, 1.49, 1.25, 1.20, and 1.04. The 1H-NMR also showed the presence of an olefinic proton at δH 6.87 (t, J = 6.4 Hz), two oxygenated methines at δH 4.54 (d, J= 6.7 Hz) and 4.13 (t, J= 6.7 Hz) in addition to two exo-methylene protons at δH 5.30 (s, 1H) and 5.13 (s, 1 H). The 13C-NMR of ganoderic acid AW1 (CD3OD, 125 MHz) showed the presence of 30 carbon resonance, three of which are ketonic carbonyl resonances at δC 216.3, 204.7, and 203.7, in addition to a carboxylic carbonyl resonance at δC 171.6. This carboxylic acid functionality was further confirmed to be attached to the Δ24 at the side chain through the HMBC correlations of the methyl group protons at δH 1.87 and the olefinic proton at δH 6.87 (t, J = 6.4 Hz) to the carboxylic carbonyl carbon at δC 171.6. The 13C-NMR along with the HSQC experiments also revealed the presence of four quaternary olefinic carbons at δC 153.6, 151.0, 151.1, and 129.0; one olefinic methine at δC 140.4; one olefinic methylene at δC 114.4; an oxygenated methylene resonance at δC 65.5, two oxygenated methines at δC 77.2 and 75.1; two methine carbons at δC 52.8 and 46.4; six methylene carbons at δC 52.4, 39.5, 38.4, 36.8, 36.3, and 36.0; four quaternary carbons at δC 55.0, 54.0, 48.4, and 40.7; as well as five methyl resonances at δC 24.9, 21.8, 19.7, 18.7, and 13.0. All these findings and based on literature data suggested that compound 1 is a highly oxygenated triterpene-related structure and it belongs to the lanostane family.
A carbonyl group at C-3 was confirmed through the HMBC correlations of H-28 methyl group at δH 1.20 to the carbonyl resonance as δC 216.3. The oxygenated methylene protons at δH 3.91 (d, J = 11.6 Hz) and δH 3.66 (d, J = 11.6 Hz)/δC 65.5 showed HMBC correlations with the C-3 carbonyl, as well as to the methyl group at δC 21.8 and the quaternary C-4 at δC 54.0. This indicate that one of the methyl groups attached to C-4, either the Me-28β or Me-29α, is hydroxylated. Comparison of our 13C-NMR chemical shifts values, δC 65.5 and δC 21.8, with dammarane triterpenoids that have similar substitution at the “A” ring, such as the C-4 isomers, caffruones A and B (Wang et al. 2018) and (20 S,24 S)-epoxy-25,28- dihydroxydammar-3-one (Yan et al. 2014), suggested that Me-29α is hydroxylated in 1. The HMBC correlations of the protons of methyl groups at C-19 (δH/C 1.49/18.7) and C-30 (δH/C 1.25/24.9) with the two quaternary olefinic carbons C-8 at δC 151.0 and C-9 at δC 153.6 suggested that a fully substituted double bond is present at the junction of rings B and C. The downfield shifted carbonyls at δC 204.7 and 203.7 suggested that those carbonyl groups are conjugated with the double bond and confirmed their positions to be at C-7 and C-11, respectively. Thus, an enedione functionality is present between rings B and C.
A hydroxy group is assigned at C-15 (δC 77.2) based on the HMBC and NOESY correlations of H-15 at δH 4.54 with C-16 (δC/H 39.5/2.40,1.91) and C-30 (δC/H 24.9/1.25), as well as the strong HMBC correlation of H-30 methyl resonance at δH 1.25 with C-15. The relative configuration of C-15 hydroxy group was determined to be alpha relative to the ring based on the NOESY correlations of H-15 with Me-30 (δC/H 24.9/1.25), H-17 (δC/H 46.4/2.97), and H-5 (δC/H 52.8/2.29) (Fig. 1b). Hydroxylation at C-15 is common in lanostane and other triterpenoids isolated from the ganoderma species (Baby et al. 2015). The HMBC correlations of the two exo-olefinic protons H-21 at δH 5.30 and 5.13 (δC 114.4) to C-20 (δC 151.1) and the oxygenated carbon at δC 75.1 (C-22) suggested the presence of a double bond between C-20 and C-21 and an allylic hydroxy group at C-22.
Based on these detailed NMR analyses and on literature data (Baby et al. 2015), compound 1 is a new lanostane-related derivative (Fig. 1a) and is named ganoderic acid AW1.
A molecular formula of C30H42O8 was proposed for ganoderic acid AW2 (2) from its HR-ESIMS data. The 1H- and 13C-NMR data of ganoderic acid AW2 (2) was very similar to the data of ganoderic acid AW1 (1) with only slight differences. Detailed analysis of 13C-NMR, HSQC, HMBC, 1H-1H-COSY, and NOESY data showed that ganoderic acid AW2 (2) has a carbonyl group at C-3 (δC 216.3), an enedione functionality between the junction of rings B and C (C-7, δC 204.8; C-8, δC 150.9; C-9, δC 153.1; and C-11, δC 203.8) and hydroxylation at C-15 (δC 76.6) and C-28 (δC 65.5). Furthermore, the characteristic α, β-unsaturated carboxylic acid functionality was observed in the side chain of ganoderic acid AW2 (2). This was clear from the characteristic carboxylic carbonyl carbon at δC 171.8 along with the methyl group at δC/H 12.6/1.85 and finally the olefinic methine at δC/H 144.1/6.81. Relative configuration of the hydroxy group at C-15 was shown to be alpha relative to the ring similar to ganoderic acid AW (1) (Fig. 1b). The first difference observed in ganoderic acid AW2 (2) is the absence of the double bond between C-20 and C-21. Instead, a hydroxylation at C-20 (δC 75.1) is observed. The second difference relative to ganoderic acid AW1 (1) is the absence of the hydroxylation at C-22. Thus, based on our detailed analyses and comparison of all the 1D and 2D-NMR data for compound 2, with those of ganoderic acid AW1(1) and literature data this compound is a new derivative of ganoderic acid and is named ganoderic acid AW2.
In an attempt to elucidate the absolute configuration of the hydroxylation of the side chain of 1 and 2, we tried to optimize the structures using gaussian 9 software. However, structures were not stable and tended to decompose. Thus, unfortunately, we could not get the calculated ECD for absolute configuration determinations.
The new triterpenes, ganoderic acid AW1 (1) and ganoderic acid AW2 (2), were tested against the chloroquine sensitive (D6) and chloroquine resistant (W2) strains of P. falciparum. Ganoderic acid AW1 (1) showed good antimalarial activity against the D6 clone with an IC50 of 257.8 nM while the activity was almost lost against the W2 clone (Table 2). However, ganoderic acid AW2 (2) was not active against both clones, D6 and W2, suggesting that the allylic hydroxy functionality at the side chain is very critical to the antimalarial activity of these structures. Interestingly, both 1 and 2 did not show any cytotoxicity up to the concentration of 9 μM, which results in a higher therapeutic window and thus better drug-like properties.
The isolated compounds did not show any activity against a panel of pathogenic bacteria and fungi including Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, Chlamydophila pneumoniae, MRS, Escherichia coli, Pseudomonas aeruginosa, and vancomycine-resistant enterococci.
Conclusion
In conclusion, two new ganoderic acid derivatives named ganoderic acid AW1 (1) and ganoderic acid AW2 (2) were isolated from the fungal fruiting bodies of the cultivated new isolate of the Egyptian Ganoderma sp. Ganoderic acid AW1 (1) showed an interesting antimalarial activity against the chloroquine sensitive strain of P. falciparum with an IC50 value of 257.8 nM with no cytotoxicity up to the concentration of 9 μM.
References
Baby S, Johnson AJ, Govindan B (2015) Secondary metabolites from Ganoderma. Phytochemistry 114:66–101
Bharate SB, Khan SI, Yunus NAM, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP (2007) Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem 15:87–96
Chen AW (2004) Growing Ganoderma Mushroom. In: Mushroom growers handbook 1. MushWorld–Heineart Inc., Haeng-oon Bldg. 150–5 Pyungchang-dong, Jongno-gu., Seul Korea, pp 110–849
Cole RJ, Schweikert MA (2003) Handbook of secondary fungal metabolites. Academic Press, New York, p 271–430
Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ (2015) Antimalarial drug resistance: literature review and activities and findings of the ICEMR Network. Am J Trop Med Hyg 93:57–68
Eastman RT, Fidock DA (2009) Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864
El-Fallal A, ElSayed AK, El-Esseily SR (2015) First record of two Ganoderma species from North East Nile Delta Egypt. Mycosphere 6:248–259
Isaka M, Chinthanom P, Mayteeworakoon S, Laoteng K, Choowong W, Choeyklin R (2018) Lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete Ganoderma australe. Nat Prod Res 32:1044–1049
Lakornwong W, Kanokmedhakul K, Kanokmedhakul S, Kongsaeree P, Prabpai S, Sibounnavong P, Soytong K (2014) Triterpene lactones from cultures of Ganoderma sp. KM01. J Nat Prod 77:1545–1553
Ma K, Ren J, Han J, Bao L, Li L, Yao Y, Sun C, Zhou B, Liu H (2014) Ganoboninketals A–C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense Pat. J Nat Prod 77:1847–1852
Nagai T, Kiyohara H, Munakata K, Shirahata T, Sunazuka T, Harigaya Y, Yamada H (2002) Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int Immunopharmacol 2:1183–1193
Nowak R, Drozd M, Mendyk E, Lemieszek M, Krakowiak O, Kisiel W, Rzeski W, Szewczyk K (2016) A new method for the Isolation of ergosterol and peroxyergosterol as active compounds of Hygrophoropsis aurantiaca and in vitro antiproliferative activity of isolated ergosterol peroxide. Molecules 21:946
Paterson RRM (2006) Ganoderma—A therapeutic fungal biofactory. Phytochemistry 67:1985–2001
Peng X, Li L, Dong J, Lu S, Lu J, Li X, Zhou L, Qiu M (2019) Lanostane-type triterpenoids from the fruiting bodies of Ganoderma applanatum. Phytochemistry 157:103–110
Satria D, Amen Y, Niwa Y, Ashour A, Allam AE, Shimizu K (2018) Lucidumol D, a new lanostane-type triterpene from fruiting bodies of Reishi (Ganoderma lingzhi). Nat Prod Res 0:1–6
Su H-G, Zhou Q-M, Guo L, Huang Y-J, Peng C, Xiong L (2018) Lanostane triterpenoids from Ganoderma luteomarginatum and their cytotoxicity against four human cancer cell lines. Phytochemistry 156:89–95
Wang X, Peng X-R, Lu J, Hu G-L, Qiu M-H (2018) New dammarane triterpenoids, caffruones A–D, from the cherries of Coffea arabica. Nat Prod Bioprospect 8:413–418
White NJ (2004) Antimalarial drug resistance. J Clin Investig 113:1084–1092
Xia Q, Zhang H, Sun X, Zhao H, Wu L, Zhu D, Yang G, Shao Y, Zhang X, Mao X, Zhang L, She G (2014) A Comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 19:17478–17535
Yan H-J, Wang J-S, Kong L-Y (2014) Cytotoxic dammarane-type triterpenoids from the stem bark of Dysoxylum binecteriferum. J Nat Prod 77:234–242
Acknowledgements
We would like to thank Dr Shabana Khan, The National Center for Natural Products Research, The University of Mississippi for the antimalarial and antibacterial activities. We also would like to thank Dr Neil Kelleher, Chemistry Department, Northwestern University, Evanston for the generous NMR analyses. We would like to thank Dr. Mohamed Helal and Dr. Khaled ElOkely for thier help with ECD calculations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wahba, A.E., El-Sayed, A.K.A., El-Falal, A.A. et al. New antimalarial lanostane triterpenes from a new isolate of Egyptian Ganoderma species. Med Chem Res 28, 2246–2251 (2019). https://doi.org/10.1007/s00044-019-02450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02450-1