Abstract
Reinforcement of the body with exogenous antioxidants have been shown to mitigate the negative effects of African trypanosomiasis on the host and contribute greatly to their survival. This study was therefore conducted to evaluate the effects of oral administration of Vitamin E on the early stage of Trypanosoma brucei brucei infection. To achieve this, parasite free healthy rats were acclimatized for 2 weeks before they were divided into three groups. Two of the groups were infected by intraperitoneal inoculation of 1 × 104 parasites/rat and monitored for the presence of Trypanosoma brucei brucei. Blood samples were collected from the infected rats from the second day post infection to detect the presence of parasites. Vitamin E treatment started day 4 post infection at the onset of parasitaemia. Parasites were monitored till the end of the study. The blood glucose level was determined using a glucometer; the lipid profile, liver and kidney biomarkers, electrolytes and protein were determined by colorimetric method using commercial kits. Haematological parameters were analysed using a sysmex haematology analyser. The results of this study showed that the infection adversely affected the biomarkers examined showing its negative effect on liver, kidney, haematological parameters and host electrolyte balance. Treatments with Vitamin E was however able to mitigate the negative effect of this infection. In conclusion, the treatment was able to ameliorate the anaemia and organ damage caused by Trypanosoma brucei brucei, extend the life span of the treated rats and greatly delay the time taken to get to the second stage of the infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
African trypanosomiasis associated with Trypanosoma brucei species is one of the major factors responsible for rural underdevelopment in many sub-Saharan African countries (Franco et al. 2018). The disease affects both human and animals with pronounce negative effect on human settlement and income-generating ability of the communities where people depend mostly on hunting, livestock rearing, farming and fishing (Tesfaye et al. 2012). This disease has also become a threat to food security in sub-Saharan Africa due to vast arable land that are rendered uncultivable (Gaithuma et al. 2019). Human African trypanosomiasis and Animal African trypanosomiasis have been linked to socio-economic burden of the affected countries where it is estimated to result in an annual economic loss of about $5 billion (Stijlemans et al. 2017; Simarro et al. 2012).
In both animals and humans, African trypanosomiasis is characterised with fever, anaemia, headache, generalized weakness, severe pruritus with scratching, skin lesions, mobile or rubbery lymphadenopathies, tenderness, oedema, malaise, weight loss, musculoskeletal pains and invasion of organs such as the brain, liver, adipose tissue, the skin and organs of the nervous system (Malvy and Chappuis 2011; Trindade et al. 2016; Capewell et al. 2014). The presence of the parasites in these organs as a result of invasion has been shown to be responsible for relapse infection of the successfully treated patients even without further exposure to tsetse fly (Trindade et al. 2016; Capewell et al. 2014).
Generation of free radicals and reactive oxygen species (ROS) by the parasite and the activities of macrophage which result in degenerative changes in cells, tissues and organs of infected animals are among the factors associated with the pathogenesis of African Trypanosomiasis (Edoga et al. 2013). The reactive oxygen species (ROS) and the free radicals generated attack the membrane lipids and proteins resulting in their destruction and exhaustion of endogenous antioxidants that provide protection for cellular macromolecules (Kobo et al. 2013). Trypanosomiasis also results in immunosuppression due to the ability of the parasite to modulate the host immune system. This in turn affects the host ability to eliminate the parasite (Akazue et al. 2019; Cnops et al 2015; Stijlemans et al. 2017).
To attain the goal of eradicating sleeping sickness as disease of public health problem by 2030 (Franco et al. 2018), stimulation of the immune response against the trypanosome may play a crucial role in attaining this goal (Akazue et al. 2019; Bouteille and Buguet 2012; Stijlemans et al. 2017). It is therefore conceivable that treatment with antioxidants such as Vitamin E which possesses immunostimulatory and antioxidant properties will improve the host immune system, boost the endogenous antioxidants, prevent oxidative damage, reverse the pathologic processes, reduce mortality and the severity of trypanosome infections (Tabel et al. 2008; Umar et al. 2008).
Materials and methods
Experimental animals
Male albino rats (Rattus Norvegious) weighing between 145 and 150 g were used in the study. They were purchased from Nigerian Institute of Trypanosomiasis Research, Vom, Plateau State, Nigeria. They were kept in clean rat cages and housed in well ventilated, fly proof experimental animal house in Bingham University Karu Nasarawa State, Nigeria. Animals were humanely cared for in compliance with the principles of laboratory Animal care as stated by Bingham University. They were fed with pelletized grower feed (Vital Feed, Jos, Nigeria) and water was available ad libitum throughout the period of study. Excess feed and water were evacuated twice daily and replaced with fresh one to avoid contamination and infection.
Parasite strain
Trypanosoma brucei brucei (Federe strain) were obtained from the Nigerian Institute of Trypanosomiasis Research, Kaduna, Kaduna state, Nigeria and maintained in the laboratory by serial blood passage in rats until when required.
Animal inoculation and determination of parasitaemia
The animals were acclimatized for two weeks before inoculation. They were infected by intraperitoneal inoculation of 1 × 104 in 0.3 ml normal saline. Parasitemia was monitored daily from the second day after the infection by bleeding the tail of each rat. To do this, about one drop of blood was collected on the slide and covered with a cover slide and then observed under × 40 magnification of a phase contrast microscope. Fields were examined and the mobile parasites counted. Trypanosomes were estimated by matching the density of parasites observed in a microscopic field with a standard (Herbert and Lunsden 1976).
Treament with Vitamin E
Treatment with Vitamin E started as soon as the parasitaemia was confirmed. The Vitamin E was administered daily to the rats orally at the dose of 300 µg/kg body weight (Adebayo et al. 2019). The treatment continued until the Vitamin E treated group stared exhibiting the symptoms of the second phase of the disease before the experiment was terminated.
Experimental design/grouping
The rats were grouped into three as shown below:
-
Group 1: Not infected with T. brucei brucei (Federe strain) and not treated with Vitamin E.
-
Group 2: Infected with T. brucei brucei (Federe strain) only and not treated with Vitamin E.
-
Groups 3: Infected with T. brucei brucei (Federe strain) and then given 300 µg/kg body weight of Vitamin E orally from the day parasitemia was confirmed till the end of the experiment when the symptoms of secon stage were observed.
Analysis of the samples
Haematological parameters were determined using the automated haematologic analyzer (Sysmex, KX-21, Japan). Other parameters were determined using commercial kits: Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Creatinine, Uric acid, Bilirubin and urea Kits were obtained from Randox; Inorganic phosphate, Sodium ion, Chloride ions and Total protein kit from Diagnostic Kit, Albumin kit from Agape Laboratories, Switzerland. High density lipoprotein, Triglycerides, Total cholesterol, Gamma Glutamyl Transferase (GGT) were obtained from VITRO SCIENT, Germany. Low density lipoprotein, Potassium ion and Carbon Dioxide kit, were obtained from Spectrum Laboratories, Germany. Blood glucose was determine using glucometer. All these kits were obtained from the retailers of these companies products in Abuja, Nigeria. The assays were performed as described by the manufacturers.
Result
In this study, generalised decrease in the packed cell volume (PCV) was observed on day 4 post infection at the onset of parasitaemia. Treatment with Vitamin E resulted in gradual improvement and restoration of the PCV of the treated rats while continuous decline was observed in the untreated rats (Table 1). Significant decrease in red blood cells and haemoglobin were also observed in the infected rats compared to the control group. Just like the PCV, treatment with Vitamin E resulted in increase in the red blood cells and haemoglobin concentrations (Table 1). There was an initial increase in white blood cells of all the rats but a sharp decrease was eventually recorded in the infected group that were not treated but increase was observed in the Vitamin E treated group till the end of the study (Table 1). Increase in lymphocytes were observed in all the infected animals irrespective of whether they were treated or not (Table 1).
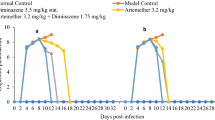
Post infection treatment with Vitamin E was observed to greatly suppress the multiplication of the parasite in the rats treated with Vitamin E. These treated rats exhibited a slower outgrowth of parasites and lower parasitemia than the infected controls (Fig. 1). The magnitude of the parasitemia was also lower in the treated group compare to the untreated group. Both groups also reached the parasitemia peak at the different time (Fig. 1). The treated group were able to control and eliminate the parasite better than the untreated group (Fig. 1). After the second parasitemia wave, there was a decrease in the parasitaemia in the treated group. However, the untreated group was not able to survive the second parasitemia wave as they all died on day 15 (Fig. 1). In general, it was obvious that treatment with Vitamin E was able confer an advantage on the treated group which makes them to live longer and prolong the period taken to get to the second phase of the infection (Fig. 1).
T. brucei brucei infection resulted in decrease in blood glucose (Table 2). The decrease in blood glucose was however, more pronounced in the infected untreated group compared to the Vitamin E treated group throughout the study period (Table 2). When the last blood glucose was taken in the untreated group, the glucose level has dropped to half of the initially blood glucose level before infection (Table 2). Even though rats treated with Vitamin E were associated with decrease in blood glucose level, the decrease was not as significant as that of the untreated group (Table 2). The result also showed that the Vitamin E treated group started recovering and displayed improvement in the blood glucose level. The initial glucose level was almost restored with the treatment before the rats started exhibiting the second stage symptoms. The consistent blood glucose level of the uninfected control group confirmed that the infection was actually responsible for the decrease in blood glucose in the infected groups. It also confirmed that Vitamin E was responsible for the restoration of the blood glucose level which was observed in the treated group (Table 2).
From the results of this study, T. brucei brucei infection resulted in significant decrease (P < 0.05) in serum cholesterol, triglycerides, high density lipoprotein (HDL) and insignificant increase in low density lipoprotein (LDL) (Table 3). This disturbance in the lipid profiles of all the infected rats could be seen when the untreated infected group was compare to the control (Table 3). This showed that the infection was responsible for the disturbance. Treatment with Vitamin E resulted in improvement of the lipid profile examined (Table 3). It was observed that serum total cholesterol, serum triglycerides, high density lipoprotein (HDL) and low density lipoprotein (LDL) were restored with the Vitamin E treatment (Table 3). They were all significantly higher (P < 0.05) than infected untreated group. Comparison of the total serum cholesterol and high density lipoprotein (HDL) of the Vitamin E treated group and the control showed no significant difference (P < 0.05). However, serum triglycerides and low density lipoprotein (LDL) of the treated group were significantly (P < 0.05) higher than the control (Table 3).
T. brucei brucei infection induced significant increase (P < 0.05) in the serum concentration of hepatic biomarkers (AST, ALT, ALP and GGT) assessed compare to the uninfected control group except bilirubin (Table 4). The increase in the serum concentration of these hepatic biomarkers may be as a result of the damage to the liver by the trypanosomes which probably resulted in the leakage of these biomarkers from the liver cells cytosol into bloodstream. However, treatment with Vitamin E resulted in significant reduction (P < 0.05) in GGT, ALT, AST, ALP and Bilirubin compare to the infected group that was not treated with Vitamin E (Table 4). When Vitamin E treated group was compared with the uninfected control group, there was a significant reduction (P < 0.05) in the serum concentration of GGT and bilirubin but significant increase in AST and ALP. ALT was the only liver biomarker examined that was not significantly different from the uninfected control group at the end of the study (Table 4).
Significant increase (P < 0.05) in serum total protein was observed in the infected rats compare to the control (Table 5). However, Vitamin E treatment resulted in significant decrease in the serum total protein compare to the infected group that were not treated. Significant difference was also observed between the control and the treated group (Table 5). On the other hand, the infection resulted in significant decrease (P < 0.05) in serum albumin but treatment with Vitamin E resulted in increase in albumin concentration compare with the untreated group which showed a severe decrease in the serum albumin (Table 5). The significant difference between the serum albumin of the control group and the untreated infected group showed that the infection was responsible for the loss in serum albumin observed in the infected group and the gradual restoration of serum protein and albumin may be due to Vitamin E treatment (Table 5).
Significant increase in the serum concentrations of all the kidney biomarkers examined were observed after T. brucei brucei infection in all the infected rat compare with the control group (Table 6). Treatment with the Vitamin E however, resulted in rapid decrease in the serum concentration of the kidney biomarkers examined (Table 6). At the end of the study, there was no significant difference between the control and the Vitamin E treated group. The increase in the serum concentration of the kidney biomarkers examined were however significant between the untreated and the Vitamin E treated group (Table 6). This clearly showed that the Vitamin E was responsible for the difference in the serum levels of the kidney biomarkers examined.
T. brucei brucei infection resulted in significant decrease in serum bicarbonate, inorganic phosphate, chloride, potassium and sodium compared to the uninfected control (Table 7). Treatment with Vitamin E, however, resulted in improvement in the serum bicarbonate, inorganic phosphate, chloride, potassium and sodium (Table 7). That is, generalised decrease in the serum electrolytes were reversed by the treatment. This is supported by significant increase observed in the Vitamin E treated group compare to the untreated group. This showed that Vitamin E was able to reverse the negative effect of the infection on serum electrolytes (Table 7).
Discussion
The packed cell volume (PCV), haemoglobin levels and red blood cell (RBC) count reduced significantly with the onset of parasitaemia in the infected groups compared to uninfected rats. The initial increase observed in white blood cells and lymphocytes was not sustained as they both fell as the disease progresses. These observations indicates the characteristics of anaemia and immunosuppression which are the main features of African Trypanosomiasis (Adieme et al. 2014; Ekanem and Yusuf 2008; Akanji et al. 2009; Ukpai and Nwabuko 2014; Longdet et al. 2014; Samuel et al. 2016). The trypanosome induced anaemia and immunosuppression have been linked to compromise in host immune capability and oxidative stress induced by trypanosome and macrophageal activities (Taylor and Authie 2004; Mbaya et al. 2009; Eze et al. 2013). The induced oxidative stress resulted in production of large amounts of peroxides and free radicals which depletes the endogenous antioxidant reserves of the animals. This deprived the cells of the antioxidant protection and predisposed the membrane lipids and proteins to oxidation resulting in their destruction. This is probably the basis for the observed oxidative haemolysis of the erythrocytes and hence the anaemia (Eze et al. 2013; Adamu et al. 2015). The free radicals have also been linked to the immunosuppression in the infected animals besides the well-known exhaustion of immune system by the ever changing variable surface glycoprotein of the trypanosomes (Abubakar et al. 2005). Treatment of the infected animals with Vitamin E, however, resulted in the improvement of the haematological parameters examined. This is an indication of recovery from the anaemia induced by the T. brucei brucei infection (Oparah et al. 2017). The recovery may be attributable to the antioxidant activity of Vitamin E which resulted in the scavenging of the trypanosomes and macrophageal -generated free radicals. The scavenging of free radicals by vitamin E might have protected the cell membranes from oxidation by stabilizing and maintaining their selective permeability (Herrera and Barbas 2001; Traber and Atkinson 2007). The increase in white blood cells in the treated group also indicates improved immunological response in Vitamin E treated rats. This probably enhanced their ability to fight the invading parasites and eliminate the free radicals that are responsible for anaemia and immunosuppression (Eze et al. 2013; Adeyemi and Sulaiman 2012; Ekanem and Yusuf 2008; Abubakar et al. 2005; Umar et al. 2001a, b).
Vitamin E treated rats were less susceptible to T. brucei brucei infection compare to the untreated group. This may be linked to the enhanced host ability to eliminate the parasite from circulation as well as altering specific factors on the parasite that help in its resistance to host immune system. (Gjini, Haydon, Barry and Cobbold 2010). This resulted in a partial protection against the disease in comparison with the untreated group at the early stage of the disease. It also increase the period taken by the animal to get to the second stage of the disease.
The onset of parasitaemia on day 4 post infection led to a reduction in the blood glucose in the infected groups compare to the uninfected group, which is similar to the results reported by Abuessailla et al. (2017); Sazmand et al. (2011) and Takeet and Fagbemi (2009). This reduction may be due to the parasites’ need for glucose for their cellular metabolism since the feeding rate of the animal did not diminish at the initial stage of the disease (Opperdoes et al. 1986). This hypoglycaemia in T. brucei brucei infection has been linked to the fact, that the only source of ATP for replicating bloodstream T. brucei brucei is the catabolism of glucose through glycolysis (Smith et al. 2017; Creek et al. 2015; Mazet et al. 2013). In fact, it has been observed in some studies that the rate of glucose utilisation in mammal by T. brucei brucei is about 50-fold higher than that of mammalian tissues (Mazet et al. 2013; Nwagwu and Opperdoes 1982). This may be responsible for the hypoglycaemia observed in this study(Abubakar et al. 2016; Mishra et al. 2017). Bloodstream form (BSF) of the parasite isolated from the mammal are known to die within few minutes of glucose starvation which shows the importance of glucose to their survival and the establishment of infection (Seyfang and Duszenko 1991). The hypoglycaemia observed in this study may not be ascribed to increased metabolic rate in the host as proposed by Takeet and Fagbemi (2009). This is because liver and kidney biomarkers examined in this work clearly showed compromise of liver and kidney functions. However, treatment with Vitamin E resulted in improved blood glucose compare to the untreated infected group. This can be linked to the ability of Vitamin E to boost the immune system and therefore improve the capacity of the host to clear the parasite. The reduce parasite load in the circulation make more glucose available to the host (Abubakar et al. 2016; Mishra et al. 2017). On the other hand, the glucose level of the untreated group continues to fall until they started showing signs of the second stage of the disease they were sacrificed. The treatment with Vitamin E however extended the period before the symptom of the second was observed to over 40 days before the research was terminated.
T. brucei brucei infection resulted in significant decrease in serum cholesterol, triglycerides, high density lipoprotein(HDL) and low density lipoprotein (LDL) which is in conformity with the findings of Adamu et al. (2008), Bala et al. (2012), Adam et al. (2009) and Abdulazeez et al. (2013). The significant decrease in serum lipids observed in this study may be due to continuous utilization of host lipid molecules by the trypanosomes which eventually resulted in decreased serum lipids and cholesterol (Abdulazeez et al. 2013). The host lipid has been shown to serve as an important source of energy for T. brucei brucei (Van Hellemond and Tielens 2006; Abdulazeez et al. 2013) and play significant roles in its ability to establish infection in animals (Van Hellemond and Tielens 2006; Abdulazeez et al. 2013). Hypoglycemia observed in this study might have also resulted in increased catabolism of lipids molecules to meet the energy demand of the host (Van Hellemond and Tielens 2006; Abdulazeez et al. 2013). Imbalance between radical-generating and radical scavenging activity of the host as a result of altered host’s antioxidant defence by the trypanosomes (Igbokwe et al. 1996; Umar et al. 2007) might have also resulted in increased formation of oxidants (Saleh et al. 2009; Ogunsanmi and Taiwo 2001) which can destroy Lipid (Mishra et al. 2017; Igbokwe 1994; Ogunsanmi and Taiwo 2001). However, treatment with Vitamin E resulted in improved serum lipid profile compare to the untreated group. This may be due to its ability to degrade peroxides and free radicals responsible for the lipid peroxidation by promoting the host radical-scavenging activity which prevent them from peroxidative damage by inhibiting and destroying endogenous peroxides (Umar et al. 1999a, b).
T. brucei brucei infection resulted in significant increase in serum AST, ALT ALP, GGT and bilirubin compare to the control group. This may be due to hepatocellular injury or increase red blood cells haemolysis by the trypanosomes. Similar increase in AST and ALT activities during Trypanosoma brucei brucei infection have been reported by Adah et al. (1992), Kaneko et al. (2008) and Allam et al. (2011), Yusuf et al, (2012) and Umar et al (2000a, b) in different animals. The increase in total serum bilirubin in infected rats in this study further indicates liver damage by the trypanosomes (Adeyemi and Sulaiman 2012). This may be due to the inability of the liver to conjugate bilirubin (Kapoor 2011; Adeyemi and Sulaiman 2012; Abdulazeez et al. 2013). Increased serum activities of ALP and GGT compare with the uninfected control group is a further confirmation of active hepatocellular damage by the parasites since corresponding increase in activities were also observed for serum ALT and AST. This showed that T. brucei brucei infection resulted in the alteration of membrane permeability; which resulted in cell destruction leading to the release of these enzymes into the blood which further support the earlier findings by Ekanem and Yusuf (2008) and Ngure et al. (2008) that T. brucei brucei infection result in tissue and organ damage. Tissue and organ damage in African Trypanosomiasis has been linked to attack on the cells and cellular macromolecules by the free radicals generated by the trypanosomes (Vray et al. 1991). The trypanosomes are also known to invade vital organs in the body such as the brain, liver and kidney (Akpa et al. 2008). The presence of the parasites in these vital organs can result in the destruction of their cells. This will result in the release of their cellular contents especially the enzymes and biomarkers specific to the organ into the bloodstream resulting in their elevation (Ngure et al. 2008). Treatment with Vitamin E reduced the liver biomarker in the serum significantly which suggests that the Vitamin E protected the liver against the free radicals generated during the disease. This may be due to its ability to protect the plasma membrane and other cellular target of the oxidative agents (Abdulazeez et al. 2013) and enhance the ability of the animal to clear the parasite from the host body(Umar et al. 1999b, 2008).
The disease resulted in a significant increase in the serum total protein of the infected group before the treatment commenced compared to that of the uninfected control. The same result was also observed by Orhue et al. (2005), Ekanem and Yusuf (2008) and Sow et al. (2014) in rats and rabbit infected with T. brucei brucei. The increase in serum protein observed in this study may be due to release of tissue and organ protein arising from destruction of their cells by the parasites resulting in the release of their cellular content especially protein into the circulation (Orhue et al. 2005; Ngure et al. 2008; Sulaiman et al. 2012). Haemolysis of erythrocytes which is the main feature of T. brucei infection might have also contributed to the increase serum protein (Igbokwe and Muhammed 1992; Omotainse and Anosa 1995). Although, the increased serum protein might have resulted from the destruction of parasites by the host immune system or be from mass of parasite proteins as a result of increased parasitaemia (Sulaiman et al. 2012). There was a significant decrease in serum albumin of the infected group, this agreed with the reports of Arora and Pathok (1995) and Samia et al. (2004) who discovered a decrease in serum albumin of infected rats and Hussain et al. 2016 who observed the same in camels. The decrease in serum albumin may be as a result of decreased hepatic biosynthesis (Eze et al. 2013) or hepatocellular damage (Eze et al. 2013; Bruijn et al. 1987; Mishra et al. 2017). Utilisation of albumin-bound fatty acids and lipoproteins (Vickerman and Tetley 1979) might have also contributed to the decrease in plasma albumin concentrations observed. Treatment of the infected rats with Vitamin E resulted in decrease in total serum protein and increase in serum albumin. This is in agreement with the finding of Umar et al. (2001a, b) who reported previously that supplementation with Vitamin E alleviate the organ damage associated with trypanosomiasis probably due to initiation of the immune response and synthesis of immunoglobulins to clear the parasites out of circulation and minimise their negative effects (Abuessailla et al. 2017; Katunguka-Rwakishaya et al. 1999).
The increase in creatinine observed in this study may be due to invasion of the skeletal muscle by the parasites resulting in muscle degeneration or destruction of kidney cells which might have impaired the ability of the kidneys to dispose the creatinine. This result was also observed by Ezeokonkwo et al. (2012) and Abdulazeez et al. (2013). These changes especially in the kidneys have been ascribed to the toxins produced by the parasite and other metabolites produced by the host in response to the invading parasites such as oxygen radicals and peroxides (Morrison et al. 1981) which are dangerous cellular toxins that can damage connective tissue, destroy biological membranes, inactivate enzymes and cause damage of nucleic acids (Ogunsanmi and Taiwo 2001; Saleh et al. 2009). The decrease in serum urea, Uric acid and creatinine when the rats were given vitamin E showed its ability to protect the kidneys during the disease. This was also observed by Umar et al. (1999a, b). This effect could be as a result of its ability to act as an antioxidant and degrades free radicals and peroxides produced during this infection (Gutteridge 1995). This greatly reduce the imbalance between the radical-generating and radical-scavenging activity during the disease and therefore greatly reduced extent of tissues and organs degeneration. Enhanced ability of the body to eliminate the parasites might have have also contributed to the result obtained (Gutteridge 1995; Ismaila et al. 2000; Umar et al. 2008).
T. brucei brucei infection resulted in generalised decrease in the serum electrolyte examined which is contrary to the observation of Karaye et al. (2017) in Trypanosoma congolense and Trypanosoma brucei infected red Sokoto bucks. This however agrees with physiological observation that increases in plasma Na+ concentration is always accompanied with fall in K+ ions. Decrease in Cl− in the infected rats in this study agrees with observation of Sackey (2011) in T. brucei and T. congolense infected Savannah Brown goat but contrast the observation of Fiennes et al. (1946). It does follow the trend that decrease in Cl− concentration always result in decrease in Na + concentration as observed by Karaye et al. (2017). The decrease in HCO−3 concentration in this study agrees with Karaye et al. (2017) but contrast the observation of Ogunsanmi et al. (1994) in infected sheep. The disproportionate changes in the concentration of chloride ion with respect to sodium; inorganic phosphate and bicarbonate ions is significant enough to be inferred that Trypanosoma brucei brucei can result in an acid–base disturbance or metabolic acidosis (Oparah et al. 2017). Treatment with Vitamin E however restored most of these electrolyte almost to the concentration observed in the control.
Conclusion
In conclusion, it was observed that treatment with Vitamin E was able to reduce the parasitaemia, reduced the severity of anaemia associated with T. brucei brucei infection and enhanced immune system. This was evident in the improved packed cell volume, reduced parasites and increase in the white blood cells of the Vitamin E treated group compared to untreated infected group. Treatment with Vitamin E also improved the blood glucose concentration probably due to the enhance immune capability to eliminate the trypanosomes which resulted in reduction in the number of parasites consuming the glucose. The reduction in excessive consumption of host glucose by T. brucei brucei, made more glucose available to the host thereby preventing excessive catabolism of lipids for energy needs which resulted in the restoration of the host serum lipids. The treatment with Vitamin E also corrected the acid–base disturbance and metabolic acidosis induced by the infection by restoring the serum electrolytes. Finally, the treatment was able to ameliorate organ damage caused by Trypanosoma brucei brucei, extend the life span of the treated rats and greatly delay the time taken to get to the second stage of the infection. It can therefore be suggested that vitamin E could be a useful agent in management of Trypanosomiasis.
References
Abdulazeez MA, Ibrahim AB, Edibo ZY, Sidali JO, Idris HO (2013) Anti-trypanosomal effect of Peristrophe bicalyculataextract on Trypanosoma brucei brucei-infected rats. Asian Pac J Trop Biomed 3(7):523–531
Abubakar A, Iliyasu B, Yusuf AB, Igweh AC, Onyekwelu NA, Shamaki BA, Afolayan DO, Ogbadoyi EO (2005) Antitrypanosomal and haematological effects of selected Nigerian medicinal plants in Wistar rats. Biokemistri 17(2):95–99
Abubakar AS, Onaolapo MH, Abdullahi YA, Kabir SS, Danladi O (2016) The metabolism of African trypanosomes in relation to pathogenic mechanisms: a review. J Pharm Biol Sci 11(2):68–72
Abuessailla A, Ismail AA, Agab H, Shuaib YA (2017) Serum biochemical and histopathological changes in rats experimentally infected with trypanosoma evansi isolated from dromedary camels in Sudan. Int J Life Sci Sci Res 3(3):1075–1084. https://doi.org/10.21276/ijlssr.2017.3.3.19
Adah MI, Otesile EB, Joshua RA (1992) Changes in levels of transaminases in goats experimentally infected with Trypanosoma congolense. Revue Elev Med Vet Pays Trop 45:284–286
Adam S, Barde N, Abenga JN, Useh NM, Ibrahim NDG, Esievo KAN (2009) Experimental Trypanosoma brucei infection-induced changes in the serum profiles of lipids and cholesterol and the clinical implications in pigs. J Cell Anim Biol 3(2):15–20
Adamu S, Ige AA, Jatau ID, Neils JS, Useh NM, Bisalla M et al (2008) Changes in the serum profiles of lipids and cholesterol in sheep experimental model of acute African trypanosomosis. Afr J Biotechnol 7(12):2090–2098
Adamu O, Haruna MK, Ovbagbedia RP, Uzowuru M, Eyitope MG, Anichebe F (2015) Effects of immunace and iron dextran on anemia and immunosuppression of T. Brucei infected rats. Int. J. Curr. Res. Chem. Pharm. Sci. 2(8):1–6
Adebayo AO, Ibitoroko GOM, Emechete EM (2019) Effect of vitamin E on hydrogen peroxide and nitrogen levels in male wistar albinorats infected with trypanosomaBrucei Brucei. Int J Biomed Adv Res 10(01):e4934
Adeyemi OS, Sulaiman FA (2012) Biochemical and morphological changes in Trypanosoma brucei brucei-infected rats treated with homidium chloride and diminazene aceturate. J Basic Clin Physio Pharm 23(4):179–183
Adieme IC, Ezeh IO, Ugochukwu EI, Romanus CE (2014) Effect of diminazene aceturate, levamisole and vitamin C combination therapy in rats experimentally infected with Trypanosoma brucei brucei. Asian Pac J Trop Med 7:438–445
Akanji MA, Adeyemi OS, Oguntoye SO, Sulyman F (2009) Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J 8:148–154
Akazue PI, Ebiloma GU, Ajibola O, Isaac C, Onyekwelu K, Ezeh CO, Eze AA (2019) Sustainable elimination (Zero Cases) of sleeping sickness: how far are we from achieving this goal? Pathogens 8(135):1–18
Akpa PO, Ezeokonkwo RC, Eze CA, Anene BM (2008) Comparative efficacy assessment of pentamidine isethionate and diminazene aceturate in the chemotherapy of Trypanosoma brucei brucei infection in dogs. Vet Parasitol 151:139–149
Allam L, Ogwu D, Agbede RIS, Sackey Allam AKB, Ogwu LD, Agbede RIS, Sackey AKB (2011) Hematological and serum biochemical changes in gilts experimentally infected with Trypanosoma brucei. J Vet Arhiv 81(5):597–609
Arora JK, Pathok KML (1995) Clinico-haematological and biochemical changes associated with T. evansi infection in dogs. Indian J Anim Health 34(1):33–38
Bala AY, Adamu T, Abubakar U, Ladan MJ (2012) Amelioration of trypanosome-infection-induced alterations in serum cholesterol, triglycerides and proteins by hydro-ethanolic extract of Waltheria indica in rats. Res J Parasitol 6:127–135
Bouteille B, Buguet A (2012) The detection and treatment of human African trypanosomiasis. Res Rep Trop Med 3:35–45
Bruijn JA, Oemar BS, Ehrich JH, Foidart JM, Fleuren GJ (1987) Anti-basement membrane glomerulopathy in experimental trypanosomiasis. J Immunol 139(7):2482–2488
Capewell P, Cooper A, Clucas C, Weir W, Macleod A (2014) A co-evolutionary arms race: trypanosomes shaping the human genome, humans shaping the trypanosome genome. Parasitology 142(S1):1-12. https://doi.org/10.1017/S0031182014000602
Cnops J, Magez S, De Trez C (2015) Escape mechanisms of African trypanosomes: why trypanosomosis is keeping us awake. Parasitology 142(3):417–427
Creek DJ, Mazet M, Achcar F, Anderson J, Kim DH, Kamour R, Morand P, Millerioux Y, Biran M, Kerkhoven EJ, Chokkathukalam A (2015) Probing the metabolic network in bloodstream-form Trypanosoma brucei using untargeted metabolomics with stable isotope labelled glucose. PLoS Pathog 11(3):e1004689
Edoga CO, Njoku OO, Okeke JJ, Ani CE (2013) Effect of vitamin C treatment on serum protein, albumin, beta globulin profiles and body weight of Trypanosoma Brucei-infected Rattus Norvegicus. Anim Res Int 10(1):1685–1688
Ekanem JT, Yusuf OK (2008) Some biochemical and haematological effects of black seed (Nigella sativa) oil on T. brucei-infected rats. Afr J Biomed Res 11:79–85
Eze JI, Okeke MC, Ngene AA, Omeje JN, Abonyi FO (2013) Effects of dietary selenium supplementation on parasitemia, anemia and serum proteins of Trypanosoma brucei brucei infected rats. Exp Parasitol 135:331–336
Ezeokonkwo RC, Ezeh IO, Onunkwo JI, Onyenwe IW, Iheagwam CN, Agu WE (2012) Comparative serum biochemical changes in mongrel dogs following single and mixed infections of Trypanosoma congolense and Trypanosoma brucei brucei. Vet Parasitol 190(1–2):56–61
Fiennes RTW, Jones ER, Laws SG (1946) The course and pathology of T. congolense (brooden) disease of cattle. J Comp Pathol 56:1–27
Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, Simarro PP, Zhao W, Argaw D (2018) Monitoring the elimination of human African trypanosomiasis: update to 2016. PLOS Neglect Trop Dis 12(12):e0006890
Gaithuma AK, Yamagishi J, Martinelli A, Hayashida K, Kawai N, Marsela M, Sugimoto C (2019) A single test approach for accurate and sensitive detection and taxonomic characterization of Trypanosomes by comprehensive analysis of internal transcribed spacer 1 amplicons. PLoS Neglect Trop Dis 13(2):e0006842
Gjini E, Haydon DT, Barry JD, Cobbold CA (2010) Critical interplay between parasitesdifferentiation, host immunity, and antigenic variation in trypanosome infections. Am Nat 176(4):424–439
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41:1819–1828
Herrera E, Barbas C (2001) Vitamin E, action, metabolism and perspectives. J Physiol Biochem 57:43–56
Herbert WJ, Lumsden WH (1976) Trypanasoma brucei: a rapid matching method for estimating the host's parasitaemia. Exper Parasitol 40:427–431
Hussain R, Khan A, Abbas RZ, Ghaffar A, Abbas G, Rahman T, Ali F (2016) Clinico-hematological and biochemical studies on naturally infected camels with trypanosomiasis. Pak J Zool Soc 48(2):311–316
Igbokwe IO (1994) Mechanisms of cellular injury in African trypanosomiasis. Vet Bull 64:611–620
Igbokwe IO, Muhammed A (1992) Some plasma biochemical changes in experimental Trypanosoma brucei infection in Sokoto red goats. Rev Elev Med Pays Trop 45(3–40):287–290
Igbokwe IO, Umar IA, Omage JJ, Ibrahim NDG, Kadima KB, Obagaiye OK, Saror DI, Esievo KAN (1996) Effect of acute Trypanosoma vivax infection on cattle erythrocyte glutathione and susceptibility to in vitro peroxidation. Vet Parasitol 63:215–224
Ismaila AU, Zipporah AT, Funnilayo II, Lawan AG, BB, (2000) The role of vitamin C administration in alleviation of organ damage in rats infected with Trypanosoma brucei. Clin Biochem Nutr 28:1–7
Kaneko JJ, Harvey JW, Bruss LM (2008) Clinical biochemistry of domestic animals, 6th edn. Elsevier, Hoboken, pp 854–858
Kapoor S (2011) Hepascore: a new liver function test. J Clin Diagnos Res 5(1):155–156
Karaye GP, Sackey AKB, Tekdek LB, Lawan IA (2017) Biochemical response of red sokoto bucks experimentally infected with Trypanosoma congolense and Trypanosoma bruceitreatment and relapse. Saudi J Biomed Res 2(4):86–90
Katunguka-Rwakishaya E, Murray M, Holmes PH (1999) The influence of energy intake on some blood biochemical parameters in Scottish Blackface sheep infected with Trypanosoma congolense. Vet Parasitol 84:1–11
Kobo PI, Ayo JO, Tagang Aluwong T, Zezi AU, Maikai VA (2013) Haematological changes in Trypanosoma brucei brucei infected wistar rats treated with a flavonoid mixture and/or diminazene aceturate. Biol Med J 6:213. https://doi.org/10.4172/0974-8369.1000213
Longdet IY, Achemu HU, Okanlawon CN (2014) Potentials of methanolic extract of N. latifolia stem bark against T. congolense infection in experimental rats. J Agric Sci Policy Res 4:29–41
Malvy D, Chappuis F (2011) Sleeping sickness. Clin Microbiol Infect 17:986–995
Mazet M, Morand P, Biran M, Bouyssou G, Courtois P, Daulouède S, Millerioux Y, Franconi JM, Vincendeau P, Moreau P, Bringaud F (2013) Revisiting the central metabolism of the blood stream forms of Trypanosoma brucei: Production of acetate in the mitochondrion Is essential for parasiteviability. PLoS Neglect Trop Dis 7(12):e2587
Mbaya AW, Aliyu MM, Nwosu CO, Egbe-Nwiyi T (2009) The relationship between parasitaemia and anaemia in concurrent Trypanosoma brucei and Haemonchus contortus infections in red fronted gazelles (Gazella rufifrons). Vet Arhiv 79(5):451–460
Mishra RR, Senapati SK, Sahoo SC, Das MR, Sahoo G, Patra RC (2017) Trypanosomiasis induced oxidative stress and hemato-biochemical alteration in cattle. J Entomol Zool Stud 5(6):721–727
Morrison WI, Murray M, Sayer PD, Preston JM (1981) The pathogenesis of experimentally induced Trypanosoma brucei infection in dog. Am J Pathol 102:182–194
Ngure RM, Ndungu JM, Ngotho JM, Nancy MK, Maathai RG, Gateri LM (2008) Biochemical changes in the plasma of vervet monkeys (Chlorocebus aethiops) experimentally infected with Trypanosoma brucei rhodesiense. J Cell Anim Biol 2(7):150–157
Nwagwu M, Opperdoes FR (1982) Regulation of glycolysis in Trypanosoma brucei: hexokinase and phosphofructokinase activity. Acta Trop 39:61–72
Omotainse SO, Anosa VO (1995) Leucocyte and thrombocyte response in dogs experimentally infected with Trypanosoma brucei. Rev Elev Med Vet Pays Trop 48(3):254–258
Ogunsanmi AO, Taiwo VO (2001) Pathobiochemical mechanisms involved in the control of the disease caused by Trypanosoma congolense in African grey duiker (Sylvicapra grimmia). Vet Parasitol 96:51–63
Ogunsanmi AO, Akpavie SO, Anosa VO (1994) Serum biochemical changes in West African Dwarf sheep experimentally infected with Trypanosoma brucei. Revue d’Elevage et de medicine veterinaire des pays tropicaux 47(2):195–200
Oparah NQ, Sackey KBA, Lawal AI, Abdullahi SU (2017) Haematological indices in Trypanosoma brucei brucei (Federe isolate) infected Nigerian donkeys (Equus asinus) treated with homidium and isometamidium chloride. Mac Vet Rev 40(1):73–82. https://doi.org/10.1515/macvetrev-2017-0014
Opperdoes FR, Hart DR, Baudhain P (1986) Biogenesis of glycosome (microbodies) in the trypanosomatidae T. brucei. Eur J Cell Biol 41:30
Orhue N, Nwanze E, Okafor A (2005) Serum total protein, albumin and globulin levels in Trypanosoma brucei-infected rabbits: effect of orally administered Scoparia dulcis. Afr J Biotechnol 4:1152–1155
Sackey AKB (2011) Hematological and serum biochemical changes in gilts experimentally infected with Trypanosoma brucei. J Vet Arhiv 81(5):597–609
Saleh MA, Bassam MA, Sanousi SA (2009) Oxidative stress in blood of camels (Camelus dromedaries) naturally infected with Trypanosoma evansi. Vet Parasitol 162:192–199
Samia HA, Elmalik KH, Khalid HS, Shamat AMA, Khojali SME (2004) Biochemical changes in rats experimentally infected with T. evansi. J Anim Vet Adv 3(7):483–486
Samuel FU, Adamu S, Bisalla M, Chiezey NP, Mohammed AK, Bello TK et al (2016) Effect of T. congolense on haematological parameters in experimentally infected donkeys. J Anim Prod Res 28(1):14–24
Sazmand A, Rasooli A, Nouri M, Hamidinejat H, Hekmatimoghaddam S (2011) Serobiochemical alterations in subclinically affected dromedary camels with Trypanosoma evansi in Iran. Pak Vet J 31(3):223–226
Seyfang A, Duszenko M (1991) Specificity of glucose transport in Trypanosoma brucei. Effective inhibition by phloretin and cytochalasin B. Eur J Biochem 202:191–196
Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Fèvre EM, Mattioli RC, Jannin JG (2012) Estimating and mapping the population at risk of sleeping sickness. PLoS Neglect Trop Dis 6:e1859
Smith TK, Bringaud F, Nolan DP, Figueiredo LM (2017) Metabolic reprogramming during the Trypanosoma bruceilife cycle. F1000Research 6:683
Sow A, Sidibé I, Kalandi M, Bathily A, Ndiaye NP, Ouédraogo M, Mouiche MM, Sawadogo GJ (2014) Biochemical changes induced by natural infection of trypanosomosis in Burkinabese local donkey breeds. Comp Clin Pathol 23:103–109
Stijlemans B, Radwanska M, De Trez C, Magez S (2017) African trypanosomes undermine humoral responses and vaccine development: link with inflammatory responses? Front Immunol 8:582
Sulaiman AF, Akanji MA, Yakubu MT (2012) Effect of administration of Ibuprofen on the levels of parasitaemia, albumin and total protein concentration in rats infected with Trypanosoma bruce brucei. Afr Sci 13(1):57–63
Tabel H, Wei G, Shi M (2008) T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev 225:128–139
Takeet MI, Fagbemi BO (2009) Haematological, pathological and plasma biochemical changes in rabbits experimentally infected with Trypanosoma congolense. Sci World J 4(2)
Taylor K, Authie EM (2004) Pathogenesis of animal trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA (eds) The trypanosomiasis. CAB International, London, pp 331–353
Tesfaye D, Speybroeck N, De Deken R, Thys E (2012) Economic burden of bovine trypanosomosis in three villages of Metekel zone, northwest Ethiopia. Trop Anim Health Prod 44(4):873–879
Traber MG, Atkinson J (2007) Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43:4–15
Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, Bento F, Young SA, Pinto A, Van Den Abbeele J, Ribeiro RM (2016) Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe 19(6):837–848
Ukpai OM, Nwabuko OP (2014) Effects of Trypanosoma brucei brucei on haematological parameters and pathology of internal organs of Trypanosoma brucei brucei infected albino rats. Niger J Biotechnol 27:8–13
Umar IA, Wuro-Chekke AU, Gidado A, Igbokwe IO (1999) Effects of Combined parental vitamin C and E administration on the severity of anaemia, hepatic and renal damage in T. brucei-infected rabbits. Vet Parasitol 85:43–47
Umar IA, Toh ZA, Igbalajobi FI, Igbokwe IO, Gidado A (1999) The effect of orally administered vitamins C and E on the severity of anaemia in T. brucei-infected rats. Trop Vet 18:71–77
Umar IA, Toh ZA, Igbalajobi FI, Igbokwe IO, Gidado A (2000) The effects of orally administered vitamins C and E on severity of anaemia in Trypanosoma brucei- infected rats. Trop Vet 18:71–77
Umar IA, Toh ZA, Igbalagobi FI, Gidado A, Buratai LB (2000) The role of vitamin C administration in alleviation of organ damage in rats infected with the T. brucei. J Clin Biochem Nutr 28:1–7
Umar IA, Igbalajobi FI, Toh ZA, Gidado A, Shugaba A et al (2001) Effect of repeated daily doses of vitamin E (alpha-tocopherol) on some biochemical indices of rats infected with T. brucei (Basa strain). West Afr J BiolSci 12:1–7
Umar IA, Igbalajobi FI, Toh ZA, Gidado A, Shugaba A, Buratai LB (2001) Effects of oral administration of repeated doses of vitamin E (alpha tocopherol) on some biochemical indices in rats infected with T. b. brucei. West Afr J Biol Sci 12:1–7
Umar IA, Ogenyi E, Okodaso D, Kimeng E, Stancheva GI, Omage JJ, Isah S, Ibrahim MA (2007) Amelioration of anaemia and organ damage by combined intraperitoneal administration of vitamins A and C to trypanosoma brucei brucei-infected rats. Afr J Biotechnol 6(18):2083–2086
Umar IA, Rumah BL, Bulus SL, Kamla AA, Jobin A, Asueliman BI, Mazai MH, Ibrahim MA, Isah S (2008) Effects of intraperitoneal administration of vitamins C and E or A and E combinations on the severity of Trypanosoma brucei brucei infection in rats. Afri J Biochem Res 2(3):088–091
Van Hellemond JJ, Tielens AGM (2006) Adaptations in the lipid metabolism of the protozoan parasite Trypanosoma brucei. FEBS Lett 580:5552–5558
Vickerman K, Tetley L (1979) Biology and ultrastructure of trypanonomes in relation to pathogenesis. In: Losos G, Chouinard A (eds) Pathogenicity of trypanosomes. IDRC, Ottawa, pp 23–31
Vray B, Debaetselier P, Ouaisis A, Carlier Y (1991) Trypanosoma with T. evansi infection in dogs. Indian J Anim Health 34(1):33–38
Yusuf AB, Umar IA, Nok AJ (2012) Effects of methanol extract of Vernonia amygdalina leaf on survival and some biochemical parameters in acute Trypanosoma brucei brucei infection. Afr J Biochem Res 6(12):150–158
Funding
No funding was received for this research, it was funded by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declared that no conflict of interest exist.
Human and animal rights
Animals were humanely cared for in compliance with the principles of laboratory Animal care as stated by Bingham University Karu Nasarawa State, Nigeria.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ojo, R.J., Enoch, G.A., Adeh, F.S. et al. Comprehensive analysis of oral administration of Vitamin E on the early stage of Trypanosoma brucei brucei infection. J Parasit Dis 45, 512–523 (2021). https://doi.org/10.1007/s12639-020-01322-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01322-5