Abstract
Trypanosoma cruzi is transmitted to vertebrate hosts during the feeding of blood-sucking insects. After the invasion of host cells, the parasite resides within the parasitophorous vacuole until to escape to host cytoplasm and to proliferate, establishing an infection. Studies demonstrated that some intracellular parasites have to acquire all essential nutrients as well as transition metals from the host cell to be pathogenic, to maintain the homeostasis and to replicate. The present study investigated the progressive steps of the intracellular parasite development and establishment of infection in the presence of ZnCl2, CdCl2 and HgCl2. LLC-MK2 cells were infected with trypomastigotes during 6–84 h to investigate the steps of intracellular parasite development. After the host cells were infected during 12 h and treated with metals during 24 or 60 h or they were treated for 24 h and cultured for 72 h more to observe the reversibility. The results showed that the non-synchronous invasion of trypomastigotes resulted in an increasing number of intracellular parasites in intermediary forms (until 24 h post-infection), the appearance (from 36 h) and proliferation (84 h) of the amastigotes. The 24 h-treatments were not enough to impair parasite escape to the host cytoplasm and reproduction. However, 60 h of incubations led to a significant reduction in parasite numbers, as well as the reversibility assays. In conclusion, new insights about the intracellular T. cruzi development in the presence of metals were provided, and further studies should be performed to investigate the events involved in parasite death and elimination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosoma cruzi, the causative agent of Chagas’ disease, is a protozoan parasite of the Trypanosomatidae family. The parasite life cycle has different stages involving epimastigotes and metacyclic trypomastigotes in the triatomine insect vector, and blood trypomastigotes and intracellular amastigotes in vertebrate hosts, including humans. Trypomastigotes—the infective forms—adhere to the host cell surface via molecules that work as receptor-ligands, invade the cells and reside temporarily within the parasitophorous vacuole (PV) on host cytoplasm, as reviewed in (de Souza et al. 2010). After many hours, the trypomastigotes escape from the PV to cytoplasm where they undergo morphological changes to the replicative forms—amastigotes. After successive divisions, the amastigotes differentiate back to trypomastigotes, rupture the cells and reach the bloodstream from where they can invade new cells, establishing an infection (Tyler and Engman 2001).
The processes of parasite internalization, changes in parasite morphology and establishment of infection are orchestrated by metalloproteins (Alvarez et al. 2012). In addition to this, metal ions play important roles in host-parasite interactions (Weinberg 1966), but they are still poorly described.
Metals are divided into essentials and non-essentials according to their functions to the organisms (Martinez-Finley et al. 2012). The essential ones, like zinc, are present in many proteins and enzymes as structural component or co-factor, assisting for the cell homeostasis, although their concentrations are tightly regulated to avoid toxicity (Formigari et al. 2007). On the other hand, non-essential metals as cadmium and mercury have any function to the organisms, but are also present on the environment and can get the intracellular milieu through routes destined to the essential ones, causing a range of toxic effects (Martinez-Finley et al. 2012).
Currently, metal ions have been drawn attention due to pharmacological properties, and many metallo-drugs have been synthesized and tested against a wide variety of diseases, including Chagas disease (Vieites et al. 2008; Benítez et al. 2011; Martins et al. 2012). Nonetheless, deeper studies about the role of metals in host cell-parasite interactions lack, as well as about the influence of metals on parasite development and the establishment of infection. Recently, two studies investigating the role of zinc, cadmium and mercury chlorides in host-parasite interactions were published (de Carvalho and de Melo 2017), concerning the Toxoplasma gondii and T. cruzi, respectively. In the last study, extra- and intracellular proliferative T. cruzi were incubated with these metals and the results showed that all parasite forms were susceptible to metal incubations in conditions not toxic to the host cells.
In this context, this study was carried out to investigate whether early metal incubations influence the escape of intracellular parasites from the PV to the host cytoplasm and the parasite proliferation.
Materials and methods
Host cell
LLC-MK2 (kidney fibroblasts of Macaca mulatta) (the cells were provided by the Institute of Biophysic Carlos Chagas Filho, Federal University of Rio de Janeiro) were grown in plastic Falcon flasks (25 cm2) containing RPMI 1640 (Sigma®) medium supplemented with 5% fetal calf serum (FCS) (Sigma®). The cultures were treated with trypsin when the cell densities approached the monolayer. For experimental proposals, the cells were placed on Linbro 24-well plates with a sterile coverslip at a density of 3 × 104 cells per well or in medium flasks (3 × 106 cells). The cells were allowed to attach for 24 h at 37 °C in a 5% CO2 atmosphere (Gomes et al. 2012). Then, the host cells were infected during different times and submitted to treatments with ZnCl2, CdCl2 and HgCl2.
Parasite maintenance
Epimastigotes of T. cruzi (DM28 strain) were cultivated in Liver Infusion Tryptose (LIT) (Fluka®) medium supplemented with 0.4% of hemin and 10% FCS at 28 °C. Every 5 days, a 1 mL aliquot of parasite-containing medium was transferred to a new tube and the volume completed to 5 mL with fresh culture medium (de Carvalho and de Melo 2017).
Trypomastigotes of T. cruzi were obtained from transformation of epimastigotes. The epimastigotes were centrifuged at 500 g for 10 min and the pellet homogenized in RPMI 1640 supplemented with 10% FCS and incubated at 37 °C for 48 h. After this time, around 90% of the parasites were in the form of trypomastigote. For experimental purpose, the parasites were centrifuged at the same condition and homogenized in 1 mL of RPMI 1640. An aliquot of 0.1 mL was scored at Neubauer chamber and a rate of 20:1 parasite: cell was used to infected the culture. After 5–6 days of infection, the host cell lysis occurred and the trypomastigotes were released into the supernatant. So, the supernatant was collected, centrifuged as described above and new cultures were infected (de Carvalho and de Melo 2017).
Metal treatments
Dilutions of HgCl2, CdCl2 and ZnCl2 salts originated 0.1 M stock solutions in ultra-pure quality water. The final concentrations were prepared to dilute the stock solution with the medium.
These times and concentrations were based on the paper published by others (de Carvalho and de Melo 2016, 2017) where the same uninfected cells were treated at the same conditions, establishing the parameters for the next studies.
Intracellular parasite development
The host cells were infected with trypomastigotes during times ranging from 6 to 84 h to observe the progressive steps of intracellular development.
Toxicity assays
The host cells were infected during 12 h (before parasite escape) and treated with ZnCl2 at 20 µM or HgCl2 and CdCl2 at 1 µM during 24 or 60 h to investigate whether metals impair the parasite escape from the PV to the host cytoplasm and proliferation.
Reversibility assays
The host cells were infected for 12 h, treated with ZnCl2 at 20 µM or HgCl2 and CdCl2 at 1 µM during 24 h. Thus, the medium was changed to a drug-free medium and the infected cells were cultured for 24 h to observe whether metal incubations induced reversible or irreversible toxic effects to the remaining parasites.
Cell quantification and morphological analyses
After treatments, the coverslips containing cells were rinsed in PBS, fixed in Bouin’s solution for 5 min and stained with Giemsa’s solution (diluted in PBS, pH 7.2, 10%, v/v) during 6 h at room temperature. The coverslips were mounted on glass slides with Entellan (Merck®) for observation by light microscopy. The extracellular parasites were centrifuged and the pellets were suspended in formaldehyde 4% (w/v) for 30 min and rinsed with PBS, pH 7.2. An aliquot of parasites was put on glass slides. To examine all preparations a Zeiss Axioplan microscope, equipped with 20× and 40× objectives was used.The Analysis System software to obtain the images. Three random fields of each of six samples (individual treatments) of infected cells were scored to the following parameters: (1) uninfected host cells; (2) infected host cells and (3) number of intracellular parasites. The observation of morphological changes and reduction of cell and parasite numbers indicated the metal cytotoxicity (de Carvalho et al. 2013).
Ultrastructural analyses
For this purpose, infected LLC-MK2 cells were incubated with CdCl2 at 1 μM, during 24 h and fixed or cultivated during 24 h more (reversibility assay) to be processed for transmission electron microscopy. After the treatments, the samples were washed with PBS pH 7.2 at 37 °C and fixed at room temperature in a Karnovsky`s solution containing 1% (v/v) glutaraldehyde, 4% (v/v) paraformaldehyde, 5 mM CaCl2 and 5% (w/v) saccharose in cacodylate buffer 0.1 M, pH 7.2. The samples were postfixed for 1 h in a solution containing 2% (v/v) OsO4, 0.8% (v/v) potassium ferrocyanide. The samples were rinsed with 0.1 M cacodylate buffer, pH 7.2, dehydrated in graded acetone, embedded at PolyBed812 (Fluka®). After, the resin was polymerized for 2 days in 60° C. Ultra-thin sections obtained with an ultramicrotome (LEICA) were stained with uranyl acetate and lead citrate, and observed with a JEOL 1400Plus Transmission Electron Microscope at 60 kV acceleration (Carvalho et al. 2010).
Statistics
The assays were performed in quadruplicate. At least 4 different fields and 500 host cells were counted for each assay. The parameters observed were: the number of (1) uninfected cells; (2) infected cells and (3) intracellular parasites.
The statistical significance was determined using GraphPad Prism v.6 software (GraphPad Software, Inc. CA, USA). Two-way ANOVA followed by a Bonferroni post-test was used to compare the differences in cell viability relative to the control cultures (p < 0.001).
Results
Intracellular Trypanosoma cruzi
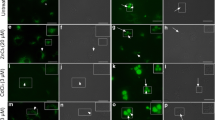
After the establishment of the host cells on the plate, they were infected with trypomastigotes-derived culture. The intracellular parasites were quantified and the parasite morphologies were also considered at the initial steps of development (Figs. 1, 2). A low number of infected host cells were observed in 6 h of infection, but this number increased until 24 h when it was established (Figs. 1a, 2a–c). At the initial periods of infection (6–24 h), the parasites were at an intermediate stage of development (punctuate and condensed morphology) (Figs. 1b—white bars, 2a–c). From 36 h the amastigote forms also appeared (Figs. 1b—grey bars, 2a, b, c).
The establishment of the intracellular T. cruzi infection. The intracellular T. cruzi was quantified and the morphologies considered to observe the initial steps of the establishment of the infection. The number of infected host cells increased up to 24 h and it was maintained at about 75% until 84 h (a—white bars). During the times of 6–24 h, only intermediary stages were observed (a—white bars). After 36 h, some parasites started to show the spread portion of cytoplasm—amastigotes, suggesting a more advanced stage of development (b—grey bars). From 84 h, the majority of parasites was spread within the cytoplasm and presented two punctuate stains (nucleus and kinetoplast), indicating the establishment of infection and proliferation steps
Optical microscopy of the development of intracellular T. cruzi during different times of infection. Intermediary forms were observed in (a, b, c). From 36 h d the parasites started to present a spread cytoplasm, a similar amastigote morphology. Black arrows: host cells nuclei. White arrows: parasites. Scale bars: 100 μm. Inserts: amplification of the selected areas
Metal toxicities on intracellular Trypanosoma cruzi
The host cells were infected during 12 h and incubated with ZnCl2 at 20 μM or CdCl2 and HgCl2 at 1 μM during 24 h to verify the effects of a low dose of metals on the initial steps of the intracellular development of T. cruzi. The 12 h period is the average time required for parasite escape from the vacuole to host cytoplasm, before the establishment of infection. In these conditions, no reduction in host cell numbers was observed but occurred a decrease of 17, 13 and 4% on parasite number after ZnCl2, CdCl2 and HgCl2 treatments, respectively (Fig. 3).
The analyses of the metal effects confirmed that any treatments cause morphological changes to host cells. Untreated (Fig. 4a) and treated cultures (Fig. 4b–d) showed the same typical features as a spread and non-vacuolated cytoplasm and nucleus. However, the number of punctual parasites decreased.
As previously described, CdCl2 and HgCl2 treatments induced similar morphological effects to parasites (de Carvalho and de Melo 2017). Then, to further investigate the morphology of both host cells and parasites, they were treated with CdCl2 at 1 µM during 24 h and processed. The untreated host cells showed a homogeneous cytoplasm and a well-established T. cruzi with its typical morphology (Fig. 5a). After CdCl2 treatment at 1 μM during 24 h, the host cells remained with is usual features but the intracellular parasites presented highly disorganized morphology (Fig. 5b).
The new metal incubations last 60 h to observe whether longer incubations can eliminate more parasites and also the progressive steps of parasite elimination. The untreated culture had 56% of infected cells, but this percentage decreased after metal incubations. After ZnCl2 treatment, the percentage of infection dropped down to 30% while with CdCl2 to 50%, with no reduction in cell number in comparison to the untreated ones. However, this longer time of incubation with HgCl2 also induced a toxic effect on the host cells and eliminated 52% of them (Fig. 6a). Also, the ZnCl2 and CdCl2 incubations decreased 61% of the number of intracellular parasites, and HgCl2 72% (Fig. 6b).
In agreement with the quantification above, the optical microscopy showed that untreated cells had typical morphology and a high number of intracellular parasites (Fig. 7a). The ZnCl2 (Fig. 7b) and CdCl2 (Fig. 7c)—treated ones had a lower number of viable parasites and any toxic effect on host cells. Nonetheless, HgCl2 treatment led to cell condensation and elimination in addition to parasite destruction (Fig. 7d). These results showed that HgCl2 has a higher and accumulative toxic effect on both host cells and parasites.
Host cells infected with T. cruzi for 12 h and incubated with metals at 1 μM during 60 h. After this longer time of incubations, a lower number of viable parasites was seen. Black arrows: host cell nuclei. White arrows: intracellular parasites. Scale bars: 100 μm. Inserts: amplification of the selected areas
Reversibility assay
A 24 h-treatment induced a low number of parasite elimination, although many morphological changed parasites remained on the culture. Then, the reversibility assays were performed to investigate whether the remained parasites were able to revert the toxic effects caused by the metals or not. For this purpose, 12 h-infected cells were treated with ZnCl2 at 20 μM or CdCl2 and HgCl2 at 1 μM during 24 h, then the medium was replaced by a drug-free medium and the cells were cultivated for additional 72 h. After this time, the untreated culture had 85% of infected cells, after ZnCl2 incubation, this number decreased to 63%, and to 70% after CdCl2 and HgCl2, with no significant reduction on host cell number (Fig. 8a). On the other hand, a greater number of intracellular parasites was eliminated on the reversibility assays in comparison to the direct toxic effect. The ZnCl2, CdCl2 and HgCl2 incubations led to reductions of 54, 69 and 66%, respectively (Fig. 8b). These results suggest that irreversible toxic effects were already triggered during the 24 h of treatment.
The analyses by optical microscopy showed the untreated cells with an established cytoplasmic infection (Fig. 9a). After the reversibility assays, a lower number of intracellular parasites was observed and the remaining parasites had modified and condensed appearance (Fig. 9b).
Reversibility assays of metal treatments in T. cruzi infected cells. The 12 h-infected cultures were incubated with metals during 24 h and cultivated for additional 72 h on a metal-free medium. After this time, a large number of inviable parasites was seen. Scale bars: 50 μm. Inserts: amplification of the selected areas
The ultrastructure analyses showed host cytoplasm with its typical morphology containing proliferative T. cruzi with the usual nucleus, kinetoplast and flagellum (Fig. 10a). After CdCl2 reversibility assay, different stages of parasites were observed, including typical and condensed parasites. In addition, many vacuoles appeared on host cytoplasm, suggesting parasite destruction and elimination (Fig. 10a).
Reversibility assay of CdCl2 treatment in T. cruzi infected cells. The 12 h-infected culture was incubated at 1 μM during 24 h, and cultivated during additional 72 h on a metal-free medium. a Untreated culture. b CdCl2 treated culture. Different stages of parasite destruction were observed after metal treatment. N: parasite nucleus. K: kinetoplast. HC: host cytoplasm. V: vesicles. Black arrows: intracellular parasites. Dotted black arrow: destroyed parasite
Discussion
The establishment of an intracellular infection and parasite propagation is dependent on host cell metabolism (Caradonna et al. 2013). For this reason, the metabolic coupling of intracellular pathogens with host cells is strictly regulated. However, many deaths have been caused annually due to the self-limiting effects of an unbalanced T. cruzi infection (Santos et al. 2012). To overcome this problem, many research groups have been using the advances in the rational design of metal-based chemotherapy to synthesize anti-pathogenic therapeutic agents, as metals are known to potentialize pharmacological properties (Vieites et al. 2008; Benítez et al. 2011; Martins et al. 2012).
Many studies demonstrated that some intracellular parasites have to acquire all essential nutrients as well as transition metals from the host cell to be pathogenic, to maintain the homeostasis and to replicate (Porcheron et al. 2013). For these reasons, metal flux control is vital to parasites. However, few studies have been published concerning the toxic effects of metal ions to intracellular protozoan parasites. Therefore, the comprehension of the antiparasitic impact of metals is crucial for improving the activity of metal-based agents (Lemire et al. 2013).
Trypanosoma cruzi, an obligate intracellular parasite, is transmitted to vertebrate hosts during the feeding of blood-sucking insects. The process of parasite internalization is triggered by molecules present in both parasite and mammalian cells that work as receptor-ligands resulting in the endocytic or phagocytosis pathways in a non-synchronic manner, as reviewed in (de Souza et al. 2010). The host cell plasma membrane and lysosomes contribute to parasitophorous vacuole formation, where the trypomastigotes initiate their intracellular cycle (Woolsey et al. 2003; Tardieux, Nathanson and Andrews 1994; Rodriguez et al. 1996). After 8–16 h post-invasion, the trypomastigotes start the process of transformation to amastigotes, while many events related to the parasitophorous vacuole destruction are activated. The parasitophorous vacuole membrane is marked with early and late endosome proteins, suggesting a process of parasite destruction through endolysosome formation and vacuole acidification (Andrews and Whitlow 1989). However, the parasite uses this host microbicide defense mechanism to escape to the cytoplasm, to replicate and to establish an infection (de Carvalho and de Souza 1989). These steps result from the Tc-Tox activity—a parasite’s peptide activated by the pH decrease after lysosome-vacuole fusion—that lead to the disintegration of the parasitophorous vacuole membrane (Andrews and Whitlow 1989; Ley et al. 1990). After T. cruzi reach the host cytoplasm, the parasites finish their transformation to amastigotes—proliferative form—and multiply in direct contact with the host cell organelles.
As the present study aimed to investigate the progressive steps of the intracellular parasite development, host cells were infected and the number of intracellular parasites and their morphology were observed. According to expectations, this paper showed that the non-synchronous invasion of trypomastigotes resulted in an increasing number of intracellular parasites in intermediary forms (until 24 h post-infection), the appearance (from 36 h) and proliferation (84 h) of the amastigotes (Fig. 1). From the confirmation that after 12 h of infection, only intermediary forms were observed on host cell indicating that the infection was not established yet, this time was used to further investigate the role of metal ions in the intracellular development. It is well-known that the metal ions play an important role in the establishment and maintenance of host-parasite interactions and an imbalance can cause severe damage to both of them (Weinberg 1966).
In this context, a previous study showed that ZnCl2, CdCl2 and HgCl2 incubations in epimastigotes, trypomastigotes and intracellular proliferative T. cruzi were able to eliminate part of the parasites without causing toxic effects to the host cells (de Carvalho and de Melo 2017). Nonetheless, this study showed that early metal incubations (ZnCl2, CdCl2 and HgCl2) at the concentration of 1 µM during 24 h was not enough to impair parasite escape to the host cytoplasm and the infection establishment (Figs. 3, 4). However, it was able to eliminate a high number of proliferative ones mainly with CdCl2 (de Carvalho and de Melo 2017). This higher survival rate of trypomastigote and intermediary forms in comparison to amastigotes can be a result of the different morphological life cycle forms and the changes in gene expression (Tyler and Engman 2001). Trypomastigotes have important survival mechanism to adapt to the environmental changes while the metabolism of the amastigote is directed to proliferation (Nardy et al. 2015). However, longer time of incubation (60 h) led to a significant reduction on parasite number after the treatments with the three metals (Figs. 6, 7), as well as the reversibility assays (Figs. 8, 9, 10).
Metal incubations, mainly non-essential ones, can induce parasite death through many pathways. Mercury, for example, can cross lipid membranes and have a high affinity for thiol groups (Girault et al. 1997), as well as cadmium that can impair intracellular signaling pathways after interacting with surface receptors (Moulis 2010). In case of zinc, an essential metal, it is also toxic in high concentrations and, for this reason, it needs to be tightly regulated (Eide 2006). Then, the misbalance of metal ions can result on the production of free radicals involving in the lipid peroxidation, modifications to DNA bases and disruption of calcium and sulphydryl homeostasis (Valko et al. 2007; Jomova and Valko 2011). Trypanosomatids, including T. cruzi, have a peculiar defense mechanism against free radicals, which includes the trypanothione (Ariyanayagam and Fairlamb 2001; Turrens 2004) and low activity of superoxide dismutase (Maya et al. 2007). Due to the lack of defenses against metal effects, the parasites were the main target for metal effects, as observed in our results.
References
Alvarez VE, Niemirowicz GT, Cazzulo JJ (2012) The peptidases of Trypanosoma cruzi: digestive enzymes, virulence factors, and mediators of autophagy and programmed cell death. Biochim Biophys Acta 1824:195–206
Andrews NW, Whitlow MB (1989) Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol Biochem Parasitol 33:249–256
Ariyanayagam MR, Fairlamb AH (2001) Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol Biochem Parasitol 115:189–198
Benítez J, Becco L, Correia I et al (2011) Vanadium polypyridyl compounds as potential antiparasitic and antitumoral agents: new achievements. J Inorg Biochem 105:303–312
Caradonna KL, Engel JC, Jacobi D et al (2013) Host metabolism regulates intracellular growth of Trypanosoma cruzi. Host Cell Microbe 13:108–117
Carvalho CS, de Melo EJT, Tenorio RP, Góes AJ (2010) Anti-parasitic action and elimination of intracellular Toxoplasma gondii in the presence of novel thiosemicarbazone and its 4-thiazolidinone derivatives. Braz J Med Biol Res 43:139–149
de Carvalho LP, de Melo EJT (2016) Essential and nonessential metal effects on intracellular Toxoplasma gondii. Eur J Biomed Pharm Sci 3:22–32
de Carvalho LP, de Melo EJT (2017) Life and death of Trypanosoma cruzi in presence of metals. Biometals 30:955–974
de Carvalho TMU, de Souza W (1989) Early events related with the behaviour of Trypanosoma cruzi within an endocytic vacuole in mouse peritoneal macrophages. Cell Struct Funct 14:383–392
de Carvalho LP, Gomes MAGB, Rocha BS, de Oliveira RR, de Melo EJT (2013) Anti-parasite effects of new thiosemicarbazones and their products thiazolidinone including cellular aspects of intracellular elimination of Trypanosoma cruzi in vitro. J Dev Drugs 3:1000126
de Souza W, de Carvalho TMU, Barrias ES (2010) Review on Trypanosoma cruzi: host cell interaction. Int J Cell Biol. https://doi.org/10.1155/2010/295394
Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763:711–722
Formigari A, Irato P, Santon A (2007) Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol 146:443–459
Girault L, Boudou A, Drfourc EJ (1997) Methyl mercury interactions with phospholipid membranes as reported by fluorescence, 31P and 199Hg NMR. Biochim Biophys Acta 1325:250–262
Gomes MAGB, de Carvalho LP, de Melo EJT, Oliveira RR, Maria EJ (2012) Evaluating anti-Toxoplasma gondii activity of new serie of phenylsemicarbazone and phenylthiosemicarbazones in vitro. Med Chem Res 22:3574–3580
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384
Ley V, Robbins ES, Nussenzweig V, Andrews NW (1990) The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med 171:401–413
Martinez-Finley EJ, Chakraborty S, Fretham SJB, Aschner M (2012) Cellular transport and homeostasis of essential and nonessential metals. Metallomics 4:593–605
Martins DA, Gouvea LR, da Gama JBD et al (2012) Copper(II)–fluoroquinolone complexes with anti-Trypanosoma cruzi activity and DNA binding ability. Biometals 25:951–960
Maya JD, Cassels BK, Iturriaga-Vásquez P et al (2007) Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol 146:601–620
Moulis JM (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23:877–896
Nardy AF, Freire-de-Lima CG, Morrot A (2015) Immune evasion strategies of Trypanosoma cruzi. J Immunol Res. https://doi.org/10.1155/2015/178947
Porcheron G, Garénaux A, Proulx J et al (2013) Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90
Rodriguez A, Samoff E, Rioult MG et al (1996) Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. J Cell Biol 134:349–362
Santos D, Parajón-Costa B, Rossi M et al (2012) Activity on Trypanosoma cruzi, erythrocytes lysis and biologically relevant physicochemical properties of Pd(II) and Pt(II) complexes of thiosemicarbazones derived from 1-indanones. J Inorg Biochem 117:270–276
Tardieux I, Nathanson MH, Andrews NW (1994) Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J Exp Med 179:1017–1022
Turrens JF (2004) Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Asp Med 25:211–220
Tyler KM, Engman DM (2001) The life cycle of Trypanosoma cruzi revisited. Int J Parasitol 31:472–481
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Vieites M, Otero L, Santos D et al (2008) Platinum-based complexes of bioactive 3-(5-nitrofuryl) acroleine thiosemicarbazones showing anti-Trypanosoma cruzi activity. J Inorg Biochem 103:411–418
Weinberg ED (1966) Roles of metallic ions in host-parasite interactions differential metallic ion growth requirements of virulent and avirulent bacterial strains. Bacteriol Rev 30:136–151
Woolsey AM, Sunwoo L, Petersen CA et al (2003) Novel Pl 3- kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J Cell Sci 116:3611–3622
Acknowledgements
FAPERJ (Fundação de Amparo à Pesquisa do Rio de Janeiro) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (Grant No. E-26/010002612/2014).
Author information
Authors and Affiliations
Contributions
Lais Carvalho performed all assays while Edésio Melo organized the results and he also wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pessanha de Carvalho, L., Tenório de Melo, E.J. Intracellular development of Trypanosoma cruzi in the presence of metals. J Parasit Dis 42, 372–381 (2018). https://doi.org/10.1007/s12639-018-1010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-018-1010-2