Abstract
Sarcocystis species are cyst-forming intracellular protozoan parasites. Cattle are mainly infected with Sarcocystis cruzi, Sarcocystis hominis and Sarcocystis hirsuta. Water buffaloes are intermediate hosts for Sarcocystis fusiformis, Sarcocystis levinei (S. cruzi-like species), Sarcocystis dubeyi, Sarcocystis sinensis (S. hominis-like species) and Sarcocystis buffalonis (S. hirsuta- like species). The aim of this study was Identification of Sarcocystis spp. in slaughtered cattle and water buffaloes in Ahvaz, Khuzestan province by PCR-restriction fragment length polymorphism. Meat inspection was done on 124 cattle and 147 water buffaloes. From each animal tissue samples (each 50 g) from heart, esophagus, diaphragm and intercostal muscle were collected during meat inspection. Samples examined with digestion method. Genomic DNA of 80 positive samples was extracted and their 18S rRNA gene was amplified. PCR products were digested by restricted enzymes (FokI, SspI and DraI). S. cruzi in cattle and S. fusiformis in water buffaloes were identified. Our study clarified that sarcocystosis in cattle in Ahvaz district may be results acute infection according to determined species, but in buffaloes as S. fusiformis was detected we may expect only economic loss follow up slaughterhouse inspection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cyst-forming coccidian parasites, Sarcocystis spp. (Apicomplexa: Sporozoa), have obligatory two-host life cycle involving carnivorous as definitive hosts and herbivorous or omnivorous as intermediate hosts. These organisms cause sarcocystosis in domestic animals and sometimes human and each intermediate and definitive host may be infected with more than one Sarcocystis species (Dubey et al. 1989). Cattle and water buffaloes are the intermediate hosts of some important species of Sarcocystis, that is, they may harbor macro or micro sarcocystis on their muscles. Intermediate hosts become infected with the parasite via ingesting sporocysts or sometimes sporulated oocysts existed in the food or water (Dubey and Lindsay 2006).
The parasites are most prevalent in livestock animals all around the world which the infection rate in cattle reported up to 100 percent in many countries (Dubey et al. 1989). Cattle are mainly infected with Sarcocystis cruzi, Sarcocystis hominis and Sarcocystis hirsuta (Dubey and Lindsay 2006) and water buffaloes are usually infected with Sarcocystis fusiformis, Sarcocystis levinei, Sarcocystis dubeyi and Sarcocystis buffalonis (Dubey et al. 1989). Some Sarcocystis species induce weight loss, general weakness, fever, anorexia, abortion and death in domestic animals but, macrocyst inducing Sarcocystis species are often considered as economic loss producers in slaughterhouses (Dubey et al. 1989).
The conventional tools for species diagnosis of Sarcocystis spp. were based on transmission electron microscopy, structure of the cyst wall in the striated muscles of the intermediate host or information about the lifecycle of the parasite (Jehle et al. 2009). However, because of showing the morphologic variations in these procedures they are not exactly reliable at the species-specific identification. On the other hand, electron microscopy is not a choice for wide and extensive detective studies (McManus and Bowles 1996).
In recent times, various molecular techniques such as PCR and its variants based on sequence changes have been used regarding the sensitivity and rapidity to determine genetic diversity among many parasites, phylogenetic and taxonomic studies and in epidemiological mapping (González et al. 2006; Maurer 2011).
Currently, PCR-restriction fragment length polymorphism (PCR–RFLP) based on variable regions of the small subunit ribosomal RNA sequences is considered and used widely as a rapid, inexpensive and accurate molecular approach to discriminate different protozoa as well as Sarcocystis spp. (Ellis et al. 1995; Heckeroth and Tenter 1999a, b; Motamedi et al. 2011).
As cattle and water buffaloes are rearing as the strategic animals in livestock industry in Iran, so, the aim of this study was to determine the Sarcocystis species of these two economically important animals in Khouzestan by PCR–RFLP in basis of amplification of 18S rRNA gene.
Materials and methods
Samples collection
The presented study was conducted on 123 and 147 inspected cattle and water buffaloes respectively in Ahvaz slaughterhouse from Khouzestan, Iran. After inspection of carcasses, at least 50 g from esophagus, diaphragm, intra-costal, abdomen and leg muscles from cattle and water buffaloes were sampled. Sample collection continued until we reached to 40 microcyst positive cattle and 40 positive water buffaloes (18 microcysts and 22 macrocysts) that confirmed by digestion method as described below.
Digestion method
The method of Dubey et al. (1989) with some modifications was utilized for digestion of muscles. Briefly, mixture of collected muscles from each animal was minced and digested for 24 h at 37 °C in 100 ml of digestion medium containing 6 g of pepsin (Merck), 10 ml of HCl, and 600 ml of distilled water. After digestion, the mixture were centrifuged 3 min at 2,500×g and the sediment was then stained with giemsa and examined by optical microscope at 400× magnification for detecting bradyzoites. Thereafter, the sediments containing bradyzoites washed two times with normal saline and TE solution respectively and preserved at −20 °C until molecular examinations.
DNA extraction
DNA was extracted using the genomic DNA extraction Kit (Cinnagen, Iran) according to manufacturer instructions. Next, the extracted DNA was stored at −20 °C until PCR performance.
PCR Amplification and RFLP
Species determination was carried out by PCR–RFLP according to amplification of 18S rRNA gene. PCR protocol and primer selection were adopted according to the previously described by Jehle et al. 2009.
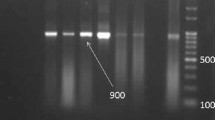
Briefly, amplification of the 18S rRNA gene was carried out in 25 μl reaction volumes containing 5 μl of DNA template, 15 pmol of each reverse and forward primers, 5 mM MgCl2, 2.5 μl 10 × PCR Buffer, 10 mM of each dNTP and 0.5 U Taq DNA Polymerase. Forward and reverse genus-specific primer sequences used in this study were (18S1H): 5′ GGC AAA TGC TTT CGC AGT AG 3′ and (18S9L): 5′ GGA TAA ACC GTG GTA ATT CTA TG 3′ respectively. The thermal program of PCR was as follows: 94 °C for 5 min, 40 cycles of 94 °C for 2 min, annealing at 57 °C for 40 s, and 72 °C for 2 min, followed by a final extension step at 72 °C for 5 min. To verify the results, 10 μl of each PCR product was electrophoresed in a 1 % agarose gel, stained with ethidium bromide and visualized on a UV transilluminator. The PCR products were identified by size using a 100 base pair ladder (Fermentas). The expected PCR product had a length of 900 bp.
To determine the possibility of the cross reaction with related protozoans, Neospora caninum and Toxoplasma gondii, the whole tachyzoites of these two parasites were also analyzed by mentioned primers. The amplified products were analyzed with RFLP using SspI, HindII, FokI and DraI restriction enzymes. Digested PCR products were resolved on a 2 % agarose gel and visualized with safe stain under ultraviolet light.
Results
Follow up slaughterhouse inspections there was no macro cyst in cattle, but after digestion, all of the samples were infected with micro cysts.
PCR amplification of T. gondii and N. caninum tachyzoites with mentioned primers revealed no electrophoretic bands but, in the same situation our isolates demonstrated a Sarcocystis specific 900 and 850 base pairs band for cattle and water buffaloes respectively (Figs. 1 and 2).
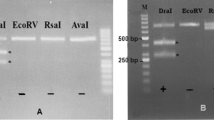
Gel electrophoresis of the PCR–RFLP by amplification of 18S rRNA gene from all isolates of micro sarcocysts from cattle showed that restriction with DraI enzyme produced 412 and 444 base pairs fragments and also, FokI produced 321 and 535 base pairs fragments which clearly representing the S. cruzi (Fig. 3).
Also, examination on 40 water buffaloes micro and macrocysts apparently revealed that restriction with SspI enzyme produced 266 and 613 base pairs fragments and DraI produced 99 and 768 base pairs fragments which represent the S. fusiformis (Fig. 4).
Discussion
The results of our work approximately repeated other data prevalence of Sarcocytis infections in cattle or water buffaloes in Iran (Ghorbanpoor et al. 2007; Nourollahi Fard et al. 2009; Hamidinejat et al. 2010) and also support the importance of this parasite in this district.
Exact detection of etiological agents of infectious diseases is critically important and has central role on control and prevention purposes. Many conventional procedures such as trichinoscopy, staining with methylene blue, dob-smear, digestion and histology have been employed for diagnosis of sarcocystosis in meat samples. These methods are genus specific and just performable on slaughtered carcasses (Williams et al. 1990; Verhasselt et al. 1992; Holmdahl et al. 1993). On the other hand, some serological assays including enzyme linked immunosorbent assay (ELISA) and indirect fluorescent antibody test, based on bradyzoites derived from sarcocysts have been assessed for serological diagnosis of sarcocystosis in sheep as well as some other animals (Uggla and Buxton 1990; Moré et al. 2008). Since, the bradyzoites of different Sarcocystis spp. have very antigenic similarities and therefore they have considerable cross reactions with other Sarcocystis spp. and other related parasites (Moré et al. 2008), it is obvious that these serological methods have serious limitations for species diagnosis as well as the genus determination.
To our knowledge there is not distinct molecular data in Iran to determine the species of micro and macro sarcocysts in both cattle and water buffaloes accurately and simultaneously.
Three species of Sarcocystis, S. cruzi, S. hominis and S. hirsuta (Dubey and Lindsay 2006) have detected in cattle. Among these species S. cruzi and S. hominis produce microscopic cysts, whereas water buffaloes are usually infected with microcyst forming S. levinei and S. dubeyi as well as S. fusiformis and S. buffalonis which produce macrocysts (Dubey et al. 1989). Microcyst have pathogenic subsequences as an acute disease presented by abortion, fever, anemia and anorexia in early period of infection and then some chronic disorders maybe develop. On the other side, although macrocyst forming species are considered nonpathogenic, they can affect the meat quality and marketing and subsequently cause economic loss (Tenter 1995; Pescador et al. 2007). From the other point of view, another aspect of differences between macro and microcyst producers is that the definitive hosts of these two types of Sarcocystis spp. always are different in which cats often the final hosts of Sarcocystis spp. macrocyst producers and dogs play this role for microcyst forming Sarcocystis spp. By inference, exact determination of the species has central role for control and prevention purposes.
In recent years, molecular diagnostic techniques have been assessed for specific determination of Sarcocystis spp. (Yang and Zuo 2000). Among various genomic targets, the highly conserved 18s ribosomal subunit is used widely for species-specific detection of different protozoa as well as Sarcocystis spp. due to presentation of highly variable regions (Yang and Zuo 2000). On the other hand, many authors confirmed the 18S rRNA for firmly species-specific discriminating of sarcocysts (Yang et al. 2001; Tenter et al. 1992, 1994). Thus, the aim of our study was to determine the Sarcocystis species of cattle and water buffaloes in Khouzestan province, Iran, using PCR–RFLP in basis of amplification of 18S rRNA.
This work not detected any cross reactions with T. gondii and N. caninum. Reported by Homan et al. (2000), no cross reaction exists between T. gondii and Sarcocystis spp. or N. caninum in PCR results.
Since our work demonstrates the presence of S. cruzi in cattle we can conclude that probably acute clinical sarcocystosis is considerably important in differential diagnosis of cattle disorders. On the other hand, detected species in water buffaloes, S. fusiformis, which is not considered as illness producing agent is cause of many economical loss in slaughterhouses according to its effect on meat quality. The definitive hosts of these two species are cats, so we must concentrate on both of them for control and prevention programs of Sarcocystis infection in the area of our study especially when it appears that another species are not present in the quarter at yet. Otherwise, this goal is proper to prohibit of probable transmission of other Sarcocystis species to herds (Adriana et al. 2008).
These conclusions clearly reveal and support the importance of molecular investigations to identify the etiology of different diseases, especially when these methods are more accurate and economically superior in compare with other methods (Pescador et al. 2007).
References
Adriana T, Mircean V, Blaga R, Bratu CN, Cozma V (2008) Epidemiology and etiology in sheep sarcocystosis. Bull UASVM Vet Med 65:49–54
Dubey JP, Lindsay DS (2006) Neosporosis, toxoplasmosis, and sarcocystosis in ruminants. Vet Clin Food Anim 22:645–671
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. C.A.C. Press Inc, Boca Raton, pp 105–145
Ellis JT, Luton K, Baverstock PR, Whitworth G, Tenter AM, Johnson AM (1995) Phylogenetic relationships between Toxoplasma and Sarcocystis deduced from a comparison of 18S rDNA sequences. Parasitol 110:521–528
Ghorbanpoor M, Hamidinejat H, Nabavi L, Khadjeh GH, Razi Jalali M (2007) Evaluation of an ELISA for the diagnosis of sarcocystosis in water buffaloes. Bull Vet Inst Pulawy 51:229–231
González LM, Villalobos N, Montero E, Morales J, Álamo Sanz R R, Muro A, Harrison LJS, Parkhouse RME, Gárate T (2006) Differential molecular identification of Taeniid spp. and Sarcocystis spp. cysts isolated from infected pigs and cattle. Vet Parasitol 142:95–101
Hamidinejat H, Razi Jalali MH, Nabavi L (2010) Survey on Sarcocystis infection in slaughtered cattle in south-west of Iran, emphasized on evaluation of muscle squash in comparison with digestion method. J Anim Vet Adv 9:1724–1726
Heckeroth AR, Tenter AM (1999a) Comparison of immunological and molecular methods for the diagnosis of infections with pathogenic Sarcocystis species in sheep. Tokai J Exp Clin Med 23:293–302
Heckeroth AR, Tenter AM (1999b) Development and validation of species-specific nested PCR for diagnosis of acute Sarcocystis in sheep. Int J Parasitol 29:1331–1349
Holmdahl OJ, Mathson JG, Uggla A, Johansson KE (1993) Oligonucleotide probes complementary to variable regions of 18S rRNA from Sarcocystis spp. Mol Cell Probes 7:481–486
Homan WL, Vercammen M, De Braekeleer J, Verschueren H (2000) Identification of a 200–300 fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol 30:69–75
Jehle C, Dinkel A, Sander A, Morent M, Romig T, Luc PV, De TV, Thai VV, Mackenstedt U (2009) Diagnosis of Sarcocystis spp. in cattle (Bos taurus) and water buffalo (Bubalus bubalis) in Northern Vietnam. Vet Parasitol 23:314–320
Maurer JJ (2011) Rapid detection and limitations of molecular techniques. Annu Rev Food Sci Technol 2:259–279
McManus DP, Bowles J (1996) Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. Int J Parasitol 26:687–704
Moré G, Basso W, Bacigalupe D, Venturini MC, Venturini L (2008) Diagnosis of Sarcocystis cruzi, Neospora caninum, and Toxoplasma gondii infections in cattle. Parasitol Res 102:671–675
Motamedi GR, Dalimi A, Nouri A, Aghaeipour K (2011) Ultrastructural and molecular characterization of Sarcocystis isolated from camel (Camelus dromedarius) in Iran. Parasitol Res 108:949–954
Nourollahi Fard SR, Asghari M, Nouri F (2009) Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Trop Anim Health Prod 41:1633–1636
Pescador CA, Corbellini LG, de Oliveira EC, Bandarra PM, Leal JS, Pedroso PMO, Driemeier D (2007) Aborto ovino associado com infecc¸a˜o por Sarcocystis sp. Pesq Vet Bras 27:393–397
Tenter AM (1995) Current research on Sarcocystis species of domestic animals. Int J Parasitol 25:1311–1330
Tenter AM, Baverstock PR, Johnson AM (1992) Phylogenetic relationships of Sarcocystis species from sheep, goats, cattle and mice based on ribosomal RNA sequences. Int J Parasitol 22:503–513
Tenter AM, Luton K, Johnson AM (1994) Species-specific identification of Sarcocystis and Toxoplasma by PCR amplification of small subunit ribosomal RNA gene fragments. Appl Parasitol 35:173–188
Uggla A, Buxton D (1990) Immune responses against Toxoplasma and Sarcocystis infections in ruminants: diagnosis and prospects for vaccination. Rev Sci Tech Int Epiz 9:441–462
Verhasselt P, Voet M, Volckaert G (1992) DNA sequencing by a subcloning-walking strategy using a specific and semi-random primer in the polymerase chain reaction. Int J Parasitol 28:1053–1060
Williams JGK, Kubelik AR, Jivak KS, Rafalksi JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Yang ZQ, Zuo YX (2000) The new views of the researchers on cyst forming coccidia species including Sarcocystis by using the molecular biological techniques. Chin J Parasitol Parasit Dis 18:120–126
Yang ZQ, Zuo YX, Yao YG, Chen XW, Yang GC, Zhang YP (2001) Analysis of the 18S rRNA genes of Sarcocystis species suggests that the morphologically similar organisms from cattle and water buffalo should be considered the same species. Mol Biochem Parasitol 115:283–288
Acknowledgments
This study is supported by the Shahid Chamran University for M.Sc. thesis. The authors wish to thank the vice-chancellor for research of the Shahid Chamran University for the research. The authors also declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamidinejat, H., Razi Jalali, M.H., Gharibi, D. et al. Detection of Sarcocystis spp. in cattle (Bos taurus) and water buffaloes (Bubalus bubalis) in Iran by PCR–RFLP. J Parasit Dis 39, 658–662 (2015). https://doi.org/10.1007/s12639-014-0426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-014-0426-6