Abstract
Purpose
Acute pain after open abdominal hysterectomy limits the function of patients in the postoperative period, but data regarding the analgesic efficacy of a low dose of pregabalin (75 or 150 mg) have been conflicting. This study was performed to determine if a low dose of pregabalin could decrease postoperative opioid use following abdominal hysterectomy when compared with placebo.
Methods
American Society of Anesthesiologists I-II patients older than 18 yr and scheduled for open elective abdominal hysterectomy were recruited for participation and randomized to one of three groups: pregabalin 75 mg (P75), pregabalin 150 mg (P150), or placebo. The study drug was administered two hours prior to surgery and 12 hr following the initial dose. Anesthetic technique and postoperative analgesia were standardized. Postoperative pain was managed using patient-controlled analgesia with morphine. Pain at rest and movement as well as nausea were assessed with an 11-point numeric rating scale.
Results
One hundred and one patients were recruited, and 89 patients completed the study. Mean (SD) cumulative morphine consumption at 24 hr postoperatively was 54.0 (26.2) mg for the placebo group, 53.1 (22.7) mg for the P75 group, and 44.3 (20.9) mg for the P150 group. Independent Student’s t tests indicated no difference between the placebo group and either the P75 group (95% confidence interval [CI]: −11.75 to 13.44; P = 0.8937) or the P150 group (95% CI: −2.74 to 22.15; P = 0.1238).
Conclusions
At the doses used in this study, pregabalin treatment may not be effective in reducing opioid use up to 24 hr postoperatively following abdominal hysterectomy. This trial was registered at www.ClinicalTrials.gov: NCT00781131.
Résumé
Objectif
La douleur aiguë faisant suite à une hystérectomie par voie abdominale limite les fonctions des patients au cours de la période postopératoire, mais les données concernant l’efficacité analgésique d’une faible dose de prégabaline (75 ou 150 mg) ont été contradictoires. Cette étude a été menée pour déterminer si une faible dose de prégabaline pouvait diminuer l’utilisation postopératoire des morphiniques après hystérectomie par voie abdominale, comparativement au placebo.
Méthodes
Des patientes, âgées de plus de 18 ans, de type I-II selon les critères de l’American Society of Anesthesiologists, et devant subir une hystérectomie programmée par voie abdominale ont été recrutées et randomisées dans l’un des trois groupes suivants: prégabaline 75 mg (P75), prégabaline 150 mg (P150), ou placebo. Le médicament étudié a été administré deux heures avant l’intervention chirurgicale et 12 h après la dose initiale. La technique anesthésique et l’analgésie postopératoire ont été standardisées. La douleur postopératoire a été gérée en utilisant une analgésie par morphine contrôlée par le patient. La douleur au repos et au mouvement ainsi que les nausées ont été évaluées au moyen d’une échelle d’évaluation numérique de 11 points.
Résultats
Cent une patientes ont été incluses et 89 patientes ont terminé l’étude. Les consommations cumulées moyennes (ET) de morphine à 24 heures en postopératoire étaient de 54,0 (26,2) mg pour le groupe placebo, 53,1 (22,7) mg pour le groupe P75 et 44,3 (20,9) mg pour le groupe P150. Des tests t de Student indépendants n’ont pas fait apparaître de différence entre le groupe placebo et soit le groupe P75 (intervalle de confiance [IC] à 95 %: −11,75 à 13,44; p = 0,8937), soit le groupe P150 (IC à 95 %: −2,74 à 22,15; p = 0,1238).
Conclusions
Aux doses utilisées dans cette étude, le traitement par prégabaline ne semble pas être efficace dans la réduction de la consommation de morphine à 24 heures postopératoires après hystérectomie par voie abdominale. Cette étude a été enregistrée sur le site www.clinicaltrials.gov: NCT00781131.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute pain after abdominal hysterectomy limits the functioning of patients in the postoperative period. Additionally, for those few patients who suffer from chronic pain after surgery, this pain can become a lifetime burden. Chronic pain has been reported to occur in 4.7-31.9% of females following hysterectomy, including 22.9% who indicated that pain affected their daily living.1,2 Currently, opioids continue to play a major role in postoperative pain management despite contributing to increased in-hospital morbidity and costs.3,4 In this context, postoperative patients may be at significant risk for opioid-related adverse effects (postoperative nausea and vomiting, sedation, sleep disturbances, urinary retention, and respiratory depression).5
Use of the α2-δ subunit calcium channel ligands (gabapentin and pregabalin) has been effective in neuropathic pain,6 diabetic neuropathy,7 post-herpetic neuralgia,8 and reflex sympathetic dystrophy.9 Gabapentin reduced pain scores and postoperative opioid use after abdominal hysterectomy.10-13 Pregabalin has been shown to have greater analgesic efficacy in rodent models of neuropathic pain and exhibits linear pharmacokinetics across its therapeutic dose range.14 Physicians are currently using gabapentin and pregabalin to treat acute postoperative pain in an “off-label” manner using anecdotal evidence. Data on the efficacy of using a low dose of pregabalin are limited and conflicting.15,16 We therefore performed this study and hypothesized that the use of lower doses of pregabalin would decrease postoperative opioid use following abdominal hysterectomy when compared with placebo.
Methods
The protocol was reviewed and approved by the Izaak Walton Killam (IWK) Health Centre Research Ethics Board (REB) (December 11, 2007, IWK Health Centre REB# 4101). After REB approval, patients scheduled to undergo open elective abdominal hysterectomy at the IWK Health Centre were recruited for participation. Inclusion criteria included females older than 18 yr who were American Society of Anesthesiologists physical status I-II. Patients were excluded if they had a known or suspected allergy, sensitivity, or contraindication to pregabalin or any of the standardized medications used in the study; a history of a seizure disorder; current therapy with pregabalin, gabapentin, or any opioid; or renal dysfunction (CrCl < 60 mL·min−1). Following their introduction to the patients by members of the care team, the research coordinators explained the study to the patients and discussed their participation. The research coordinators enrolled the patients in the study once they had provided informed written consent to participate.
Patients were randomized to one of three groups: pregabalin 75 mg (P75), pregabalin 150 mg (P150), or placebo (PL). Participants were allocated using consecutively numbered sealed opaque envelopes that corresponded to a computer-generated randomization table created by the Pharmacy Research Unit at the IWK Health Centre. Randomization was done in blocks of 15 with five participants per group. A screening log, based on the suggested format given in the CONSORT Statement, was maintained to document the number of participants approached for study enrolment and the reasons for refusal. Trial capsules were compounded and prepared by the pharmacy such that all study medications were identical in appearance, and randomization codes were concealed from investigators, nurses, and patients until the end of the study and data analysis.
Blinded research staff administered the study capsules two hours prior to the start of surgery and 12 hr after the initial dose. Each patient received a standardized anesthetic consisting of induction with propofol 0.5-2.0 mg·kg−1 and fentanyl 1-3 μg·kg−1 and maintenance with desflurane 0.6-1.4 MAC and fentanyl 25-100 μg boluses for > 30% increase in heart rate or blood pressure from baseline values. Muscle relaxation was at the discretion of the anesthesiologist. Nausea prophylaxis consisted of dexamethasone 4-8 mg after induction of anesthesia and ondansetron 4 mg 30 min prior to emergence. At the conclusion of surgery, each patient received ketorolac 30 mg iv. In the postanesthesia care unit, participants received boluses of morphine (1-2 mg q5min) to achieve ≤ 3/10 on a numerical rating scale (NRS) where 0 = no pain and 10 = worst possible pain. Subsequently, postoperative pain was managed with patient-controlled analgesia with morphine set to deliver 1 mg of morphine with a lockout interval of five minutes and a four-hour maximal dose of 35 mg. The incremental dose was increased to 1.5 mg if analgesia was inadequate after two hours. Patients also received naproxen 500 mg pr/po every 12 hr for 48 hr as part of their analgesia regimen beginning 12 hr after the intraoperative ketorolac.
The primary outcome of this study was cumulative morphine consumption at 24 hr postoperatively. For an improved understanding of the patient’s experience with this intervention, we included a number of pre-specified secondary outcomes which were all measured up to 24 hr postoperatively: pain at rest, pain after movement, nausea, vomiting, pruritus, urinary retention, sedation, respiratory depression, and functional ability.
A blinded research coordinator collected all data at two, four, and 24 hr postoperatively. Pain at rest (supine), pain after movement (supine log roll), and nausea were assessed using an 11-point NRS (0 = no pain/nausea; 10 = worst possible pain/nausea).18 Vomiting episodes were recorded separately; retching was recorded as vomiting. Pruritus was assessed at 24 hr with a four point scale; 0 = absent; 1 = mild (restricted to one area and not troubling to the subject, usually noted after prompting); 2 = moderate (affecting a larger area or multiple sites, but not troubling to the subject and not requiring treatment); and 3 = severe (extensive or generalized pruritus, troubling to subject and/or requiring treatment).19 Functional ability was assessed at 24 hr for each of three functions, i.e., resting in bed, sitting on the edge of the bed, and walking in the room. The effect of pain on the overall functional ability was quantified as the sum of the scores for all three functions (0-5 each: 0 = no difficulty; 5 = unable to participate; 0-15 total score).20 Sedation was assessed with the modified Ramsay scale.21 Respiratory depression was defined as a respiratory rate < 8 breaths·min−1 and/or oxygen saturation < 90% at any time during the study. Urinary retention was defined as the need for a urinary catheter after the first 24 hr.
Forty-eight hours postoperatively, the participants were asked about their satisfaction with postoperative pain relief using an 11-point NRS (0 = completely dissatisfied; 10 = completely satisfied). Investigators contacted patients at home via telephone 30 days and six months after the day of the operation and asked the patients to complete the five-minute SF-36® survey,22,23 which is considered the most commonly used generic measure of health-related quality of life.
The primary outcome of the study was cumulative morphine consumption at 24 hr postoperatively. Quality improvement audit data from our institution over a two-month period showed that patients’ mean (SD) cumulative consumption of morphine was 35.5 (14.1) mg at 24 hr following an abdominal hysterectomy. The sample size calculation was based on this audit of opioid consumption which was clinically similar to that of Fassoulaki et al.24 Assuming α = 0.05 and 80% power, an a priori sample size calculation indicated 28 patients in each group were required to detect a 30% reduction in opioid consumption in the first 24 hr after surgery for either dose of pregabalin (GPower 2.0). Morphine consumption data were analyzed using independent Student’s t tests to compare the placebo group with the P75 and P150 groups. All other data collected were not subjected to inferential statistical testing. Analysis was performed using SAS® version 9.0 (SAS Institute, Cary, NC, USA). All reported P values are two-sided.
Results
Patients
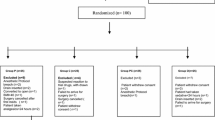
One hundred one patients were recruited for this study from May 2008 to July 2010. Twelve patients were excluded from the study for the following reasons: errors in timing of drug administration (n = 2), second dose of study drug not administered (n = 2), early discharge from the hospital (n = 2), requiring additional surgical intervention (n = 4), and declining to participate after consent (n = 2) (Figure). Consequently, 89 patients were included in the final analysis. There were no clinically relevant differences in demographic characteristics between groups (Table 1).
Morphine consumption
Independent Student’s t tests performed on morphine consumption data indicated no differences between the placebo group and the P75 group (95% confidence interval [CI]: −11.75 to 13.44; P = 0.8937) or the P150 group (95% CI: −2.74 to 22.15; P = 0.1238) at 24 hr postoperatively. There were no other clinically relevant differences between groups at any other time points (Table 2).
Pain
The mean pain scores at rest and after movement were consistently numerically higher in the placebo group compared with both pregabalin doses; however, this difference might not be clinically meaningful (Table 3).
Side effects
The proportion of individuals with low nausea scores was numerically higher in the P75 and P150 groups than in the placebo group at two and four hours following surgery, but there were no other clinically meaningful differences between groups at any individual time points (Table 4). Occurrences of vomiting were at two hours [placebo: 1 (3.3%), P75: 0 (0%), P150: 0 (0%)], four hours [placebo: 2 (6.7%), P75: 0 (0%), P150: 0 (0%)], and 24 hr postoperatively [placebo: 5 (16.7%), P75: 1 (3.2%), P150: 5 (17.9%)]. There were also no apparent clinically relevant differences in postoperative pruritus or urinary retention between groups.
Sedation and functional ability
Sedation scores at two, four, and 24 hr following surgery are listed in Table 4. The vast majority of participants were a modified Ramsey score of 2 (cooperative, oriented, and tranquil). There were no apparent clinically relevant differences between groups in functional ability at 24 hr postoperatively (Table 4).
Patient satisfaction and SF-36
All groups reported comparable mean (SD) patient satisfaction on the 11-point NRS at 48 hr follow-up [placebo: 8.8 (1.4); P75: 9.0 (1.8); P150: 9.0 (1.3)]. Further, mean (SD) results of the SF-36 health survey were comparable between groups at both the 30-day [placebo: 63.8 (12.8); P75: 59.3 (13.9); P150: 61.7 (14.5)] and the six-month follow-up [placebo: 89.7 (10.9); P75: 87.7 (14.3); P150: 83.4 (18.8)].
Discussion
Existing reports on the analgesic efficacy of a low dose of pregabalin are limited and marred by conflicting results.15,16,25-27 Higher pregabalin doses of 300 mg may intensify side effects, including somnolence and sedation.17 Accordingly, this study sought to determine if two lower doses of pregabalin could decrease postoperative opioid use compared with placebo following abdominal hysterectomy. Our results showed no difference in cumulative morphine consumption between groups at 24 hr postoperatively. While the P150 group tended to consume less opioid up to 24 hr into recovery, wide variability among patients rendered this result not statistically significant. Among our secondary outcomes measured, there appeared to be some transient benefits of pregabalin treatment on pain and nausea early in recovery with no apparent increase in sedation.
Previous reports of premedication with pregabalin 300 mg for hysterectomy have shown more robust and consistent reductions in morphine consumption compared with placebo than the modest improvements seen in the current study with a reduced dosage.16,28,29 Only Yucel et al.27 have reported consistently reduced morphine consumption compared with placebo across a 24 hr period with a single 150 mg dose of preoperative pregabalin. Further, they showed a dose-response effect where their 300 mg group had greater improvements above the 150 mg group.27 In the current study, while the 150 mg group had reduced total morphine consumption at 24 hr postoperatively, neither group showed a statistically significant difference in morphine consumption compared with placebo. Therefore, pregabalin treatment with the regimens used in this study does not appear to reduce morphine consumption in the postoperative period following hysterectomy.
Pregabalin appears to enhance analgesia during the early stages of recovery, as the P75 group reported numerically, what appears to be, lower pain at rest at two and four hours postoperatively. Similarly, the P150 group reported numerically lower pain at rest four hours postoperatively. Improved pain management within the first few hours following hysterectomy with preoperative pregabalin has been previously shown with 300 mg dosages of pregabalin, a substantially greater dose than the 75-150 mg doses administered here.16,29,30 Albeit, any analgesia produced here with lower doses of pregabalin appears to be shorter in duration and unable to improve pain consistently as recovery progresses. Although analgesia has been shown with premedication pregabalin 150 mg for cholecystectomy31 and gynecological laparoscopic surgery in the first few hours following surgery,15 we have observed reduced pain scores early in recovery with as little as 75 mg of pregabalin. Possible explanations for the differences between our findings and previous reports could be related to alternative opioid use (fentanyl)15 or co-interventions such as concomitant paracetamol administration32 or infusion of 0.5% hyperbaric bupivacaine 0.3 mg·kg−1.33
While existing literature reveals general inconsistency among side effects, an increase in side effects with higher doses of pregabalin emphasizes the need for dose-related research.17 Both study groups receiving pregabalin reported numerically lower nausea scores at two and four hours postoperatively compared with placebo. Interestingly, our observations implicate that as little as 75 mg of pregabalin may reduce nausea in the early recovery period despite the lack of morphine sparing.15,25,28,30,33 This is in agreement with similar reports in this area, although there continues to be inconsistency on this measure.15,32,34,35 All our groups reported comparable sedation at two, four, or 24 hr postoperatively, an important finding considering our goal of having patients return to a functional state as efficiently as possible following surgery. This may suggest that lower doses might be useful in the ambulatory surgery setting where enhancement of early postoperative recovery would be clinically relevant. These results are in agreement with previous research, although several studies have reported increased sedation with pregabalin treatment, including evidence that this phenomenon occurs in a dose-dependent manner.17,27,29,30,33 All groups appeared similar in terms of pruritus, vomiting, and urinary retention, with relatively low rates of each overall; findings which may help resolve currently disparate data regarding the influence of pregabalin on these general outcomes.27,29,32,35
Patient satisfaction was similar between groups, likely resulting from the lack of sustained benefit from pregabalin administration. With previous reports that patient satisfaction is higher,29 lower,27 or unchanged15 with pregabalin treatment, it seems probable that the impact of pregabalin on patient satisfaction is diluted by the wide variety of other factors at play. No dissimilarities were observed between groups on any measure of the SF-36 health survey, including total score at either 30 days or six months, further emphasizing the limited impact of reduced pregabalin dosages on later stages of patient recovery.
Limitations of the study include the homogeneity of our patient population, indicating that our results might not be generalizable to other patient populations. Adding a study group receiving 300 mg doses of pregabalin in an attempt to replicate the findings of previous research could have strengthened this work; however, the questions we have attempted to answer with this study did not explicitly require a fourth treatment group. All patients in our study received two doses of pregabalin; therefore, we are unable to comment if there was any benefit from the second dose of pregabalin. Another limitation is that we did not complete data collection on some of the enrolled patients where some protocol deviations occurred, and therefore, we did not include those patients in an intention-to-treat analysis.
In conclusion, administration of two doses of pregabalin, 75 mg or 150 mg, does not appear to reduce total morphine consumption at 24 hr compared with placebo. Both doses were observed to improve pain scores at rest up to four hours postoperatively but not in the later postoperative period. Nevertheless, there was no observed improvement in functional ability with the pregabalin regimens used. While we observed very modest benefits with pregabalin treatment, there was no detrimental impact on the patient treated with a low dose of pregabalin in terms of adverse side effects; in particular, there was no increased sedation with the doses used.
References
Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology 2007; 106: 1003-12.
Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS. Chronic pain after hysterectomy. Acta Anaesthesiol Scand 2008; 52: 327-31.
Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003; 25: 276-83.
Philip BK, Reese PR, Burch SP. The economic impact of opioids on postoperative pain management. J Clin Anesth 2002; 14: 354-64.
Taylor S, Voytovich AE, Kozol RA. Has the pendulum swung too far in postoperative pain control? Am J Surg 2003; 186: 472-5.
Dickenson AH. Neurophysiology of opioid poorly responsive pain. Cancer Surv 1994; 21: 5-16.
Fishman S, Borsook D. Opioids in pain management. In: Benzon H, Raja SN, Borsook D, Molloy RE, Strichartz G, editors. Essentials of Pain Medicine and Regional Anesthesia. 1st ed. New York: Churchill Livingstone; 1999. p. 51-4.
Rosner H, Rubin L, Kestenbaum A. Gabapentin adjunctive therapy in neuropathic pain states. Clin J Pain 1996; 12: 56-8.
Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 1998; 280: 1831-6.
Dierking G, Duedahl TH, Rasmussen ML, et al. Effects of gabapentin on postoperative morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind trial. Acta Anaesthesiol Scand 2004; 48: 322-7.
Fassoulaki A, Melemeni A, Stamatakis E, Petropoulos G, Sarantopoulos C. A combination of gabapentin and local anaesthetics attenuates acute and late pain after abdominal hysterectomy. Eur J Anaesthesiol 2007; 24: 521-8.
Gilron I, Orr E, Tu D, O’Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain 2005; 113: 191-200.
Turan A, Karamanlioglu B, Memis D, Usar P, Pamukcu Z, Ture M. The analgesic effects of gabapentin after total abdominal hysterectomy. Anesth Analg 2004; 98: 1370-3.
Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661-9.
Jokela R, Ahonen J, Tallgren M, Haanpaa M, Korttila K. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth 2008; 100: 834-40.
Przesmycki K, Wiater-Koziol E, Kotarski J, et al. Effect of pre-emptive pregabalin on pain intensity and morphine requirement after hysterectomy (Polish). Anestezjol Intens Ter 2011; 43: 14-7.
White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg 2009; 108: 1140-5.
Gan TJ, El-Molem H, Ray J, Glass PS. Patient-controlled antiemesis: a randomized, double-blind comparison of two doses of propofol versus placebo. Anesthesiology 1999; 90: 1564-70.
Horta ML, Morejon LC, da Cruz AW, et al. Study of the prophylactic effect of droperidol, alizapride, propofol and promethazine on spinal morphine-induced pruritus. Br J Anaesth 2006; 96: 796-800.
Carvalho B, Riley E, Cohen SE, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg 2005; 100: 1150-8.
Carrasco G. Instruments for monitoring intensive care unit sedation. Crit Care 2000; 4: 217-25.
Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000; 25: 3130-9.
Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res of 2001; 10: 405-13; discussion 415-20.
Fassoulaki A, Stamatakis E, Petropoulos G, Siafaka I, Hassiakos D, Sarantopoulos C. Gabapentin attenuates late but not acute pain after abdominal hysterectomy. Eur J Anaesthesiol 2006; 23: 136-41.
Jokela R, Ahonen J, Tallgren M, Haanpaa M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain 2008; 134: 106-12.
Paech MJ, Goy R, Chua S, Scott K, Christmas T, Doherty DA. A randomized, placebo-controlled trial of preoperative oral pregabalin for postoperative pain relief after minor gynecological surgery. Anesth Analg 2007; 105: 1449-53.
Yucel A, Ozturk E, Aygogan MS, Durmus M, Colak C, Ersoy MO. Effects of 2 different doses of pregabalin on morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind clinical trial. Curr Ther Res 2011; 72: 173-83.
Fassoulaki A, Melemeni A, Tsaroucha A, Paraskeva A. Perioperative pregabalin for acute and chronic pain after abdominal hysterectomy or myomectomy: a randomised controlled trial. Eur J Anaesthesiol 2012; 29: 531-6.
Ittichaikulthol W, Virankabutra T, Kunopart M, Khamhom W, Putarawuthichai P, Rungphet S. Effects of pregabalin on post operative morphine consumption and pain after abdominal hysterectomy with/without salphingo-oophorectomy: a randomized, double-blind trial. J Med Assoc Thai 2009; 92: 1318-23.
Ghai A, Gupta M, Hooda S, Singla D, Wadhera R. A randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomy. Saudi J Anaesth 2011; 5: 252-7.
Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008; 101: 700-4.
Mathiesen O, Jacobsen LS, Holm HE, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 2008; 101: 535-41.
Kohli M, Murali T, Gupta R, Khan P, Bogra J. Optimization of subarachanoid block by oral pregabalin for hysterectomy. J Anaesthesiol Clin Pharmacol 2011; 27: 101-5.
Chang SH, Lee HW, Kim HK, Kim SH, Kim DK. An evaluation of perioperative pregabalin for prevention and attenuation of postoperative shoulder pain after laparoscopic cholecystectomy. Anesth Analg 2009; 109: 1284-6.
Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth 2011; 106: 454-62.
Disclosures
This study was completed at the IWK Health Centre and supported by Nova Scotia Health Research Foundation. The authors have no relevant conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Ronald B. George, Dolores M. McKeen, and Ashraf S. Habib contributed to the study design and to drafting the manuscript. Ronald B. George and Dolores M. McKeen contributed to data collection and analysis. Ronald B. George, Dolores M. McKeen, and Ashraf S. Habib contributed to the interpretation of results. Ronald B. George, Dolores M. McKeen, Pantelis Andreou, and Ashraf S Habib provided feedback. Pantelis Andreou contributed to statistical interpretation and analysis of results.
Rights and permissions
About this article
Cite this article
George, R.B., McKeen, D.M., Andreou, P. et al. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anesth/J Can Anesth 61, 551–557 (2014). https://doi.org/10.1007/s12630-014-0147-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0147-4