Abstract
Purpose
To determine if preoperative pregabalin could decrease 24-h postoperative morphine consumption after spinal anesthesia with intrathecal morphine compared with placebo.
Methods
A randomized, double-blind, controlled trial was performed in the tertiary care center. Patients aged between 18 and 65 years who were American Society of Anesthesiologists class I–II and scheduled for abdominal hysterectomy with or without salpingo-oophorectomy were randomly allocated to a placebo or a pregabalin group. Patients received pregabalin 150 mg or placebo 1 h prior to anesthesia. Spinal anesthesia was achieved with 0.5% hyperbaric bupivacaine with morphine 0.2 mg. Intravenous patient-controlled analgesia morphine was provided postoperatively. Postoperative morphine consumption at 6, 12, and 24 h, time to first analgesic rescue, pain scores, adverse effects, and patient satisfaction were evaluated at 24 h after the operation.
Results
One hundred twenty-five patients were recruited and 119 patients (placebo N = 58, pregabalin N = 61) were included in the analysis. Forty-seven (81.0%) patients in the placebo group and 53 (86.9%) patients in the pregabalin group required morphine in the first 24 h. Median [IQR] 24-h morphine consumption was 4.0 [1.8, 10.0] mg in the placebo group and 5.0 [2.0, 11.0] mg in the prebagalin group, p = 0.60. There were no differences in cumulative morphine consumption at 6, 12, and 24 h postoperatively. The two groups also did not differ in time to first analgesic rescue, pain scores at rest and on movement, and side effects.
Conclusion
A single preoperative dose of pregabalin 150 mg did not reduce 24-h postoperative morphine consumption or pain scores or prolong the time to first analgesic rescue in spinal anesthesia with intrathecal morphine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multimodal analgesia combining various analgesics plays an important role in acute postoperative pain management due to its potential to reduce opioid consumption and minimize opioid side effects [1, 2]. Pregabalin is a structural analogue of gamma-aminobutyric acid (GABA) that binds potently to the alpha2-delta subunit of presynaptic, voltage-dependent calcium channels. It modulates the calcium influx at nerve terminals, resulting in the reduced release of several neurotransmitters [3]. The Food and Drug Administration has approved pregabalin as a medication for neuropathic pain and fibromyalgia as well as an adjunctive therapy for seizure and anxiety disorder [4]. In recent years, multiple meta-analyses have suggested that preoperative pregabalin is an effective adjunct for postoperative analgesia in terms of opioid-sparing effect and improved pain scores [5,6,7,8]. However, doses and administration regimens are still to be determined at the expense of some increased adverse effects.
Previous studies of the application of preoperative pregabalin in abdominal hysterectomy performed under general or spinal anesthesia reported a reduction in opioid consumption at 24 h [9,10,11,12] as well as a prolonged time to first analgesic rescue in spinal anesthesia [13, 14]. Intrathecal morphine is a well-known potent adjunct to spinal anesthesia, but the use of perioperative pregabalin in the context of intrathecal morphine has not been investigated. Due to the lack of data, we chose to investigate single-dose preoperative administration of pregabalin instead of multiple postoperative administration. We hypothesized that the administration of preoperative pregabalin in abdominal hysterectomy under spinal anesthesia with intrathecal morphine would decrease opioid consumption during the first 24 h postoperatively. Secondary outcomes were time to first analgesic rescue and potential adverse effects.
Materials and methods
Study center and population
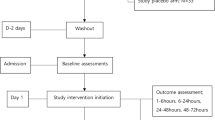
This double-blinded randomized controlled trial was conducted at the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand, which is a tertiary care center. This trial was registered at http://www.ClinicalTrials.gov: NCT02285010. After institutional review board approval (Si594/2014, 14 October 2014), patients who were scheduled to undergo abdominal hysterectomy with or without salpingo-oophorectomy under spinal anesthesia were recruited for participation. Female patients aged between 18 and 65 years who were American Society of Anesthesiologists physical status I–II were included. Patients agreed and understood how to use intravenous patient-controlled analgesia (IV-PCA). Exclusion criteria were a known or suspected allergy to gabapentinoids or any medications used in the study; a history of chronic usage of analgesic drugs, a psychiatric medication, or alcohol or drug addiction; or a diagnosis of kidney disease (creatinine clearance <60 mL/min/1.73 m2), liver disease, seizures, or a psychiatric disorder. Patients were admitted and approached 1 day prior to surgery by the research investigators (SS and PC). After discussing their participation and obtaining their written informed consent, the patients were enrolled in the study.
Randomization
The patients were randomly allocated to a pregabalin (150 mg) group or a placebo group by computer randomization in blocks of 10. Trial capsules were compounded and prepared by the Siriraj Hospital Pharmacy Department; they looked identical, were sealed in opaque individual packages, and were labeled with the appropriate randomization numbers. Randomization codes were concealed from patients, nurses, surgeons, anesthesiologists, recovery room personnel, and investigators until the end of the study.
Intervention
The appropriate study capsule was given to each patient 60 min prior to the start of anesthesia by a nurse who was blinded to the patient group assignments. After standard monitoring was carried out, including electrocardiography (ECG), noninvasive blood pressure, and pulse oximetry , each patient was preloaded with Ringer’s lactate solution 15 mL/kg. Spinal anesthesia was achieved with 0.5% hyperbaric bupivacaine and morphine 0.2 mg (total volume: 3.0–3.6 mL). If needed, the patient could be sedated with fentanyl 1–2 mcg/kg IV and/or pethidine <1 mg/kg IV and/or midazolam 0.02–0.07 mg/kg IV and/or propofol infusion 4–8 mg/kg/hr. In cases of prolonged surgery, the anesthesiologist could convert to general anesthesia provided that the agent was within the aforementioned dosage, including volatile anesthetic agents. Withdrawal criteria were (1) a change in the treatment plan such that the patient did not undergo abdominal hysterectomy or spinal anesthesia and (2) failure of spinal anesthesia such that the required anesthetic level could not be achieved or the level was inadequate for surgery. In the post-anesthesia care unit, IV-PCA (Sapphire pump, Q CORE Medical Ltd., Israel) with morphine was set to deliver 1 mg with a 5-min lockout interval and a 4-h limit of 35 mg. Other nonsteroidal anti-inflammatory drugs (NSAIDs) and local anesthetic infiltration were prohibited. As soon as a patient was able to accept a liquid diet, acetaminophen 500 mg PO every 6 h was initiated.
Outcome
The primary outcome of this study was postoperative cumulative morphine consumption at 24 h. The secondary outcomes were measured up to 24 h after operation and included time to first analgesic rescue, pain scores, and side effects. Accumulative morphine consumption and pain scores were measured at 6, 12, and 24 h. Pain scores at rest and on movement were measured by an 11-point numerical rating scale (NRS, 0 = no pain, 10 = worst possible pain). Side effects, including pruritus, dizziness, visual disturbance, nausea, vomiting, and patient satisfaction, were measured after 24 h on a 4-point scale (no, mild, moderate, severe). Heart rate, respiratory rate, oxygen saturation, and sedation score (scored on a 5-point scale: 0 = awake, 1 = mild sleepiness, 2 = moderate sleepiness, easy to arouse, 3 = extreme sleepiness, difficult to arouse, S = sleep) were monitored hourly by nurses.
Power analysis
The required sample size was calculated based on Niruthisard’s study, which showed that the mean (SD) 24-h morphine consumption in Thai patients who underwent abdominal hysterectomy under spinal anesthesia with intrathecal morphine 0.2 mg was 13.1 (12.7) mg [15]. We anticipated a 50% reduction in morphine consumption at 24 h postoperatively [9]. Assuming an alpha value of 0.05, the required sample size was calculated to be 60 patients in each group for a power of 80%.
Statistical analysis
Patient demographics and characteristics were expressed as number and percentage, median (interquartile range), and mean (standard deviation, SD), and were analyzed using the chi-square test for categorical variables and the independent t test for normally distributed continuous variables. The Mann–Whitney U test was used to analyze continuous variables that were not normally distributed: cumulative morphine consumption, time to first analgesic rescue, and blood loss. Dot–box graphs were analyzed using Tukey’s hinges method. The survival as a function of time to first analgesic rescue was analyzed using the Kaplan–Meier estimator. Ordinal variables such as side effects were analyzed using the chi-square test for trend. These statistical analyses were performed using PASW Statistics (SPSS) for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
Result
Patients, surgical, and anesthesia characteristics
We approached 142 patients between December 2014 and January 2016. Of these, 17 patients were found to be ineligible or they declined to participate, which resulted in the recruitment of 125 patients into the study. Six patients were withdrawn from analysis after randomization due to a change in surgical or anesthesia intervention: one from the pregabalin group and five from the placebo group. Two patients had a myomectomy instead of a hysterectomy, and four patients received general anesthesia due to a large tumor size, a difficult surgical approach, or at the surgeon’s request. No patient was withdrawn due to failed spinal anesthesia or refusal. Consequently, 119 patients (placebo N = 58, pregabalin N = 61) were included in the final analysis (Fig. 1).
Patient characteristics and intraoperative data, including anesthesia-related and surgical information, are presented in Table 1. It is evident that there was no difference in these parameters between the two groups.
Outcome
The primary outcome was 24-h morphine consumption, which was analyzed using the Mann–Whitney U test. Forty-seven (81.0%) patients in the placebo group and 53 (86.9%) patients in the pregabalin group required IV-PCA morphine in the first 24 h. The results indicated that there was no difference in cumulative morphine consumption between the placebo and pregabalin groups at 6, 12, and 24 h postoperatively. At 24 h, median [IQR] morphine consumption was 4.0 [1.8, 10.0] mg in the placebo group and 5.0 [2.0, 11.0] mg in the prebagalin group, p = 0.60. The mean (SD) 24-h morphine consumption was 7.4 (8.4) mg in the placebo group and 8.0 (8.8) mg in the pregabalin group (difference: −0.6; 95% confidence interval was −3.8 to 2.5). Time to first analgesic rescue did not differ between the two groups (Table 2; Figs. 2, 3). Pain scores at rest and on movement did not differ between the two groups at 6, 12, and 24 h (Table 3; Figs. 4, 5).
Side effects and patient satisfaction
No statistically significant difference was observed between the groups in terms of pruritus, dizziness, visual disturbance, nausea, vomiting, and patient satisfaction (Table 4). There were no apparent clinically relevant differences between the groups in sedation scores at 6, 12, and 24 h following surgery (Table 5).
Discussion
The use of pregabalin 150 mg preoperatively in patients undergoing abdominal hysterectomy with or without salpingo-oophorectomy under spinal anesthesia with intrathecal morphine and postoperative oral acetaminophen did not lead to greater analgesic efficacy compared to the placebo group in terms of postoperative morphine consumption, time to first analgesic rescue, and pain scores at rest and on movement at 6, 12, and 24 h. Side effects and patient satisfaction were also similar for the two groups. These results differed from those of a previous meta-analysis, which indicated that pregabalin reduced 24-h morphine consumption and 24-h pain scores in gynecologic surgery, open hysterectomy, laparoscopic cholecystectomy, orthopedic surgery, spine surgery, and miscellaneous procedures [8]. Categorized by surgical model, pregabalin 150–300 mg/day decreased 24-h opioid consumption in both pronociceptive and nonpronociceptive pain surgical models [6]. Categorized by dose and administration regimen, pregabalin at any of the doses considered (≤75, 100–150, and 300 mg) resulted in a reduction in 24-h morphine consumption; there was also no significant difference in pain outcome between single or multiple doses [7]. According to the aforementioned meta-analyses, single preoperative pregabalin 150 mg is an effective dose for postoperative pain control after abdominal hysterectomy. However, those studies did not monitor choice of anesthesia (general vs regional anesthesia) or the use of analgesic adjuncts such as nonsteroidal anti-inflammatory drugs (NSAIDs) and intrathecal morphine.
Our result differed from previous studies which focused on surgeries conducted under spinal anesthesia and showed a reduction in postoperative narcotic administration and a prolongation of time to first analgesic rescue in abdominal hysterectomy [13, 14], gynecologic surgery [16], vaginal hysterectomy [17], and urogenital surgery [18]. Intrathecal morphine is a potent analgesic adjunct that may influence the opioid-sparing effect of pregabalin. Our results are consistent with Niruthisard’s study, in which it was found that pregabalin 150 mg or celecoxib 400 mg or both did not reduce 24-h postoperative morphine consumption after total knee arthroplasty under spinal anesthesia with intrathecal morphine 0.2 mg [19]. Gabapentin, a predecessor of pregabalin that binds to the same receptor, did not reduce 24-h opioid consumption after cesarean section under spinal anesthesia with intrathecal morphine 0.1 mg in two studies [20, 21]. In both studies, each patient received ketorolac 30 mg IV and an acetaminophen suppository at the end of the surgery; moreover, in Monks’ study, oral diclofenac 50 mg was provided every 8 h and acetaminophen 1 gm every 6 h for 72 h. The addition of oral pregabalin to intrathecal morphine resulted in neither a reduction in opioid consumption nor a prolonged time to first analgesic rescue, as expected.
The results for perioperative pregabalin in the context of multimodal analgesia are inconsistent. Pregabalin 150 mg reduced 24-h opioid consumption after total knee arthroplasty under general anesthesia with celecoxib 400 mg preoperatively and 200 mg every 12 h until discharge and periarticular injection, p = 0.009 [22]. On the other hand, a study [23] compared pregabalin 75 and 150 mg and placebo in abdominal hysterectomy under general anesthesia, and found no difference in 24-h morphine consumption. Each patient received ketorolac 30 mg IV at the end of surgery and naproxen 500 mg PO 12 h after ketorolac administration. Another study [24] compared pregabalin 75 mg and 150 mg and diazepam 5 mg in gynecological laparoscopic surgery and found that pregabalin did not decrease opioid consumption in the recovery room or analgesic requirement in the first 24 h. Each patient received ibuprofen 800 mg PO in the evening of the day of surgery and the next day. Adding multiple doses of perioperative pregabalin did not reduce 24-h opioid consumption in ankle surgery and total knee arthroplasty under a combination of spinal-epidural anesthesia, peripheral nerve block, and oral oxycodone/acetaminophen [25, 26]. However, perioperative pregabalin was observed to be effective in a pronociceptive pain surgical model such as spine surgery at reducing analgesic rescue and pain scores, even when NSAIDs were implemented in a multimodal analgesia regimen [27, 28]. It is clear that type of surgery [6, 8] and dose of pregabalin [7] can influence postoperative analgesic effects, which may suggest that multimodal analgesia is another factor that can influence pregabalin efficacy.
Our study did not find any differences in adverse effects between the groups. In a meta-analysis of preoperative pregabalin, risk of visual disturbance was reported to be higher in a pregabalin group than in a control group (risk ratio 3.11, 95% confidence interval 1.34–7.21, p = 0.008) [29]. No difference was reported in sedation, dizziness, or headache between the control and pregabalin groups: p = 0.85, 0.56, and 0.54, respectively. In terms of nausea and vomiting, preoperative pregabalin was associated with a significant reduction in PONV (risk ratio 0.53, 95% confidence interval 0.39–0.73, p = 0.0001). Those authors postulated that the antiemetic mechanism is likely multifactorial, arising from either the signaling pathway itself or from the reduction in narcotic administration. Given the fact that pregabalin potentially has an adverse effect on visual disturbance, is expensive, and shows inconsistent efficacy, appropriate patient and operation selection is necessary to maximize analgesic efficacy and minimize undesired outcomes.
There are limitations of this study. First, we did not perform an intention-to-treat analysis. When the treatment plan was changed, the patient was withdrawn from the study. Second, we investigated the use of just one preoperative dose of pregabalin 150 mg. In the context of multimodal analgesia, multiple doses or a larger dose might be beneficial. Third, we did not evaluate functional recovery, which is one of the factors to consider in the context of early postoperative recovery. The homogeneity of the population and the procedure used meant that this study is not generalizable to the general population. Future research on perioperative pregabalin could focus on the influence of the administration regimen (single vs. multiple doses of pregabalin) and on chronic postsurgical pain. Another area of interest is the role of perioperative pregabalin in the context of multimodal analgesia.
In conclusion, preoperative single administration of pregabalin 150 mg did not reduce 24-h postoperative morphine consumption after abdominal hysterectomy with or without salpingo-oophorectomy under spinal anesthesia with intrathecal morphine. No differences were found in regard to time to first analgesic rescue, pain scores at rest and movement, adverse effects, or patient satisfaction.
References
Zukowski M, Kotfis K. The use of opioid adjuvants in perioperative multimodal analgesia. Anaesthesiol Intensive Ther. 2012;44(1):42–6.
Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22(5):588–93. doi:10.1097/ACO.0b013e328330373a.
Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119(5):1215–21. doi:10.1097/ALN.0b013e3182a9a896.
Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805–15. doi:10.1213/01.ane.0000287643.13410.5e.
Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106(4):454–62. doi:10.1093/bja/aer027.
Eipe N, Penning J, Yazdi F, Mallick R, Turner L, Ahmadzai N, Ansari MT. Perioperative use of pregabalin for acute pain—a systematic review and meta-analysis. Pain. 2015;156(7):1284–300. doi:10.1097/j.pain.0000000000000173.
Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10–31. doi:10.1093/bja/aeu293.
Lam DM, Choi SW, Wong SS, Irwin MG, Cheung CW. Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine (internet). 2015;94(46):e1944. doi:10.1097/MD.0000000000001944. Accessed 20 Feb 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4652811/pdf/medi-94-e1944.pdf (cited 20 Feb 2017).
Ittichaikulthol W, Virankabutra T, Kunopart M, Khamhom W, Putarawuthichai P, Rungphet S. Effects of pregabalin on post operative morphine consumption and pain after abdominal hysterectomy with/without salphingo-oophorectomy: a randomized, double-blind trial. J Med Assoc Thai. 2009;92(10):1318–23.
Yücel A, Ozturk E, Aydoğan MS, Durmuş M, Colak C, Ersoy MÖ. Effects of 2 different doses of pregabalin on morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind clinical trial. Curr Ther Res Clin Exp. 2011;72(4):173–83. doi:10.1016/j.curtheres.2011.06.004.
Ghai A, Gupta M, Hooda S, Singla D, Wadhera R. A randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomy. Saudi J Anaesth. 2011;5(3):252–7. doi:10.4103/1658-354X.84097.
Eman A, Bilir A, Beyaz SG. The effects of preoperative pregabalin on postoperative analgesia and morphine consumption after abdominal hysterectomy. Acta Medica Mediterr. 2014;30:481–5.
Kohli M, Murali T, Gupta R, Khan P, Bogra J. Optimization of subarachanoid block by oral pregabalin for hysterectomy. J Anaesthesiol Clin Pharmacol. 2011;27(1):101–5.
Talikoti AT, Dinesh K, Nanda A, Kumar P, Somasekharam P. Optimizing the dose of preemptive oral pregabalin for postoperative pain control after abdominal hysterectomy under spinal anaesthesia. J Clin Biomed Sci. 2013;3(1):12–9.
Niruthisard S, Werawataganon T, Bunburaphong P, Ussawanophakiat M, Wongsakornchaikul C, Toleb K. Improving the analgesic efficacy of intrathecal morphine with parecoxib after total abdominal hysterectomy. Anesth Analg. 2007;105(3):822–4. doi:10.1213/01.ane.0000277489.87015.1d.
Bafna U, Rajarajeshwaran K, Khandelwal M, Verma AP. A comparison of effect of preemptive use of oral gabapentin and pregabalin for acute post-operative pain after surgery under spinal anesthesia. J Anaesthesiol Clin Pharmacol. 2014;30(3):373–7. doi:10.4103/0970-9185.137270.
Rajappa GC, Vig S, Bevanaguddaiah Y, Anadaswamy TC. Efficacy of pregabalin as premedication for post-operative analgesia in vaginal hysterectomy. Anesth Pain Med (internet). 2016;6(3):e34591. doi:10.5812/aapm.34591. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5018136/pdf/aapm-06-03-34591.pdf (cited 2017 Feb 20). Accessed 20 Feb 2017
Park M, Jeon Y. Preoperative pregabalin prolongs duration of spinal anesthesia and reduces early postoperative pain: a double-blind, randomized clinical CONSORT study. Medicine (Baltimore). 2016;95(36):e4828. doi:10.1097/MD.0000000000004828. Accessed 20 Feb 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5023921/pdf/medi-95-e4828.pdf.
Niruthisard S, Earsakul A, Bunburaphong P, Chinda P, Anutinmanee R, Prapreuttham S, Tunprayoon A, Werawatganon T. Preoperative pregabalin and/or celecoxib for pain management after total knee arthroplasty under intrathecal morphine: a randomized controlled trial. Asian Biomed. 2013;7(4):579–85. doi:10.5372/1905-7415.0704.215.
Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg. 2011;112(1):167–73. doi:10.1213/ANE.0b013e3181fdf5ee.
Monks DT, Hoppe DW, Downey K, Shah V, Bernstein P, Carvalho JC. A perioperative course of gabapentin does not produce a clinically meaningful improvement in analgesia after cesarean delivery: a randomized controlled trial. Anesthesiology. 2015;123(2):320–6. doi:10.1097/ALN.0000000000000722.
Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with COX-2 inhibitor: a trial in total knee arthroplasty. J Arthroplasty. 2015;30(1):38–42. doi:10.1016/j.arth.2014.04.004.
George RB, McKeen DM, Andreou P, Habib AS. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anaesth. 2014;61(6):551–7. doi:10.1007/s12630-014-0147-4.
Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth. 2008;100(6):834–40. doi:10.1093/bja/aen098.
Yadeau JT, Paroli L, Kahn RL, Jules-Elysee KM, Lasala VR, Liu SS, Lin E, Powell K, Buschiazzo VL, Wukovits B, Roberts MM, Levine DS. Addition of pregabalin to multimodal analgesic therapy following ankle surgery: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2012;37(3):302–7.
YaDeau JT, Lin Y, Mayman DJ, Goytizolo EA, Alexiades MM, Padgett DE, Kahn RL, Jules-Elysee KM, Ranawat AS, Bhagat DD, Fields KG, Goon AK, Curren J, Westrich GH. Pregabalin and pain after total knee arthroplasty: a double-blind, randomized, placebo-controlled, multidose trial. Br J Anaesth. 2015;115(2):285–93. doi:10.1097/AAP.0b013e31824c6846.
Kim JC, Choi YS, Kim KN, Shim JK, Lee JY, Kwak YL. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine. 2011;36(6):428–33. doi:10.1097/BRS.0b013e3181d26708.
Khurana G, Jindal P, Sharma JP, Bansal KK. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine. 2014;39(6):E363–8. doi:10.1097/BRS.0000000000000185.
Grant MC, Betz M, Hulse M, Zorrilla-Vaca A, Hobson D, Wick E, Wu CL. The effect of preoperative pregabalin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 2016;123(5):1100–7. doi:10.1213/ANE.0000000000001404.
Acknowledgements
We wish to thank Mr. Suthipol Udompunturak, statistician from the Office for Research and Development, for helping with the statistical analysis, and Ms. Nichapat Sooksri, Department of Anesthesiology, for administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was supported by the Faculty of Medicine, Siriraj Hospital, Mahidol University [Grant number (IO) R015839006].
About this article
Cite this article
Kiatchai, T., Sanansilp, V., Triyasunant, N. et al. Effects of pregabalin on postoperative pain after hysterectomy under spinal anesthesia with intrathecal morphine: a randomized controlled trial. J Anesth 31, 861–868 (2017). https://doi.org/10.1007/s00540-017-2406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2406-3