Abstract
Background
EWGSOP2 criteria for sarcopenia recommends the use of either handgrip strength (GS) or 5-times repeated chair stand test (RCS) as a muscle strength measure. We aim to compare the impact of different muscle strength definitions on sarcopenia prevalence and predictive validity for 2-year outcomes, using the EWGSOP2 clinical algorithm.
Methods
We studied 200 community-dwelling older adults, comparing sarcopenia prevalence using three muscle strength definitions: 1) maximum GS (Asian Working Group cutoffs); 2) RCS-1 (standard cutoff >15s); and 3) RCS-2 (ROC-derived cutoff >12.5s). Two-year outcomes include: 1) Incident frailty (modified Fried criteria); 2) Physical performance [Short Physical Performance Battery (SPPB) score <10]; and 3) Quality of life [EuroQol-5 dimension (EQ-5D) <25th percentile]. We performed logistic regression on 2-year outcomes adjusted for age, gender, cognition and mood.
Results
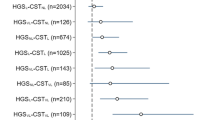
Prevalence of confirmed sarcopenia was 14.5%, 4% and 9% for GS, RCS-1 and RCS-2 respectively. For 2-year outcomes (N=183), RCS-2 predicted incident frailty (OR: 5.7, 95% CI 1.4–22.8, p=0.013), low SPPB (OR: 4.4, 95% CI 1.4–13.1, p=0.009), and trended towards predicting low QOL (OR: 2.1, 95% CI 0.9–4.9, p=0.095). In contrast, GS and RCS-1 did not predict frailty nor low QOL, but predicted low SPPB only (GS: OR 3.8, 95% CI 1.3–10.6, p=0.01; RCS-1: OR: 8.8, 95% CI 2.2–35.0, p=0.002).
Conclusions
Sarcopenia prevalence varies with muscle strength definitions, with GS being significantly higher vis-á-vis RCS definitions. Our results also support the use of population-specific over standard cutoffs for RCS to obtain intermediate estimates of sarcopenia prevalence and the best predictive validity for two-year outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder that is associated with increased risk of falls, fractures, disability and mortality (1). To diagnose sarcopenia, early definitions emphasized loss of muscle mass as a key criterion (2). However, decreased muscle mass alone does not adequately explain adverse outcomes (3). Consequently, recent definitions of sarcopenia combine loss of muscle mass with loss of strength and physical performance.
In the updated definition proposed by the European Working Group on Sarcopenia in Older People (EWGSOP2) (1), muscle strength is elevated to the forefront of the diagnostic framework. To measure muscle strength, handgrip strength (GS) and 5-times repeated chair stand (RCS) are recommended. While both are convenient and reliable surrogates for arm and leg strength, the impact of different muscle strength definitions on sarcopenia prevalence and longitudinal adverse outcomes is unknown. Furthermore, EWGSOP-recommended cutoffs reference non-Asian populations. Therefore, we aim to compare the impact of different muscle strength definitions on (1) sarcopenia prevalence; and (2) the predictive validity for adverse outcomes of physical performance, frailty and quality of life at 2 years in an Asian cohort.
Methods
Participants and setting
The “Longitudinal Assessment of Biomarkers for characterization of early Sarcopenia and predicting frailty and functional decline in community-dwelling Asian older adults Study” (GERI-LABS) is a prospective cohort study involving 200 participants [4]. We included cognitively intact and functionally independent older adults aged 50 years and above residing within the community. We excluded older adults who have history of dementia and living in sheltered or nursing homes.
We obtained ethics approval from the Domain Specific Review Board of the National Healthcare Group and written informed consent from participants.
Measurements
Sarcopenia assessment
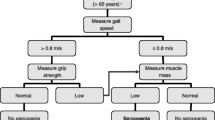
We measured GS using a hydraulic hand dynamometer (North Coast™). Two trials of GS for each hand were obtained and the highest value was used. We assessed RCS by measuring the time needed to rise five times from a seated position with arms folded across the chest. We obtained lean mass measures via dual-energy X-ray absorptiometry (DXA) (Discovery™ APEX 13.3; Hologic, Bedford, MA, USA). Relative appendicular skeletal muscle mass (ASM) was calculated using the Asian Working Group for Sarcopenia (AWGS) recommended gender-specific cutoff values for height-adjusted ASM (ASM/height2) (5). Gait speed was assessed based on the time taken to walk 3 meters, with ≤0.8 m/s cutoff indicative of severe sarcopenia. Poor physical performance, defined by a Short Physical Performance Battery (SPPB) cutoff score of <10 (6, 7) also indicated severe sarcopenia.
Sarcopenia was diagnosed by the AWGS and EWGSOP2 criteria, employing the different muscle strength measures, viz. GS and RCS. Low GS was defined according to published Asian cutoffs as <26 kg for men and <18 kg for women (5). For RCS, we used 2 cutoffs points: the EWGSOP2-recommended cutoff of >15 s for 5 rises (RCS-1) (1); and an empirically derived cutoff of >12.5 s (RCS-2). RCS-2 was obtained using Receiver Operating Characteristic (ROC) analysis in the same cohort with AWGS-defined impaired maximum GS as the outcome variable. The area under the curve was 0.61 (95% CI 0.51–0.72). The optimal cutoff was determined by the maximum value of Youden index, yielding a sensitivity of 32.5% and specificity of 90.4% (Appendix 1).
Two-year outcomes
Adverse outcomes assessed included incident frailty, physical performance and quality of life (QOL) at 2 years. A modified Fried criteria (8) was used to measure frailty, comprising 5 domains: low GS, slow gait speed, body mass index (BMI) <18.5, low physical activity and fatigue. Published Asian cutcoffs were used to define low GS and slow gait speed (maximum GS <26 kg for males and <18 kg for females; and gait speed <0.8 m/s) (5). Low physical activity was defined using the pentile cutoff of <29 on the Frenchay Activities Index (9). We modified 2 questions from the Center for Epidemiologic Studies-Depression Scale to assess fatigue. Participants answered yes/no to the following: (a) I felt that everything I did was an effort, and (b) I could not get “going”. The frailty sub-domain of fatigue was considered positive if either question was endorsed. A participant was considered frail if ≥3 out of 5 domains were positive. Poor physical performance was defined as a Short Physical Performance Battery (SPPB) cutoff score of <10. Poor QOL was indicated by values below the lowest quartile of EuroQol-5 dimension (EQ-5D) utility scores (<0.854) (10, 11).
Covariates
Baseline data included age, gender, ethnicity and comorbidities. Physical function was assessed using the modified Barthel index (MBI) (12) for activities of daily living (ADL) and the Lawton Index for Instrumental Activities of Daily Living (IADL) (13). Cognitive function was assessed using a locally-validated modified Chinese version of the mini-mental state examination (CMMSE) (14) and depressive symptoms by the 15-item Geriatric Depression Scale (GDS) (15).
Statistical analysis
Sarcopenia prevalence according to AWGS and EWGSOP2 criteria are presented as percentages. For adverse outcomes, we only included subjects who completed 2-year follow-up. Additionally, for the outcome of incident frailty, we excluded subjects who fulfilled frailty criteria at baseline. We performed logistic regression analysis to examine the relationship between GS, RCS-1 and RCS-2 with the adverse outcomes of incident frailty, physical performance and QOL at 2 years, adjusted for age, gender, baseline CMMSE and GDS scores. Two-sided tests with a significance level of P <0.05 were applied. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY).
Results
We included 200 subjects in the study. Baseline characteristics are presented in Table 1.
Sarcopenia prevalence (Table 1)
The prevalence of sarcopenia according to AWGS criteria was 15.5%. Using EWGSOP2 criteria with different muscle strength measures, the prevalence of probable sarcopenia was 21% (GS), 6.5% (RCS-1), 15.5% (RCS-2) and 14.5% (GS), 4% (RCS-1) and 9% (RCS-2) for confirmed sarcopenia. Using gait speed as an indicator, the prevalence of severe sarcopenia was 1.5% (GS), 1.5% (RCS-1) and 2% (RCS-2) respectively. Using SPPB, the prevalence was 2% (GS), 2.5% (RCS-1) and 3% (RCS-2).
Adverse outcomes (Table 2)
We analyzed 183 (91.5%) subjects who completed 2-year follow up. Age, gender, baseline MBI, IADL and SPPB did not differ between excluded subjects (N=17) and completers. For the outcome of incident frailty, we excluded a further eight subjects with baseline frailty, with 175 (87.5%) subjects included in the regression analysis.
Nine (5.2%) cases of incident frailty were observed at 2 years. In adjusted analysis, low baseline GS did not predict incident frailty (OR 4.3, 95% CI 0.9–21.0). For participants with impaired baseline RCS, RCS-1 cutoff did not predict incident frailty, unlike RCS-2 in which significantly increased odds were observed (OR 5.7, 95% CI 1.4–22.8). For the outcome of physical performance at 2 years, both GS and RCS predicted SPPB<10 in adjusted models (GS: OR 3.8, 95% CI 1.3–10.6; RCS-1: OR 8.8, 95% CI 2.2–35.0; RCS-2: OR 4.4, 95% CI 1.4–13.1). For 2-year QOL outcomes, low baseline GS and RCS-1 were not predictive, whereas the RCS-2 cutoff approached statistical significance (OR 2.1, 95% CI 0.9–4.9).
Discussion
Our study contributes to the extant body of evidence by demonstrating how different muscle strength definitions impact on sarcopenia prevalence and predictive validity for adverse outcomes. Specifically, sarcopenia prevalence was higher when GS was used vis-á-vis RCS definitions. We obtained intermediate estimates of sarcopenia prevalence using cohort-specific over standardized cutoffs for RCS. Using different muscle strength measures (GS, standardized and cohort-specific RCS cutoffs) also differentially predict adverse health outcomes of incident frailty, poor physical performance and low QOL at 2 years.
Our finding of a higher sarcopenia prevalence with GS compared to RCS, regardless of cutoff used, is similar to a previous study which showed an overall prevalence of 9.3% with GS and 7.9% with RCS (16). While limited evidence exists on the difference in prevalence between case-finding approaches using GS and RCS, the wide range of sarcopenia prevalence (4–14.5%) in our study suggests that the choice of muscle strength measures and attendant cutoffs is likely to have a significant impact on prevalence figures. This is germane in light of the elevation of muscle strength to the forefront of the EWGSOP2 diagnostic framework.
GS is easy to perform, incurs little cost and is a good predictor of disability and mortality. Thus, it is recommended as the preferred muscle strength measure, with RCS used only when GS is unavailable or impractical (17). However, our results indicate that muscle strength measures may not be interchangeable. Besides the wide variation in sarcopenia prevalence depending on choice of GS or RCS measures, RCS predicted incident frailty at 2 years whereas GS did not. Earlier studies report low to moderate correlation of GS with RCS and lower extremity strength. Furthermore, knee extensor and flexor strength, but not GS, are correlated with usual gait speed (18). A possible explanation is the association between increased weight and presence of knee osteoarthritis with declines in lower extremity performance (19). In our cohort, the mean BMI (24.0+3.7 kg/m2) crosses the Asian threshold for “overweight” (20), and while knee osteoarthritis prevalence estimates are unavailable, it is a common problem in the older adult (21). Therefore, in our cohort, lower extremity muscle strength, measured by RCS may better predict incident frailty. Taken together, both strength measures may not be interchangeable, and the choice of which test to use depends on the population to be screened.
Different RCS cutoffs also result in varying sarcopenia prevalence estimates. Recommended cutoff times, including EWGSOP2, are higher than in existing studies conducted in Asian cohorts (22). For example, in Japanese community-dwelling older adults, the optimal cutoff time for the development of disability is 9 seconds (23). In contrast, a 17-second cutoff categorized a high-risk group for mobility limitation in another cohort with different sociodemographic characteristics (24). Comparative studies of physical performance measures, including the chair stand test, show differences between Asian and non-Asian populations. These differences may be ascribed to variation in body size (height, weight and BMI), physical activity level or living in rural/urban settings (25). Thus, our study further highlights the need for specific cutoffs to account for population differences.
The strengths of the present study include the comprehensive follow-up of a representative healthy community-dwelling cohort at baseline. This provides new insight into applying the EWGSOP2 criteria to predict important longitudinal outcomes. However, several limitations exist. Firstly, the relatively small sample size and short follow-up may have accounted for the lower rates of incident frailty and poor physical performance in our cohort of well-functioning older adults and the wide confidence intervals observed for incident frailty and SPPB. The small sample size also precludes further subgroup analysis by gender. Secondly, GS and RCS are components of the outcome measures, physical frailty and SPPB. Despite this, GS does not predict incident frailty although it is part of the Fried frailty criteria, whereas RCS-2 predicts incident frailty even though it is not. Furthermore, we report a comprehensive range of 3 outcomes, encompassing clinical, physical performance and patient-reported measures.
In summary, the present study in an Asian population showed that sarcopenia prevalence among community-dwelling older adults differed according to the muscle strength measures used. Sarcopenia prevalence was higher if GS was used, compared to RCS. Choosing a population-specific cutoff for RCS yielded an acceptable prevalence estimate, intermediate between that of GS and standard RCS cutoff. This cutoff also demonstrated the best predictive validity for adverse outcomes at 2 years. Our results underscore the importance of using standardized, population-specific definitions of sarcopenia in clinical practice. Further research is required on applying standardized EWGSOP2 criteria to identify cases for interventions to prevent and treat sarcopenia.
References
Cruz-Jentoft, A.J., G. Bahat, J. Bauer, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1): 16–31.
Janssen, I., R.N. Baumgartner, R. Ross, et al. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4): 413–21.
Delmonico, M.J., T.B. Harris, M. Visser, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6): 1579–85.
Chew, J., L. Tay, J.P. Lim, et al. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J Nutr Health Aging. 2019;23(10): 979–986.
Chen, L.K., L.K. Liu, J. Woo, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2): 95–101.
Guralnik, J.M., E.M. Simonsick, L. Ferrucci, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2): M85–94.
Vasunilashorn, S., A.K. Coppin, K.V. Patel, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64(2): 223–9.
Fried, L.P., C.M. Tangen, J. Walston, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3): M146–56.
Wade, D.T., J. Legh-Smith and R. Langton Hewer. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med. 1985;7(4): 176–81.
Oh, B., B. Cho, H.C. Choi, et al. The influence of lower-extremity function in elderly individuals’ quality of life (QOL): an analysis of the correlation between SPPB and EQ-5D. Arch Gerontol Geriatr. 2014;58(2): 278–82.
Abdin, E., M. Subramaniam, J.A. Vaingankar, et al. Measuring health-related quality of life among adults in Singapore: population norms for the EQ-5D. Qual Life Res. 2013;22(10): 2983–91.
Mahoney, F.I. and D.W. Barthel. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14: 61–5.
Lawton, M.P. and E.M. Brody. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3): 179–86.
Sahadevan, S., P.P. Lim, N.J. Tan, et al. Diagnostic performance of two mental status tests in the older chinese: influence of education and age on cut-off values. Int J Geriatr Psychiatry. 2000;15(3): 234–41.
Yesavage, J.A., T.L. Brink, T.L. Rose, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1): 37–49.
Kim, M. and C.W. Won. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing. 2019;48(6): 910–916.
Bahat, G. and A.J. Cruz-Jentoft. Putting Sarcopenia at the Forefront of Clinical Practice. Eur J Geriatr Gerontol. 2019;1(2): 43–45.
Harris-Love, M.O., K. Benson, E. Leasure, et al. The Influence of Upper and Lower Extremity Strength on Performance-Based Sarcopenia Assessment Tests. J Funct Morphol Kinesiol. 2018;3(4).
Forrest, K.Y., J.M. Zmuda and J.A. Cauley. Correlates of decline in lower extremity performance in older women: A 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61(11): 1194–200.
W. H. O. Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403): 157–63.
Leung, Y.Y., S. Ma, M. Noviani, et al. Validation of screening questionnaires for evaluation of knee osteoarthritis prevalence in the general population of Singapore. Int J Rheum Dis. 2018;21(3): 629–638.
Buatois, S., D. Miljkovic, P. Manckoundia, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8): 1575–7.
Makizako, H., H. Shimada, T. Doi, et al. Predictive Cutoff Values of the Five-Times Sit-to-Stand Test and the Timed “Up & Go” Test for Disability Incidence in Older People Dwelling in the Community. Phys Ther. 2017;97(4): 417–424.
Cesari, M., S.B. Kritchevsky, A.B. Newman, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57(2): 251–9.
Aoyagi, K., P.D. Ross, M.C. Nevitt, et al. Comparison of performance-based measures among native Japanese, Japanese-Americans in Hawaii and Caucasian women in the United States, ages 65 years and over: a cross-sectional study. BMC Geriatr. 2001;1: 3.
Acknowledgements
We would like to thank all participants who contributed to this study. This study was supported by the Lee Foundation Grant 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: The authors have no conflicts of interest to disclose.
Ethical standards: Ethics approval for the study was obtained from by the Domain Specific Review Board of the National Healthcare Group and informed written consent was obtained from the participants.
Electronic supplementary material
Appendix 1
. Receiver Operating Characteristic (ROC) analysis of five-times repeated chair stand (RCS) time with Asian Working Group for Sarcopenia (AWGS)-defined impaired maximum grip strength (GS) as the outcome variable.
Rights and permissions
About this article
Cite this article
Chew, J., Yeo, A., Yew, S. et al. Muscle Strength Definitions Matter: Prevalence of Sarcopenia and Predictive Validity for Adverse Outcomes Using the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) Criteria. J Nutr Health Aging 24, 614–618 (2020). https://doi.org/10.1007/s12603-020-1371-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-020-1371-y