Abstract
Background
Malnutrition is a major determinant of the physical frailty syndrome. Dynamic transitions in frailty states over time is well documented, but few studies have documented temporal changes in nutritional states and whether they influence frailty outcomes.
Design
Longitudinal cohort study.
Setting and Participants
Community-dwelling older Singaporeans aged ≥55y with a 5-year follow-up (n=1162) in the Singapore Longitudinal Ageing Study 2 (SLAS-2).
Measurements
The Mini Nutritional Assessment Short-Form (MNA-SF) was used to determine nutritional status, and the Fried’s criteria (shrinking, weakness, slowness, exhaustion and inactivity) was used to assess physical frailty phenotype at both baseline and follow-up. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were adjusted for multiple baseline co-variables.
Results
At baseline, being at risk of malnutrition/malnourished was associated with increased odds of prevalent prefrailty (OR=2.76, 95% CI=1.86-4.10) and frailty (OR=4.10, 95% CI=1.41-11.9). Baseline robust individuals who were persistently at risk of malnutrition/malnourished showed an increased odds of conversion to being pre-frail/ frail at follow-up (OR=3.45, 95% CI=1.00-11.9). Among baseline pre-frail/frail individuals, reversion to being robust were significantly less likely among those who were persistently at risk of malnutrition/malnourished (OR=0.26, 95% CI=0.10-0.67) and those whose baseline normal nutrition worsened at follow-up (OR=0.20, 95% CI=0.06-0.74).
Conclusion
Changes in nutritional states are associated with frailty state transitions, and monitoring changes in nutritional status is recommended for the prevention and severity reduction of frailty among older people in the community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a state of increased vulnerability to stressors due to progressive multisystem physiological decline leading to increased risks of adverse health outcomes including functional decline, poor quality of life, loss of independence, hospitalization and institutionalizations, and mortality (1). Frailty is widely characterized as a physical phenotype including features of body mass loss, muscle weakness, slow gait, exhaustion and physical inactivity (2). Studies show that (protein-calorie) macronutrient and micronutrient deficiencies, malnutrition and poor quality of diet are associated with the development and severity of frailty in older people (3-6). Frailty and malnutrition are equally very common among older persons. Studies reported community prevalence of 50% prefrailty and 5% frailty (using the physical phenotype criteria) (7), and Mini Nutritional Assessment (MNA) estimates of malnutrition at 6% in the community, and 50% in the clinical and long-term care settings (8).
Frailty is a dynamic process characterized by transitions between adjacent frailty states (9, 10). Though progression typically favours a more deteriorated state of frailty, studies show that a sizeable proportion of pre-frail/frail individuals revert back to lesser frailty states (11, 12). Identifying key factors influencing frailty transitions thus has significant therapeutic implications. In contrast, little is known of the longterm changes in nutritional status over years among free-living older persons in the community (13), and there are no studies that show whether deterioration or improvement in nutritional status over time is associated with changes in frailty status.
In this study, we investigated the association between changes in nutritional states and frailty state transitions in a population-based older adult cohort in the Singapore Longitudinal Ageing Study 2 (SLAS-2) with a 5-year followup, using the Mini Nutritional Assessment Short-Form (MNASF) and the Fried’s frailty criteria to assess nutritional and frailty status at baseline and follow-up.
Methods
Study design and participants
The Singapore Longitudinal Ageing Study (SLAS) is a long-term observational prospective cohort study of ageing and health of older persons aged 55 and above in Singapore. Two cohorts were recruited from community-living older adults aged 55 and above in separate recruitment waves in 2003-2005
(SLAS-1) and 2008–2013 (SLAS-2) in different geographical areas. An extensive range of demographic, psychosocial, life style, behavioural, biomedical, physical, cognitive, functional and blood biomarkers data were collected at baseline interviews. The participants in SLAS-1 had completed two follow-ups approximately 3 years apart, and participants in SLAS-2 started the first follow-up in 2013, and is ongoing. Details of the methodology of the SLAS-1 and SLAS-2 cohorts have been described in previous papers (14). The study was approved by the National University of Singapore Institutional Review Board (NUS IRB) with informed consent collected for all participants.
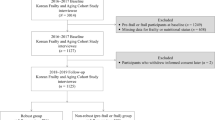
The participants in this study were selected from the total 3270 participants recruited at SLAS-2 baseline and involved 1162 participants who had been re-assessed at follow-up to date as of February 2017 (flow chart detailed in Supplementary Figure 1).
Measurements
Frailty
Participants were assessed for frailty in accordance to the Fried’s frailty criteria employed in the Cardiovascular Health Study: shrinking (unintentional weight loss), weakness, slowness, physical inactivity and exhaustion (2). Participants who presented none of the components were defined as robust, 1 to 2 components as pre-frail, and 3 or more components as frail.
Shrinking
Body mass index (BMI) of less than 18.5 kg/m2 and/or unintentional weight loss of 4.5 kg (10 pounds) or more within the past 6 months.
Weakness
Dominant knee extensions were used to evaluate leg muscle strength, with an average value from 3 trials used (in kilograms), standardized based on gender. Knee extension strength in the lowest quintile was categorized as weakness.
Slowness was determined with a 6-meter fast gait speed test, using an average of 2 measurements. A gait speed of less than 0.8 m/s was categorized as slowness.
Physical inactivity
Physical activities were determined based on time in hours (self-reported) used to conduct light (such as office work, strolling, driving a car, personal care or standing with minute motion), moderate, and vigorous activities (such as strenuous sports dancing, gardening, jogging, brisk walking or swimming) throughout the week (both weekdays and weekends). Physical inactivity was denoted using the overall amount of time used to perform moderate and vigorous activities weekly and activity time falling below the gender-specific lowest quintile.
Exhaustion was evaluated using 3 questions from the vitality domain in the Medical Outcomes Study 12-Item Short Form Healthy Survey (SF-12): “Did you feel tired?” “Did you feel worn out?” “Did you have a lot of energy?”. The total summed scores ranged from 3 to 15 with a higher score corresponding to more energy. Exhaustion was denoted with a score of less than 10.
Nutritional status
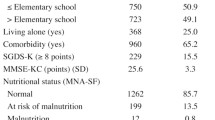
MNA-SF was used to evaluate nutritional status. The MNASF is a commonly employed nutritional screening tool for the elderly population, comprising 6 questions relating to health, cognition, mobility and nutrition (14). Total scores range between 0–14, with 12–14 indicating normal nutritional status, 8–11 indicating at risk of malnutrition and 7 or less indicating malnourished.
Baseline Covariates
Sociodemographic data included age, gender, race, marital status, education, living arrangements and housing type (an indicator of socioeconomic status). Central obesity was determined by waist circumference ≥90 cm. Medical comorbidities were evaluated based on responses to a selfreported checklist of whether participants were diagnosed and treated by a medical practitioner for 22 medical illnesses for the past year. Cognitive function was determined using the locally validated Mini-Mental State Examination (MMSE) (15). Normal cognition was denoted by a score of 24 or more, and cognitive impairment was denoted by a score of less than 24. Depressive symptoms were evaluated using the Geriatric Depression Scale (GDS), previously validated in local Singaporean populations (16). Clinically significant depression was identified as a score of 5 or more. Polypharmacy was identified as self-reported use of 5 or more medications. Hospitalization was identified as self-reported new hospitalization events for any medical conditions within the past year. The instrumental/basic activities of daily living (IADL/ADL) disability was determined by self-reported difficulty and/or requiring assistance in at least one IADL and/or ADL activity from the Lawton Instrumental Activity of Daily Living and Barthel Basic Activities of Daily Living instruments. Quality of life (QOL) was measured using the SF-12 physical component score (PCS) and mental component score (MCS), and poor QOL was determined by values below the lowest quartile of PCS score.
Statistical Analysis
The data were analysed using Stata Version 12.0 (Stata Corp LP, Texas, USA). Categorical variables were represented as count and percentage (n, %) while continuous variables were represented as mean ± standard deviation. Differences in the distribution of categorical variables and continuous variables among different groups were evaluated using Chi- Square test and Kruskall-Wallis test respectively. Statistical significance was specified by a P-value of <0.05. Multinomial logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of associations. OR estimates were adjusted for baseline age, gender, education levels, race, house type, marital status, living alone, cognitive impairment, physical activity score, social activity score, productive activity score, lifestyle activity score, PCS score, MCS score, low albumin, low cholesterol, hospitalization, IADL/ADL disability, type 2 diabetes, arthritis, dyslipidaemia, mental and sleep disorders, stroke, cardiac disease, anaemia, multi-morbidity, polypharmacy, and hearing loss.
Conclusion
Baseline characteristics of participants according to nutritional and frailty status are summarized in Table 1. As expected, pre-frailty and frailty compared to robust, and being at risk of malnutrition/malnourished compared to normal nutrition in common were significantly associated with many baseline covariate measures of adverse sociodemographic and lifestyle variables related to social deprivation, chronic medical illnesses and multiple morbidities, and poorer physical and mental health and function. Compared to participants who successfully completed follow-up in our analysis, those who were not included were more malnourished, more frail at baseline, and had worse profiles on baseline characteristics shown in Table 1 (data not shown).
Cross-sectional associations at baseline
The associations between baseline nutritional states and prevalence of pre-frailty and frailty were reported in Table 2. As there were only 3 malnourished individuals who were also pre-frail or frail, data for both “at risk of malnutrition” and “malnourished” was presented as one group. At baseline, individuals who were at risk of malnutrition/malnourished had a higher prevalence of pre-frailty (adjusted OR=2.76, 95% CI=1.86-4.10) and frailty (adjusted OR=4.10, 95% CI=1.41-11.9), in comparison to their “normal nutrition” counterparts.
Nutritional and frailty state transitions
As shown in Table 3, 89.5% of participants with normal nutrition at baseline remained unchanged with normal nutrition at follow-up, but 10.5% converted to being at risk of malnutrition/malnourished. Among participants who were at risk of malnutrition/malnourished at baseline, 46.5% remained being at risk of malnutrition/malnourished, while 53.5% reverted to normal nutrition.
Frailty state transitions among the robust at baseline were 53.2% who remained robust, while the others either progressed to pre-frailty (43.4%) or frailty (3.4%) at follow-up. Amongst individuals who were already pre-frail at baseline, 33.9% displayed improvement to being robust, while 10.9% deteriorated to being frail. Among frail individuals at baseline, 53.1% showed improvements to being pre-frail and 18.8% to being robust.
Associations between nutritional and frailty state transitions
At baseline and follow-up, robust individuals who were persistently at risk of malnutrition/malnourished showed an increased odds of conversion to being pre-frail/frail (adjusted OR=3.45, 95% CI=1.00-11.9) (Table 4). Among pre-frail/ frail individuals, reversion to being robust were significantly less likely among those who were persistently at risk of malnutrition/malnourished (adjusted OR=0.26, 95% CI=0.10-0.67) and those whose baseline normal nutrition worsened at follow-up (adjusted OR=0.20, 95% CI=0.06-0.74) (Table 4).
Discussion
Our study showed that 46.8% of robust individuals transited to poorer states of pre-frailty and frailty upon follow-up. These results reiterated previous findings of the dynamic transitions in frailty (8, 9). Interestingly, reversion to improved states of frailty was observed, which was higher than those observed in other studies. In this population, 33.9% of pre-frail individuals reverted to robust state compared to the 11.9% observed in a prior study (8), and more than half of frail individuals (53.1%) converted to pre-frailty state at follow-up. This may be attributed to the relatively younger age and longer duration of follow-up of our cohort.
We showed at the same time that there were changes in nutritional states of older people over time that were dynamically associated with corresponding frailty state transitions. Nutrition has been shown in previous studies and systematic reviews to be an important determinant of frailty development and severity. Studies show that low intake of protein, vitamins A, B6, D, E and C, folate, and polyphenols, low serum levels of carotenoids, α-tocopherol, 25-hydroxyvitamin D, and vitamin B6, and poor nutritional status were positively associated, and dietary patterns rich in milk, meat and fish, fresh fruits and vegetables, and Mediterranean-like or high quality balanced diet were inversely associated with the development and severity of frailty in older people (3-6). However, most studies were based on cross-sectional design, and the few longitudinal studies have all determined nutritional status only once at baseline. Our baseline prevalence data showed the same: individuals at risk of malnutrition or malnourished were more than 2 times likely to be pre-frail and more than 4 times likely to be frail. Notably, however, the longitudinal analysis in this study showed that changes in nutritional status strongly influenced frailty outcomes. Older persons who were persistently at risk of malnutrition or malnourished over time were 3–4 times more likely to convert from being robust to pre-frailty or frailty. Older persons who were already pre-frail or frail were less likely to revert to being robust when their normal nutritional status worsened or when they remained at risk of malnutrition or malnourished.
Although systematic reviews suggest that a high-quality diet with satisfactory energy intake, optimally high intake of quality protein (evenly distributed throughout all meals), and meals that are rich in micronutrients and antioxidants are likely important factors for preventing and delaying the onset of frailty in older adults, there are presently still very limited evidence from randomized controlled trials in support of the efficacy of nutritional interventions in reversing frailty (17, 18) and even less still in improving physical performance and functional ability (19-24).
More randomized controlled trials are needed, but despite this, interventions to improve nutrition among older persons is arguably most certain to be effective for preventing and reducing frailty and improving functional wellbeing in older adults. Malnutrition is evidently a modifiable risk factor for frailty. This longitudinal study indicated that in the naturalistic real-world setting, certain positive changes in dietary, life style or health states had evidently helped to improve the nutritional and frailty status in some study participants. On the other hand, the observation that worsening nutritional status and its association with worsening frailty status among other participants suggests that monitoring changes in nutritional status and appropriate interventions to improve nutritional health should be recommended in early interventions to prevent and reduce the severity of frailty among older people in the population.
In this regard, early interventions should consider screening, assessment and interventional measures for both malnutrition and frailty together, given the intimate relations between the two. Indeed both constructs share phenotypic similarities (25, 26) and overlapping pathophysiological pathways, though still not fully understood. However, they are not interchangeable constructs, and represent distinct geriatric syndromes (27, 28). The presence of either one or both syndromes in the same individual increases dramatically the risk of future adverse health outcomes that are preventable. More interventional studies should be conducted to identify the interventional modalities for different modifiable risk factors and their individual and combined efficacy (such as protein supplementation and exercise) in improving functional and mortality outcomes.
The design of a large representative population-based cohort of older persons makes the study results generalizable to multiethnic Asian populations of community-dwelling older persons. However, some limitations still exist in our study. First, the changes of health behaviors/status from baseline to the 5-year follow-up is not considered in our study, which may also be associated with transitions of nutritional or frailty status. The second is the younger age of cohort (≥55 years) and limited number of participants classified as frail and malnourished. Third, although the MNA is currently most widely accepted for measuring global malnutrition, given the known phenotypic similarities of malnutrition and physical frailty, it is difficult to determine the extent to which the observed close association between nutritional and frailty state transitions is due to the conflation of measurement items in the two constructs. Indeed there are non-physical measurement items in the MNA that do not overlap with physical frailty, but future studies should consider using other more specific and biological measures of nutritional status.
Conclusion
Changes in nutritional states are associated with frailty state transitions, and monitoring changes in nutritional status is recommended for the prevention and severity reduction of frailty among older people in the community.
Funding Sources: The study is supported by a research grant (No. 03/1/21/17/214) from the Biomedical Research Council, Agency for Science, Technology and Research (A*STAR).
Role of the Sponsors: The sponsors had no role in the conduct of the study or preparation of this manuscript.
Author Contributions: TPN had full access to all of the data in the study and has primary responsibility for final content. TPN formulated the hypothesis, designed the study, supervised and reviewed the data analysis, and reviewed and revised the manuscript. KW performed the data analysis, interpreted the results, and drafted and reviewed the manuscript. FST reviewed the literature, interpreted the results, and drafted and reviewed the manuscript. MSZN and QG contributed to the study design and data collection, reviewed the results and drafts of the manuscript. SLW contributed to the study design, reviewed the literature, interpreted the results, drafted and reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Disclosure: None reported.
Acknowledgments: We thank the following voluntary welfare organizations for their support: Geylang East Home for the Aged, Presbyterian Community Services, St Luke’s Eldercare Services, Thye Hua Kwan Moral Society (Moral Neighbourhood Links), Yuhua Neighbourhood Link, Henderson Senior Citizens’ Home, NTUC Eldercare Co-op Ltd, Thong Kheng Seniors Activity Centre (Queenstown Centre) and Redhill Moral Seniors Activity Centre.
Ethical standards: Appropriate approval and procedures were used concerning human subjects. The study was approved by the Institutional Review Board of the National University of Singapore.
References
Clegg A, Young J, Iliffe S et al. Frailty in older people summary. Lancet 2013;381:752–762.
Fried LP, Tangen CM, Walston J et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–156.
Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed 2010;81:37–45.
Bonnefoy M, Berrut G, Lesourd B et al. Frailty and nutrition: searching for evidence. J Nutr Health Aging 2015;19:250–257.
Lorenzo-López L, Maseda A, de Labra C et al. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr 2017;17:108.
Robinson S, Cooper C, Aihie Sayer A. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res 2012;510801.
Collard RM, Boter H, Schoevers RA et al. Prevalence of frailty in communitydwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92.
Kaiser MJ, Bauer JM, Ramsch C et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010;58:1734–8.
Gill TM, Gahbauer EA, Allore HG et al. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418–23.
Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009;55:539–49.
Asmar Mariana A, Domingues Marcos D, Figueiredo Costa L et al. Transitions in Frailty state in Community-Dwelling Older Adults. Topics in Geriatric Rehabilitation 2015;31:105–113.
Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 2012;60:652–60.
Kaiser MJ, Bauer JM, Ramsch C et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–8.
Ng TP, Feng L, Nyunt MSZ et al. Frailty in Older Persons: Multisystem Risk Factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc 2014;15:635–42.
Ng TP, Niti M, Chiam PC et al. Ethnic differences in cognitive performance on Mini-Mental State Examination in Asians. Am J Geriatr Psychiatry 2007;15:130–9.
Nyunt MS, Fones C, Niti M et al. Criterion-based validity and reliability of the Geriatric Depression Screening Scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health 2009;13:376–82.
Cameron ID, Fairhall N, Langron C et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med 2013;11:65.
Ng TP, Feng L, Nyunt MS et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med 2015;128:1225–1236.
Fiatarone M A, O’neill E F, Ryan N D et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–75.
Payette H, Boutier V, Coulombe C et al. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Diet Assoc 2002;102:1088–95.
Milne A C, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med 2006;144:37–48.
Rosendahl E, Lindelöf N, Littbrand H et al. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother 2006;52:105–13.
Smoliner C, Norman K, Scheufele R et al. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008;24:1139–44.
Kim C O, Lee K R. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a communitybased randomized controlled study. J Gerontol A Biol Sci Med Sci 2013;68:309–16.
Laur CV, McNicholl T, Valaitis R et al. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab 2017;42:449–458.
Abellan Van Kan G, Vellas B. Is the Mini Nutritional Assessment an appropriate tool to assess frailty in older adults? J Nutr Health Aging 2011;15:159–61.
Verlaan S, Ligthart-Melis GC, Wijers SLJ et al. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults-A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 2017;18:374–382.
Wei K, Nyunt MSZ, Gao Q et al. Frailty and Malnutrition: Related and Distinct Syndrome Prevalence and Association among Community-Dwelling Older Adults: Singapore Longitudinal Ageing Studies. J Am Med Dir Assoc 2017;18:1019–1028.
Author information
Authors and Affiliations
Corresponding author
Additional information
Co-first authors for this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wei, K., Thein, F.S., Nyunt, M.S.Z. et al. Nutritional and Frailty State Transitions in the Singapore Longitudinal Aging Study. J Nutr Health Aging 22, 1221–1227 (2018). https://doi.org/10.1007/s12603-018-1096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-018-1096-3