Abstract
Objectives
To study the importance of weight change with regard to mortality in older people.
Design
Prospective cohort study.
Participants
The cohort includes participants in the Hordaland Health Study, Norway, 1997-99 (N=2935, age 71-74 years) who had previously participated in a survey in 1992-93.
Measurements
Participants with weight measured at both surveys were followed for mortality through 2012. Cox proportional hazards models were used to calculate risk of death according to changes in weight. Hazard ratios (HR) with 95% confidence intervals (CIs) for people with stable weight (±<5% weight change) were compared to people who lost (≥5%) or gained (≥5%) weight. Cox regression with penalized spline was used to evaluate the association between weight change (in kg) and mortality. Analyses were adjusted for age, sex, physical activity, smoking, diabetes, hypertension, and previous myocardial infarction or stroke. Participants with cancer were excluded.
Results
Compared to those with stable weight, participants who lost ≥5% weight had an increased mortality risk (HR 1.59 [95% CI: 1.35-1.89]) while the group with weight gain ≥5% did not (HR 1.07 [95% CI 0.90-1.28]). Penalized spline identified those who lost more than about three kg or gained more than about 12 kg as having increased risk of death.
Conclusion
Even a minor weight loss of ≥5% or >3 kg were significantly associated with increased risk of mortality. Thus, weight should be routinely measured in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that by 2015 worldwide, approximately 2.1 billion adults will be overweight and that at least 700 million will be obese (1,2). While obesity due to weight gain is a risk factor for non-communicable diseases that are responsible for most deaths in developed countries, the risk associated with degree of weight change in the elderly is unclear. Even in those who are obese, weight loss includes loss of muscle mass, which may explain the relationship of weight loss to disability (3) and mortality (4.5). The increased risk of mortality associated with weight loss in older adults appears to be similar across studies in spite of variations in methods (6-12). With regard to mortality risk, it appears that weight stability may be the best option for most elderly (5-8, 10-12). High body mass index (BMI, kg/m2) and weight gain have been associated with hypertension, diabetes mellitus (13) and disability (3). However, there are only a limited number of studies that report an association between weight gain and increased mortality in older people (6, 8-10). Cheng et al recently published a meta-analysis on weight change and all-cause mortality in older adults. They concluded that the effect of weight gain deserves further exploration and that future studies should consider subgroup analysis (e.g., by baseline BMI category). Moreover, they point out that recognizing the characteristics that discern survivors from non-survivors should be helpful to guide clinicians and public health practitioners in detecting at-risk individuals for early prevention (11). We therefore aimed to investigate the effect of different degree of weight change on risk of mortality in community-dwelling older people, whose weight and height were measured in their mid-sixties and again in their early seventies, with subsequent 14 years follow up with regard to mortality.
Materials and methods
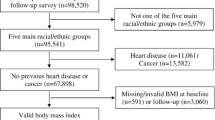
The current study includes 2935 older people who had weight and height measured in the Hordaland Homocysteine Study, Western Norway, during 1992-93 (age 65-67 years), and again in the Hordaland Health Study (HUSK) during 1997-99 (age 71-74 years) (14) (http://husk-en.b.uib.no). They were subsequently followed with regard to mortality through 2012. Six participants emigrated after the second measurement and were therefore not included in the analysis. Additionally, 369 participants diagnosed with cancer before or between the surveys in 1992-93 and 1997-99 (information obtained by linkage to the Norwegian Cancer Registry) were excluded from the present analysis, since cancer is highly associated with weight loss and mortality (15). This resulted in a study population of 1309 men and 1626 women. The mean time between the surveys was five years (range: 5-6 years) and mean follow-up after 1997-99 was 11.4 years (range: 0-14 years).
Measurements in 1992-93 and 1997-99 included height and weight using standard procedures. Height was measured without shoes to the nearest centimeter and weight was measured wearing light clothes without shoes to the nearest half-kilogram (kg) on a calibrated scale (16). Change in weight between the first and second measurements was based on weight at the first measurement and was categorized into loss (≥5%), stable (±<5%), and gain (≥5%), in agreement with prior studies (8, 17).
Self-administered questionnaires included information on myocardial infarction, stroke and diabetes in 1992-93 and 1997-99, and being under treatment for hypertension, physical activity and education in 1997-99. Physical activity was categorized as none/light or moderate/rigorous. Smokers were classified as never, former or current smokers, based on information provided in 1992-93 and 1997-99.
Outcome
Total mortality was the primary outcome. The unique 11-digit personal identification number assigned to all Norwegian residents facilitated linkage between HUSK and the Norwegian Population Register, which contains information on vital status (alive, emigrated, or dead). Participants were followed until death or December 31st, 2012.
Statistical analyses
Frequency distributions for categorical variables and means for continuous variables were calculated for 1) the different weight change groups, and 2) for survivors and non-survivors in the different weight change groups. Chi-square analyses or t-tests were applied to determine whether 1) the weight change groups differed significantly on any of the baseline characteristics compared to the stable weight group, and 2) whether the survivors and non-survivors in the different weight change groups differed significantly on any of the baseline characteristics (data from 1992-93 and 1997-99).
Kaplan-Meier cumulative hazard curves were used to estimate hazard rates and Cox proportional hazards models were used to calculate adjusted mortality hazard ratios with 95% confidence intervals (CI) associated with change in weight. Interactions were examined by adding an interaction term with each covariate one at a time. There were no significant interactions and the interaction terms were not included in the final model.
In Cox regression with penalized splines the functional form of weight change is estimated by a smoothing spline, in which the estimated smooth function can be used to plot the relative hazard of total mortality against weight change in kg (18). Participants with a weight change of more than 15 kg were excluded (N=21), in order to reduce the likelihood of including participants with weight change due to disease. The only information we have on disease among the 21 participants who were excluded are that among those who gained more than 15 kg (n=11), two had diabetes, and among those who lost more than 15 kg (n=10), one had diabetes and two had a previous myocardial infarction. Apart from self-reported diabetes, myocardial infarction and stroke, we do not have information concerning other possible diseases.
Kaplan-Meier cumulative hazard curves were calculated using STATA, version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX:StataCorp LP), Cox proportional hazard models were calculated using SPSS, version 21 (IBM SPSS Statistics for Windows, Armonk, NY:IBM), while the Cox regression with penalized spline was generated using R, version 3.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Covariates that we had data on, and that was suspected to be confounders of the association between weight change and mortality were added one at a time to the unadjusted and adjusted model to examine possible confounding of the relationship between weight change and mortality. A variable was included in the final model if it was found to influent the HR of the association between weight change and mortality, when we specified the models stepwise. Thus, variables included in the multivariable model include age, sex, physical activity, smoking, diabetes mellitus, myocardial infarction and/or stroke, and being under treatment for hypertension. BMI at the first survey and education were evaluated, but they did not influence the estimates and overall model fit, and are therefore not adjusted for. We decided to adjust for myocardial infarction, stroke and diabetes since these diseases increase the mortality risk. Multivariable analyses were performed for the total population, both with weight change in categories (%), and as a continuous variable (kg). Due to missing values in covariates (1.6-13.7%), the multivariable analysis included 2330 participants whereas the age and sex adjusted model included 2935 participants.
In order to eliminate early deaths that could be attributed to clinical or subclinical disease, two sensitivity analyses were performed after excluding persons who 1) died within two years after baseline (n=69), and 2) persons who were diagnosed with cancer within two years after baseline (n=136).
We also examined different measures of weight change in addition to percent weight change, e.g. ± 3 kg and ±2 kg weight change. In addition, ± ≥ 5% changes in BMI were examined. Results were basically the same and results of these analyses are not shown.
Ethics
Participation in HUSK was voluntary and each participant signed a written informed consent. The Regional Committee for Medical and Health Research Ethics (REK Vest) approved the study.
Results
Characteristics of the study population
Table 1 shows participant characteristics by weight change category. Median and maximum follow-up was 13 and 14 years, respectively. The average age at start of follow-up was 72.4 years in men and 72.5 years in women. Compared to the group with stable weight, people in the weight loss category (n=460, 15.7%) were more likely to have diabetes and to smoke, and less likely to engage in physical activity. Compared to the group with stable weight, people in the weight gain category (n=559, 19.0%) were more likely to have a history of use of antihypertensive medication, and less likely to have higher education and to engage in physical activity. BMI in 1992-93 and prevalence of myocardial infarction or stroke did not differ significantly between the weight change groups.
Considering the distribution of weight change, among men there were 15% who gained weight, compared to 22% of the women. Weight loss occurred in 13% of the men, compared to almost 18% of the women. Among current smokers 29% lost weight and almost 15% gained weight. Among those with diabetes 23% lost weight and 19% gained weight. Among those treated for hypertension 14% lost weight and 23% gained weight.
Association of weight change categories with total mortality
During follow-up, 648 men and 511 women died. Figure 1 displays Kaplan-Meier cumulative hazard curves for men and women stratified by weight change category. The curve for the weight loss groups increased more rapidly than the curves for the weight stable and weight gain groups, for both sexes. The curves for the weight stable and weight gain groups followed each other closely the first few years, especially among women. The Log rank test showed significant differences in hazard between the weight change groups in both sexes.
Table 2 shows the association of weight change with mortality, with adjustment for age and sex. Compared to the stable weight group, there was a significantly increased risk of mortality for those in the weight loss category, HR 1.87 (95% CI 1.61-2.16), p<0.001. Further adjustment for smoking, physical activity, myocardial infarction and/or stroke, diabetes and being under treatment for hypertension slightly attenuated the hazard ratio; HR 1.59 (95% CI 1.35-1.89), p<0.001 in the weight loss group. The excess mortality risk associated with weight loss was similar in men (HR 1.61 [95% CI 1.28-2.03], p<0.001) and women (HR 1.62 [95% CI 1.26-2.09], p<0.001) in the fully adjusted model.
Weight gain was significantly associated with increased risk of mortality (HR 1.22 [95% CI 1.05-1.42], p=0.011) after adjustment for age and sex. Further adjustment made the association no longer significant; HR 1.07 (95% CI 0.90-1.28), p=0.453. The associations between weight gain and mortality were not significant in either sex in the fully adjusted model: HR 1.05 [95% CI 0.82-1.35], p=0.68 in men and HR 1.06 [0.97 - 1.16], p=0.602 in women.
Online Supplementary Table 1 shows that among the survivors in both the weight loss and weight gain groups, the proportion of men, ever smokers, participants with diabetes and with previous myocardial infarction were significantly lower than among those who died. Among the survivors in the weight gain group there were also a significantly lower proportion of participants performing no/light physical activity and treated with blood pressure medication than among those who died.
Cox proportional hazards regression with penalized splines, The Hordaland Health Study. Distribution of hazard ratio (black line) with 95% CI (shadow) for mortality across the distribution of weight change in kg. The model includes age, sex, myocardial infarction and/or stroke, diabetes, being treated for hypertension, physical activity and smoking habits. A HR of 1 represents the average hazard in the data. Weight change in kg ranging from 0.01 kg to 15 kg is included. Subjects with cancer diagnosed before the last measurement were excluded
Stratification by BMI at first measurement
Table 3 shows the association of weight change with mortality, stratified by BMI categories according to WHO definitions (<25 kg/m2, 25-29.9 kg/m2, and ≥30 kg/m2) (19). In multivariable analyses, the increased risk of mortality associated with weight loss persisted in participants with a BMI of <25 kg/m2 and 25-29.9 kg/m2, but not in participants with a BMI of ≥30 kg/m2 (HR 1.34 [95% CI 0.85-2.12], p=0.213), which only included 71 (2.4%) participants. In multivariable analyses, the group with weight gain did not have increased risk of mortality compared to stable weight also when stratified by BMI.
Different lengths of follow-up
When analyzing the associations between weight change and mortality within three, six, and ten years, adjusting for all covariates, we found no significant results after three years (weight loss group: 32 deaths, HR=1.63 [95% CI 0.99-2.68], p=0.057). The group that lost ≥5% had significantly higher risk of death after six years (76 deaths, HR=1.44 [95% CI 1.06-1.97], p=0.022) and ten years (149 deaths, HR=1.46 [95% CI 1.17-1.82], p=0.001). Weight gain ≥5% was not significantly associated with mortality in any of these multivariable analyses.
Association of weight change as a continuous variable with total mortality
Figure 2 displays a multivariable adjusted Cox regression with penalized spline of the relationship between weight change and mortality. A HR of 1 represents the average hazard in the data. The X-axis shows weight change in kg, while the Y-axis shows the HR and 95% CI associated with weight change. The curve illustrates that both major weight gain and minor weight loss is associated with increased mortality in comparison with stable weight.
Sensitivity analysis
After excluding 69 participants who died during the first two years of follow-up, the results did not differ materially (weight loss group: HR 1.59 [95% CI 1.34-1.90], p<0.001, weight gain group: HR 1.11 [95% CI 0.92-1.33], p=0.279). After excluding 136 participants diagnosed with cancer during the first two years of follow-up, the results also did not differ materially (weight loss group: HR 1.63 [95% CI: 1.37-1.95], p<0.001, weight gain group: HR 1.06 [95% CI 0.88-1.29], p=0.519).
Discussion
Main results
In this group of community-dwelling older adults, even a minor weight loss was significantly associated with increased mortality, whereas a weight gain had to be more substantial to increase mortality risk. The weight loss result was only to a minor degree attenuated when controlling for the fact that participants in the weight loss group had higher baseline prevalence of diabetes, performed less physical activity and smoked more compared to the stable weight group. Similar results were found when we stratified by sex.
Comparison with previous studies
Our finding that people who lost weight had a higher mortality risk compared to those whose weight was stable is in line with results from previous epidemiologic studies (5-12, 17, 20-23) that have examined weight loss over short intervals (8, 10, 20, 24), or highest lifetime weight (7). Lee and Paffenbarger summarized epidemiologic studies evaluating the health effects of weight loss in 1996. Their main finding was that in the short term, weight loss leads to improved physiological parameters, although these short-term benefits do not appear to translate into improvements in longevity. However, results were mixed across studies, and different effects were seen in different BMI groups (25). A more recent review and meta-analysis by Harrington et al (4) investigated in more detail studies that distinguished between intentional and unintentional weight loss, taking the health status of subjects into account. Overall, they concluded that weight loss was associated with a small but significant increase in total mortality among subjects with unintentional weight loss (pooled RR 1.22 [95% CI 1.09-1.37]). No association with intentional weight loss in obese subjects (pooled RR 0.94 [95% CI 0.86-1.04]) were reported, while for intentional weight losers whose baseline BMI was within the normal to overweight range, the risk of mortality was increased (pooled RR 1.09 [95% CI 1.02-1.17]) (4). Cheng et.al conducted a meta-analysis of 17 observational cohort studies from both Western and Asian Countries, to examine current literature on the association between weight change and all-cause mortality among older adults (11). Overall, they observed a higher mortality risk with weight change among non-institutionalized adults 60 years and older (11). The overall pooled relative risk of all-cause mortality for the weight loss group was 1.67 (95% CI, 1.51-1.85), while for the weight gain group it was 1.21 (95% CI, 1.09-1.33) (11).
There are a limited number of studies showing an increased mortality risk with weight gain in older people (9-12). Using data from 658 older people 65-99 years old, Deeg et al reported increased risk of mortality with weight gain of ≥2.5 kg/m2, but only in the age group 65-74 years and in those with heart disease (10). Results from the Systolic Hypertension in the Elderly Program showed increased risk with weight gain of >0.5 kg/year (OR=2.4) in the elderly aged 60 years or more (9). Cao analyzed data from 12.523 participants over 50 years old from a nationally representative longitudinal dataset, the Health and Retirement Study (12). The effects of time-varying weight change on mortality were estimated, adjusting for demographic and socio-economic variables, as well as time-varying confounders including illness and smoking. They reported a HR of 1.98 (95% CI 1.67 - 2.35) for large weight gain (>10%), and a HR of 1.20 (95% CI 1.02 - 1.41) for small weight gain (5 - 10%) (12). These results may indicate that weight gain is less hazardous in the oldest and that the time span for weight gain has an impact.
When sex stratified analyses were performed, HRs were similar in men and women. Poobalan et al studied long-term effects of weight loss on all-cause mortality in overweight/obese populations (26). They conclude that there may be gender differences, and that more studies are needed on gender differences. Otsuka et al studied age-related changes in weight in community-dwelling middle-aged and elderly Japanese (27). They reported that weight started to decline in men in their mid-50s and women in their late 40s, indicating gender differences in the occurrence of weight change (27). However, a recent meta-analysis from Cheng et al reported that the association between weight change and total mortality did not vary significantly between genders (11).
Some earlier studies have examined the association between changes in body composition and mortality. Newman et al concluded that significantly more fat free mass (FFM) was lost with weight loss than was gained with weight gain, suggesting that weight loss, even with regain, could accelerate loss of FFM in older adults (28). These changes in body composition seem to contribute to decreased health with weight change in the elderly, since studies have found that increases in fat mass (FM) increases, while increases in FFM decreases, mortality risk (17, 29).
Cheng et al, in their meta-analysis of weight change and mortality in older adults, conclude that recognizing the characteristics that discern survivors from non-survivors should be helpful to guide clinicians and public health practitioners in detecting at-risk individuals for early prevention (11). In our study the proportion of men, ever smokers, participants with diabetes and with previous myocardial infarction reported at baseline, were significantly higher among those who died, in both the weight loss and weight gain group, compared to those who survived. In the weight gain group there were also a significantly higher proportion of participants reporting no/light physical activity and treatment with blood pressure medication among those who died.
Limitations in earlier studies on older people are use of self- reported weight and weight change (7, 24), that they did not evaluate weight gain (7, 24), lack of sensitivity analysis (17, 24) and wide age groups (10, 24, 30). In the present study we considered these limitations, and found significant associations with mortality for even a minor weight loss, as well as for substantial weight gain, compared to stable weight. Further, we evaluated characteristics at baseline which discern survivors from non-survivors in the two weight change groups.
Strengths and limitations
In this study, an attempt was made to consider major known confounders in the relation between weight change and mortality i.e. education as a measure of socioeconomic status, cancer, myocardial infarction and stroke, hypertension, diabetes, BMI at the first survey in 1992-93, smoking habits and physical activity, and excluding deaths during the first two years after baseline. Education and BMI before weight change were not included in the final model. We had a relatively large sample of men and women with standardized measurements of weight and height measured about five years apart and a subsequent follow-up of up to 14 years. Participants were community-dwelling, increasing the generalizability of the findings. Self-reported information on myocardial infarction or/and stroke, diabetes, smoking habits, physical activity habits and use of hypertensive treatment was available with relatively low frequency of missing values (1.6-13.7%). The sensitivity analyses showed quite similar results as the main analysis. Moreover, we had complete follow-up of all participants, decreasing the possibility of selection bias.
The current study also has limitations. As in all studies of older adults, there is probably selection bias in that those who participate are healthier than those who do not (21). All subjects who participated in 1992-93 were also invited to participate in 1997-99, attendance in 1997-99 was 77% (31). Inclusion in the study required survival at both time points, introducing possible survivor bias. Some possible confounders not measured in this study are diseases such as chronic obstructive pulmonary disease, renal disease, rheumatologic disease, endocrine disease, gastrointestinal disease, psychiatric disease and cognitive disorders (32). However, there were no participants with overt dementia. All participants were community-dwelling people who were able to come to the study site on their own at both time points, and they were able to fill out questionnaires. This study is also limited in the lack of information on potential confounders during the follow-up period after the second survey in 1997-99. The population studied is relatively homogenous in nature and confined to a small geographic area of Norway, perhaps reducing generalizability to populations of different ethnicity.
In addition, our findings may not apply to weight loss in younger age groups and in the very obese. Baseline mean age was about 72 years, none were younger than 70 years, and only 11.2% had a BMI ≥30 kg/m2. Weight was only measured at two times, about five years apart, thus we do not know when the weight loss or gain occurred, or if the weight had cycled. We also do not know if a significant weight gain or loss occurred prior to the first measurement in 1992-93. This is a limitation as weight cycling has been related to increased mortality (6, 33, 34). Further, we could not distinguish between intentional and unintentional weight change. Studies that have distinguished between the two have generally shown that intentional weight loss has shown decreased mortality rates, while unintentional weight loss has been consistently associated with increased mortality rates (35-37). However, some studies among older adults have not found a lower risk of mortality with intentional weight loss (20, 38). Information on potential changes in FM and FFM would have strengthened our study, since increases in FM increases, while increases in FFM decreases, mortality risk (17, 29). Unfortunately, such information is not available.
Future research
There is need for studies evaluating the difference between intentional and unintentional weight loss in community-dwelling older people. Also, since weight loss accelerates the loss of FFM in older adults, future studies should examine the association between changes in body composition and mortality in older people.
Conclusion
Even a small weight loss is associated with increased mortality in older adults. Due to this, weight should be routinely measured in older adults.
Acknowledgments: None.
Funding Sources: The Norwegian Institute of Public Health, and the University of Bergen financed the data collection.
Conflict of interest: The authors declare that they have no conflict of interest. Prof. Dierkes has nothing to disclose. Prof. Vollset has nothing to disclose. Prof. Nygård has nothing to disclose. Prof. Tell has nothing to disclose. Ph.d Vinknes has nothing to disclose. MD, Ph.d Sulo has nothing to disclose. BS.c Reinhard Seifert has nothing to disclose. MS.c Teresa Risan Haugsgjerd has nothing to disclose.
Ethics: The Regional Committee for Medical and Health Research Ethics (REK Vest) approved the study.
Abbreviations
- BMI:
-
Body mass index
- HUSK:
-
The Hordaland Health Study
- CI:
-
Confidence intervals
- HR:
-
Hazard ratios
- RR:
-
Relative risk
- OR:
-
Odds ratio
- FFM:
-
Fat free mass
- FM:
-
Fat mass
References
WHO. Obesity and overweight. Fact sheet N°311. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 15 March 2014
Ng M, Fleming T, Robinson M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81.
Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. JAMA 1994;271:1093–8.
Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr res rev 2009;22:93–108.
Bamia C, Halkjaer J, Lagiou P et al. Weight change in later life and risk of death amongst the elderly: the European Prospective Investigation into Cancer and Nutrition-Elderly Network on Ageing and Health study. J Intern Med 2010;268:133–44.
Reynolds MW, Fredman L, Langenberg P, Magaziner J. Weight, weight change, mortality in a random sample of older community-dwelling women. J Am Geriatr Soc 1999;47:1409–14.
Knudtson MD, Klein BE, Klein R, Shankar A. Associations with weight loss and subsequent mortality risk. Ann Epidemiol 2005;15:483–91.
Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc 2001;49:1309–18.
Somes GW. Body Mass Index, Weight Change, and Death in Older Adults: The Systolic Hypertension in the Elderly Program. Am J epidemiol 2002;156:132–8.
Deeg DJ, Miles TP, Van Zonneveld RJ, Curb JD. Weight change, survival time and cause of death in Dutch elderly. Arch Gerontol Geriatr 1990;10:97–111.
Cheng FW, Gao X, Jensen GL. Weight Change and All-Cause Mortality in Older Adults: A Meta-Analysis. J Nutr Gerontol Geriatr 2015;34:343–68.
Cao B. Estimating the Effects of Obesity and Weight Change on Mortality Using a Dynamic Causal Model. PloS one 2015;10:e0129946.
Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82:923–34.
Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygärd O, Vollset SE. The Hordaland Homocysteine Study: a communitybased study of homocysteine, its determinants, and associations with disease. J Nutr 2006;136:1731S–40S.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cachexia: an international consensus. Lancet 2011;12:489–95.
Research Group For Lifestyle Epidemiology. Institute of Global Public Health and Primary Care, University of Bergen, Norway. Rutiner for hjerte-karunders0kelser i HUSK 1997-99. http://huskb.uib.no/files/2012/07/Teknisk_protokoll1.pdf. Accessed 01 February 2014
Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, Dam TT, Lapidus JA, Cawthon PM, Ensrud KE, Orwoll ES; Osteoporotic Fractures in Men Study Groups. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc 2011; 59:233–40.
Therneau T, Grambsch PM (2000) Modeling Survival Data: Extending the Cox Model. 1st ed. Springer, New York, USA.
WHO. BMI classification. apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 15 March 2014
Wedick NM, Barrett-Connor E, Knoke JD, Wingard DL. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: the Rancho Bernardo study. J Am Geriatr Soc 2002;50:1810–5.
Dahl AK, Fauth EB, Ernsth-Bravell M, Hassing LB, Ram N, Gerstof D. Body mass index, change in body mass index, and survival in old and very old persons. J Am Geriatr Soc 2013;61:512–8.
Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2010;65:63–70.
Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, Simonsick EM, Tylvasky FA, Cawthon PM, Harris TB; Health, Aging, and Body Composition Study. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc 2014;62:1476–83.
Sahyoun NR, Serdula MK, Galuska DA, Zhang XL, Pamuk ER. The epidemiology of recent involuntary weight loss in the United States population. J Nutr Health Aging 2004;8:510–7.
Lee IM, Paffenbarger RS, Jr. Is weight loss hazardous? Nutr Rev 1996;54:S116–24.
Poobalan AS, Aucott LS, Smith WCS, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obesity reviews 2007;8:503–513.
Otsuka R, Kato Y, Nishita Y, Tange C, Tomida M, Nakamoto M, Imai T, Ando F, Shimokata H. Age-related changes in energy intake and weight in community dwelling middle-aged and elderly japanese. J Nutr Health aging 2016;20:383–390.
Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8.
Allison DB, Zannolli R, Faith MS, Heo M, Pietrobelli A, Vanltallie TB, Pi-Sunyer FX, Heymsfield SB. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord 1999;23:603–11.
Pamuk ER, Williamson DF, Madans J, Serdula MK, Kleinman JC, Byers T. Weight loss and mortality in a national cohort of adults, 1971-1987. Am J Epidemiol 1992;136:686–97.
The Hordaland Health Studies. Forskningsprotokoll. http://husk.b.uib.no/ files/2012/06/Protokoll_150208_utenvedlegg1.pdf. Accessed 10 March 2014
Wong CJ. Involuntary Weight Loss. Med Clin North Am 2014;98:625–43.
Lissner L, Odell PM, D’Agostino RB, Stokes J 3rd, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med 1991;324:1839–44.
Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol 2007;22:665–73.
Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Trying to lose weight, losing weight, and 9-year mortality in overweight U.S. adults with diabetes. Diabetes care 2004;27:657–62.
Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol 1995;141:1128–41.
Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in overweight white men aged 40-64 years. Am J Epidemiol 1999;149:491–503.
Yaari S, Goldbourt U. Voluntary and involuntary weight loss: associations with long term mortality in 9,228 middle-aged and elderly men. Am J Epidemiol 1998;148:546–55.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12603_2016_866_MOESM1_ESM.docx
Association between weight change and mortality in community living older people followed for up to 14 years. The Hordaland Health Study (HUSK)
Rights and permissions
About this article
Cite this article
Haugsgjerd, T.R., Dierkes, J., Vollset, S.E. et al. Association between weight change and mortality in community living older people followed for up to 14 years. The Hordaland Health Study (HUSK). J Nutr Health Aging 21, 909–917 (2017). https://doi.org/10.1007/s12603-016-0866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-016-0866-z