Abstract
Objective To investigate the effect of weight change and weight fluctuations on all-cause-mortality in men. Methods Within a prospective population-based cohort of 1160 men aged 40–59 years at recruitment, complete weight change patterns from baseline and three follow-up examinations during a period of 15 years of follow-up was used to categorize the 505 men into stable obese, stable non-obese, weight gain, weight loss and weight fluctuation groups. For these men (age range: 55–74 years at start time of survival analysis) further survival was analyzed during the subsequent 15 years. Results Overall, 183 deaths were observed among the 505 men. Only weight fluctuations had a clear significant impact on all-cause mortality. Adjusted hazard rate ratio (HRR (95%-CI)) was 1.86 (1.31–2.66) after adjustment for age group, pre-existing cardiovascular disease or diabetes mellitus, smoking and socio-economic status. The risk rate due to weight loss was borderline significant (HRR = 1.81 (0.99–3.31)). Risk of death due to weight gain (HRR = 1.15 (0.70–1.88)) or stable obesity (HRR = 1.16 (0.69–1.94)), however, were not significantly increased compared to men staying non-obese for the first 15 years after cohort recruitment. Conclusion Weight fluctuations are a major risk factor for all-cause mortality in middle aged men. Moreover, stable obesity does not increase further mortality in men aged 55–74 years in long-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of overweight and obesity in adults has reached alarming proportions during the last decades in particular in the western world [1, 2]. The rising burden of health consequences of this development are realized consistently in many studies [3].
A reduction in weight by dieting and physical activity is often thought to be useful to reduce detrimental effects of obesity on health. However, some prospective studies have found an increased risk of all-cause and cardiovascular related mortality due to weight loss and weight fluctuations in middle-aged or older men [4–9]. Others found an enhanced mortality risk only for weight loss or weight gain [10], whereas other studies mainly ascribe the negative effect of weight loss and weight fluctuations (cycling) on mortality to adverse lifestyle factors or pre-existing disease [11–14]. We examined the effect of patterns of weight change and weight fluctuations identified during a 15 years period of observation on further survival in the subsequent 15 years.
Methods
Study design and population
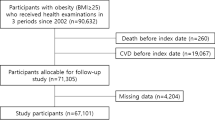
The Erfurt Male Cohort Study (ERFORT Study) is a population-based prospective cohort study of (invited) 1600 men with a target age of 40–59 years drawn by a random sample of the population born 1915–1934 in the city of Erfurt. The study design has been described in detail previously [15]. Briefly, a random sample was established by residential registry and electoral rolls (ADREMA) of the former German Democratic Republic (i.e. East-Germany before the reunification in 1990). From the 1600 invited men 46 were out of the target age range of 40–59 years. From these 1554 men 1160 could actually be recruited for participation in the study cohort (participation rate at baseline 74.6%). The study of the recruited cohort of 1160 men started between September 20, 1973 and August 26, 1975 with an extended medical examination and accompanying standardized questionnaire based survey, continued by three follow-up examinations and surveys every 5 years. The study was finished by the final mortality follow-up on September 30, 2003, when life status was determined by residential and death registries for all participants recruited in 1973–1975.
Because weight change categories (defined below) are determined from the first 15 years of follow-up, survival is not analyzed over the total follow-up time of 30 years, but only over the second 15 years of follow-up. This further follow-up period will be called ‘further survival’ (defined in detail below) and starts with the end of the weight change category definition period. Thus the analyzed population comprises all participants, who took part in all four examinations up to 1990 and are alive at the start of further follow-up. The final study population consists of 505 men being 55–74 years of age at start of the survival analysis (Fig. 1).
Study design, cohort recruitment and study population in the ERFORT-Study. aNote that 46 of these 1600 invited men were out of the target age range of 40-59 years. Thus 1554 men were actually the source population. bResponse rate is calculated as (1160/1554)*100. cResponse rate is calculated as (907/1160)*100. dResponse rate is calculated as (740/1160)*100. eResponse rate is calculated as (609/1160)*100
Body mass index
Height and weight was measured during medical examinations. All medical examinations were conducted between 7.00 and 9.00 a.m. Height was determined to the nearest centimeter by means of a fixed vertical scale with a crossbar placed on top of the head, while the participant stood upright, without shoes, heels together and eyes directed straight ahead. Weight was assessed by a calibrated level balance to the nearest 100 g. Body mass index (BMI), was derived from these measures as weight in kilogram (kg) per height in meters (m) squared.
Definition of weight change categories
The following five weight change categories were defined and calculated according to Diaz et al. [5]: Stable weight-non-obese, stable weight-obese, weight gain, weight loss, weight fluctuations. In brief, calculations are based on the combination of measures of stability of weight over the four examinations within the first 15 years of observation and BMI-defined obesity. Stability of weight was defined by the following criteria: (i) Initial and final BMI in the first 15 years of observation differed by <3 kg/m2. (ii) The sum of the absolute deviations of each of the four individual BMI-values over time from the individual average BMI was less than the average sum of these deviations in the entire population (≤3.49). Obesity was defined as initial BMI ≥ 30 kg/m2.
The weight categories of ‘stable weight-non-obese’ and ‘stable weight-obese’ were specified by the combination of both stability criteria and the respective status of obesity. Weight gain and weight loss was defined as an increase, respective decrease of ≥3 BMI-units from initial to final BMI measurements in the first 15 years of observation. Weight fluctuations were ascribed to those respondents, whose initial and final BMI differed by <3 kg/m2 and whose sum of absolute deviations were >3.49 BMI-units. Note that, no information on intentionality of weight changes was recorded.
Measures of pre-existing disease
All used measures of pre-existing disease were gathered by physician led standardized interviews and medical examinations at the 15 years follow-up at the start of the analysis of further survival.
Ischemic heart disease was assessed by a physician administered interview in relation to myocardial infarction, angina pectoris and angina pectoris symptoms and by clinical diagnosis relying on ECG measurements. A history of ischemic heart disease, myocardial infarction, stroke or diabetes mellitus was assessed by study doctors and was confirmed by hospital medical records. Hypertension was defined as being on anti-hypertensive drugs or by having an averaged diastolic blood pressure of ≥95 mmHg or a systolic blood pressure of ≥160 mmHg during medical examination. Blood pressure readings were collected with a random zero Sphygmomanometer in a sitting position after 10 min unchanged rest. Two readings were averaged.
Mortality related co-factors
Respondents who reported any smoking at any of the four data collection periods were classified as smokers. Attainment of a university or college degree was used as an indicator for high socio-economic status. Age was grouped by 5 years intervals to age cohorts to enhance ease of interpretability.
Statistical analysis and further survival time
Further survival time (i.e. the outcome) was calculated as the difference of the respondent’s date of death (expressed in years) or the end of the study at September 20, 2003 and the respondent’s date of examination in the fourth data collection period in 1988–1990. Date of death was obtained by active follow-up and consultation of residential and death registries. Further survival time and the influence of weight change categories and adjustment factors on survival, were analyzed by Cox-proportional hazard regression [16] using procedure ‘proc phreg’ of the statistical software package SAS 9.1.3. All analysis models were adjusted for the age-cohorts by dummy coding to which respondents belong at the start of the survival time axis (55–59 years (reference category), 60–64 years, .65–69 years, 70–74 years). In addition some models were also adjusted for the combined variable ‘pre-existing disease’ (at least one positive response to any of the five disease indicators), for smoking status during the first 15 years of follow-up and for socio-economic status (education).
Results
Overall, 183 deaths were observed among the 505 men of the studied population. Table 1 shows the characteristics of the study population by weight change categories. Mean age is somewhat higher in the weight loss group. Myocardial infarction, stroke, diabetes and ischemic heart disease in the group of those, who lost weight is also more prevalent. Note further, that in all weight change groups and in particular in the stable obese and weight gain group hypertension is of high prevalence. Moreover, the percentage of participants, who smoked during the first 15 years of follow-up ranged between 55% and 75%. The percentage of participants with a University or College degree was quite low in this study. However, this percentage was generally lower in East-Germany compared to West-European countries at these times.
Table 2 depicts the mean values and variability of BMI and mean change of BMI from baseline to 15-years follow-up for the five weight change categories.
The stable non-obese and stable-obese groups show the smallest increase in BMI over the 15 years time period (0.3 kg/m2) and have also the smallest variability. That is, BMI in the stable non-obese group changed from 25.1 kg/m2 at baseline to 25.4 kg/m2 at start of further follow-up and variability of BMI ranged between 2.3 and 2.4 standard deviations. In the stable obese group BMI changed from 30.4 to 30.7 kg/m2 with variability ranging between 1.4 and 1.7 standard deviations. The group of weight gainers steadily increase mean weight for height every 5 years by more than 1 kg/m2 with an average BMI of 26.4 kg/m2 at baseline. The weight gainers have the largest increase over time with 4.9 kg/m2. The weight loss group loses about 4.2 kg/m2 over the first 15 years follow-up period with an average BMI of 30.3 at baseline and a BMI of 26.1 at start of further survival analysis.
The weight fluctuations group shows only 0.9 kg/m2 change in mean BMI from baseline with a BMI of 26.9, over 26.6 and 27.8 to 27.8 kg/m2 at the 15 years follow-up and large variability over time.
Weight fluctuations significantly enhance the risk of death adjusted for age at start of the analysis of further survival (Table 3). The effect of weight loss was clearly statistically significant only when non adjusted, while the relative risk declined from 1.91 to 1.81 after adjustment for pre-existing disease, like cardiovascular disease or diabetes mellitus and adjustment for socio-economic status and any smoking. The risk of death for respondents with weight fluctuations remained nearly similar (HRR was 1.92 to 1.86) and persists highly significant after further adjustment for pre-existing disease, smoking and socio-economic status. Neither weight gain of more than 3 kg/m2 over a period of 15 years, nor stable obesity enhanced the risk of death substantially above those respondents, who stayed non-obese for the 15 years of follow-up before the start of the survival analysis. High educational attainment does not significantly contribute to further risk of survival after the other variables are taken into account. However, age, pre-existing disease and smoking enhanced the risk of death independently from weight change patterns.
The four age-specific panels in Figs. 2 and 3 illustrate the adjusted survival curves of the five weight change categories.
Survival curves of men without (Panel A, C) and with pre-existing disease and smoking history for weight change categories (Panel B, D) for respondents with lower education by age cohort in the ERFORT study (n = 504), Part 1. Legend:___1 Stable non-obese, - -2 Stable obese; - -3 Weight gain, ....4 Weight loss, - -5 Weight fluctuations
Survival curves of men without (Panel E, G) and with pre-existing disease and smoking history for weight change categories (Panel F, H) for respondents with lower education by age cohort in the ERFORT study (n = 504), Part 2. Legend:___1 Stable non-obese, - -2 Stable obese; - -3 Weight gain, ....4 Weight loss, - -5 Weight fluctuations
Panel A and C of Fig. 2 and panel E and G from Fig. 3 depict the estimated survival curves for each of the fours age cohorts, who do not have attained higher education, any positive measure of pre-existing disease and who do not have any smoking history during the period of weight change definition years. Panel B and D of Fig. 2 and panel F and H of Fig. 3 display the respective survival curves for those, who do not have attained higher education either, however have at least one of the mentioned risk factors. Weight fluctuations and weight loss clearly reduce further survival more than for the other three weight change categories for each age cohort. Note in particular, although the estimated survival probability of respondents with pre-existing disease and smoking history decreased faster than for respondents without those risk factors, the order and course of the curves by weight change category remains essentially the same for all age-cohorts (stable non-obese, stable obese and weight gain followed by weight loss and weight fluctuations). This illustrates the results from Table 3, showing that the effect of weight fluctuations on the risk of death is a risk factor independent of pre-existing disease or smoking.
To strengthen this result we have redone the analysis by stratifying for smoking status and adjusting for pre-existing disease (Table 4).
The adjusted effect on further survival for the weight loss group was substantial and significant in the stratum with at least some smoking during the first 15 years of follow-up (HRR = 2.26 (1.15–4.44)). However, this was not the case in the stratum of respondents, who did not smoke during the 15 years period used for identification of weight change patterns (HRR = 0.85 (0.19–3.75)). In contrast, the effect on further survival for respondents with weight fluctuations was substantial and significant in both strata. The effect in respondents, who did not smoke in the first 15 years before the start of the survival analysis was even stronger (HRR = 2.12 (1.09–4.12)) than the effect in the stratum, who did smoke (HRR = 1.71 (1.11–2.62)).
The risk of death was substantially and significantly enhanced with increasing age in both strata. In contrast the risk of death is enhanced by pre-existing disease mainly in the stratum of participants, who smoked during the first 15 years of follow-up. High educational attainment does not significantly contribute to further risk of survival after the other variables are taken into account.
Discussion
In this analysis of the effect of weight change patterns defined in a 15 years period and subsequent 15 years follow-up we found that weight fluctuations are the main risk for death aside of age, pre-existing disease and smoking. Weight loss reduces further survival as well. However, the effect of weight loss on mortality is mostly due to pre-existing cardiovascular disease or diabetes and the adverse health effects of smoking. Stable obesity has a smaller influence on further survival independent from age or pre-existing disease and smoking, than assumed. However, to avoid misinterpretation, obesity may enhance the risk to develop cardiovascular risk factors and diabetes and these risks clearly add to the risk of death as illustrated by the survival curves in Figs. 2 and 3.
There are several studies which specifically have assessed the impact of weight fluctuations on all-cause mortality in men [4–14], but were criticized of its shortcomings. Furthermore, most studies were conducted in the USA. In Europe only few studies have been conducted. One in the UK [14], one in Sweden [7] and a multi-center cohort study in Finland, The Netherlands, Italy, Greece and Yugoslavia [9].
Our results are partly in line and partly not consistent with these previous studies.
Six of these eleven studies have also found that weight fluctuations enhance all-cause mortality risk, even after adjustment for confounding factors like pre-existing disease or smoking [4–9]. The most recent of these studies the NHANES I epidemiologic follow-up study (NHEFS) is based on a large cohort of 8479 US-American men recruited in 1971–1974 and re-examined in 1982–1984 and 1987 [5]. Weight change categories (the ones we adopted for our analyses) were determined by one measurement at the initial examination and by two reported and two recalled weight and height values in the two follow-up surveys, resulting in five weight change definition periods. The cohort consisted of an age range of 25–74 years at recruitment and 40–90 years at start of further 6 years follow-up. The weight fluctuation group had a smoking status adjusted HRR of 1.8l (1.35–2.44) and a HRR of 1.83 (1.25–2.69) after exclusion of respondents being incapacitated or in poor health condition. These estimates are rather similar to the HRR we found in our analyses. However, further survival in the weight loss group was substantially more affected in their analysis, than in ours. This might be due to a shorter follow-up period of 6 years compared with the 15 years in this study or might be related to small numbers of the weight loss group in our study. Moreover, their results of no significant effects of stable obesity on all cause mortality (adjusted for smoke status or analyses restricted to healthy participants) are also in line with our analyses. In addition, Diaz et al. did not find a significant effect of weight gain on all cause mortality neither in smoking adjusted nor in analyses excluding participants with pre-existing diseases. The Seven Country Study, a prospective cohort study of 6441 men (age range 40–59 years) in Finland, The Netherlands, Italy, Greece and Yugoslavia with a follow-up time of 15 years also found an enhanced risk of weight fluctuation on all cause mortality (HRR = 1.2 (1.0–1.4)) [9]. Weight gain did not significantly affect (like in the present study) all cause mortality. However, weight loss was clearly a risk factor for all cause mortality.
Some of the previous studies [4–8] supporting our results have been criticized, that these results are based on recalled weights only, too few measurements or a too short follow-up period [10, 17]. We like to emphasize that our results are based on four equally spaced medical measurements defining weight change over a period of 15 years and still comprise a subsequent 15 years follow-up period for the analysis of further survival.
In the other five studies an effect of weight cycling on all-cause mortality was not observed at all [12, 13], did not show up in 25 years long-term follow-up [10] or the effect vanished after pre-existing disease was taken into account [11, 14].
The two prospective cohort studies, which did not find any adverse effect of weight fluctuations on mortality are based on recalled weight and a definition of weight cycling by two time points only, like in the Charleston Heart Study [13] or on weight measurements, however comprise only three time points as in the Baltimore Longitudinal Study [12].
We lack information beyond 15 years of further survival and hence can not exclude the possibility that in an even longer subsequent survival analysis the effect of weight fluctuations would no longer persist as in the 25 years follow-up of a cohort of men in the Chicago Western Electric Company study from Dyer et al. [10]. However, as the age range of the cohort at start of analysis of subsequent survival in Dyer et al’s study is 48–66 years and in the present study is 55–74 years, the age range of our study after 15 years of subsequent follow-up for survival results in a rather similar age range than that of Dyer et al after 25 years (i.e. 70–89 years and 73–91 years, respectively).
Both, an analysis of a male cohort of the British Regional Study conducted by Wannamethee et al. [14] and an analysis of a male cohort of the Honolulu Heart study conducted by Iribarren et al. [11] found that the effect of weight loss and weight fluctuations are not risk factors for mortality, if pre-existing disease and lifestyle factors like smoking were taken into account. However, in our analysis we do not see this large reduction in the risk of death in the weight fluctuation group, neither in “just” adjusted, nor in stratified analyses. In our analysis this explanation of a weight change effect entails only to the weight loss group. Although we cannot rule out completely that our analyses of a much smaller sample may have lead to sample selection bias, we do not have any hint on that. Moreover, the findings of Wannamethee et al. may in part reflect the ill-health status of the study participants as those were sampled from patients of general practices and not from a representative population of men as in our study. Furthermore, Wannamethee et al.’s study has a similar weight change definition period of 12–14 years as in the present study, however a subsequent follow-up period of only 8 years. As reported above, Dyer et al. have shown that the length of the follow-up period may influence the observable pattern of weight change [10]. Moreover, the definition of weight fluctuations in the analysis of Iribarren et al. is restricted to only three examinations. Hence, weight cycling is restricted to just one cycle by study design. In addition, subsequent follow-up for further survival is limited to only 6 years, which may be too short to reveal adverse effects of weight fluctuations on mortality. Moreover, the genetically and lifestyle related risks of mortality in this specific population (men of Japanese-American descent) may not be directly comparably to those in European societies or US-Americans of European decent.
Previous studies showing that weight fluctuations are an independent risk factor for mortality have been questioned, because these studies did not give a biological plausible explanation by which mechanism weight fluctuations may induce adverse health effects and mortality beyond pre-existing disease [17]. A recent study on weight cycling and immunocompetence may give some hint in this respect [18, 19]. Shade et al. showed in a study comprising 114 women that those women reporting ever having lost more than 10 pounds of weight had lower natural killer cell cytotoxicity, than those, who had not. Moreover, this reduction in immune function was enhanced by increased frequency of intentional weight loss, which could be taken as a proxy-indicator of weight cycling. In addition some recent studies have found, that weight cycling is associated with lower HDL-C in women [20], which is known to be associated with cardiac events and enhance the risk to develop hypertension [21]. However, these results are still controversial, e.g. Petersmarck et al. [22] did not find adverse effects of weight cycling neither on blood pressure, nor on blood lipids in a cohort study comprising 6 years of follow-up.
Limitations of this study
The sample size for some weight change categories is small in the ERFORT study, in particular in the weight loss group. Hence, the non significant effect of the weight loss group may be due to a power problem of statistical testing.
Lack of reliable information on intentional and unintentional weight loss is a further limitation of the present study. Whilst, we are not aware of studies that have analyzed the effect of intentional and unintentional weight loss and weight fluctuations on all cause mortality simultaneously, we think that future studies should collect data on intentional weight change. Some studies found that weight loss is hazardous only if it was not intended [23, 24], however, we are not aware of any study, showing that this result entails also explicitly to weight cycling.
A further restriction of this study is the lack of information on cancer. Occult malignant disease may influence weight change patterns. However, we think it unlikely that the found effect of weight fluctuations is mainly due to cancer, as we have a long period of 15 years of weight change definition before the starting point of the survival analysis.
Strengths of this study
Despite the limitations mentioned above, this study has several strengths we want to emphasize. Contrary to most studies on weight fluctuations being based on self-reported or just recalled weight and height the present study is based on anthropometric measurements. Furthermore information on cardiovascular risk factors and data on pre-existing disease related to mortality is gathered in medical examinations by physicians and not just by self-report.
Moreover, with the exception of the studies of Dyer et al. and Peters et al. this is the only study on weight fluctuations we are aware of, which has both a long enough period (15 years) to define weight fluctuations and also a long enough period of follow-up (15 years) to allow analysis of long-term effects of weight fluctuations on all-cause mortality.
In addition, this study further adds to the research on weight change and weight fluctuations on mortality in European populations, because only few prospective studies regarding this topic have been conducted in non-American populations [7, 9, 14].
Conclusion
Weight fluctuations are a major risk factor for all-cause mortality in middle and older aged men. Weight fluctuations are recognized as a warning signal for an increased risk of death in elder men. Moreover, stable obesity does not increase further mortality in the present study. Reducing obesity in middle aged and older men may be of less importance than assumed previously, as long as weight can be maintained quite stable and does not have already led to severe cardiovascular impairments or diabetes.
Abbreviations
- BMI:
-
Body mass index
- ERFORT study:
-
Erfurt Male Cohort Study
- HRR:
-
Hazard rate ratio
- 95%-CI:
-
95%-Confidence interval
References
Lobstein T, Rigby N, Leach R. EU. Platform on diet, physical activity and health. International Obesity Task Force 2005 http://www.iotf.org/media/euobesity3.pdf.
Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv. Data 2004;1–17.
Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin Dermatol 2004;22:296–302.
Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the Multiple Risk Factor Intervention Trial. Ann Intern Med 1993;119:749–57.
Diaz VA, Mainous AG III, Everett CJ. The association between weight fluctuation and mortality: results from a population-based cohort study. J Community Health 2005;30:153–65.
Hamm P, Shekelle RB, Stamler J. Large fluctuations in body weight during young adulthood and twenty-five-year risk of coronary death in men. Am J Epidemiol 1989;129:312–8.
Lissner L, Bengtsson C, Lapisus L, Larsson B, Bengtsson B, Brownell K. Body variability and mortality in the Gothenburg Prospective Studies of men and women. In: Björmtorp P, Rossner S, editors. Obesity in Europe 88: Proceedings of the first European congress on obesity. London: Libbey; 1989. pp. 55–60.
Lissner L, Odell PM, D’Agostino RB, Stokes J III, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med 1991;324:1839–44.
Peters ET, Seidell JC, Menotti A, Arayanis C, Dontas A, Fidanza F, et al. Changes in body weight in relation to mortality in 6441 European middle-aged men: the seven countries study. Int J Obes Relat Metab Disord 1995 Dec;19(12):862–8.
Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol 2000;152:324–33.
Iribarren C, Sharp DS, Burchfiel CM, Petrovitch H. Association of weight loss and weight fluctuation with mortality among Japanese American men. N Engl J Med 1995;333:686–92.
Lissner L, Andres R, Muller DC, Shimokata H. Body weight variability in men: metabolic rate, health and longevity. Int J Obes 1990;14:373–83.
Stevens J, Lissner L. Body weight variability and mortality in the Charleston Heart Study. Int J Obes 1990;14:385–6.
Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med 2002;162:2575–80.
Meisinger C, Wolke G, Brasche S, Strube G, Heinrich J. Postload plasma glucose and 30-year mortality among nondiabetic middle-aged men from the general population: the ERFORT study. Ann Epidemiol 2006;16:534–9.
Cox D. Regression models and life tables (with discussion). J R Stat Soc B 1972;34:187–220.
Jeffery R. Does weight cycling present a health risk? Am J Clin Nutr 1996;63(Suppl):452S–5S.
Nebeling L, Rogers CJ, Berrigan D, Hursting S, Ballard-Barbash R. Weight cycling and immunocompetence. J Am Diet Assoc 2004;104:892–4.
Shade ED, Ulrich CM, Wener MH, Wood B, Yasui Y, Lacroix K, Potter JD, McTiernan A. Frequent intentional weight loss is associated with lower natural killer cell cytotoxicity in postmenopausal women: possible long-term immune effects. J Am Diet Assoc 2004;104:903–12.
Olson MB, Kelsey SF, Bittner V, Reis SE, Reichek N, Handberg EM, Merz CN. Weight cycling and high-density lipoprotein cholesterol in women: evidence of an adverse effect: a report from the NHLBI-sponsored WISE study. Women’s Ischemia Syndrome Evaluation Study Group. J Am Coll Cardiol 2000;36:1565–71.
Schulz M, Liese AD, Boeing H, Cunningham JE, Moore CG, Kroke A. Associations of short-term weight changes and weight cycling with incidence of essential hypertension in the EPIC-Potsdam Study. J Hum Hypertens 2005;19:61–67.
Petersmarck KA, Teitelbaum HS, Bond JT, Bianchi L, Hoerr SM, Sowers MF. The effect of weight cycling on blood lipids and blood pressure in the Multiple Risk Factor Intervention Trial Special Intervention Group. Int J Obes Relat Metab Disord 1999;23:1246–55.
Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med 2003;138:383–9.
Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med 2005;165:1035–40.
Acknowledgements
The professional fieldwork of this study is deeply acknowledged. The representatives of the study physicians are the PD Dr. Hartmut Holtz and PD Dr. Klaus-Dieter Dück, who examined together with the PI (G. S.) of the study a large fraction of the study populations and who were involved also in the follow-up programs. The blood tests were conducted by supervision of Prof. Dr. Peter Koehler. The program on psychosocial factors were supervised and conducted by Prof. Dr. M. Geyer, Dr. E. Kunzendorff, Edeltraud Bartel, Klaus Andrzejak, Dr. Katja Limpert, Dr. Günter Stübe, and Helma Bürger. Furthermore, we thank the study nurses Margrit Ullmann, Margot Langelotz, Anke Binding, and Siglinde Litzrodt as well as the team assistant Margret Remke and data manager Elke Siegemund, Hubert Schneller and Claudia Greschik for their extremely cautious work. The authors acknowledge the institutional support of the directors of the department of the Erfurt Medical School, Department for Preventive Cardiology, Prof. Dr. Joachim Knappe, and of the GSF-Institute of Epidemiology, Prof. Dr. Dr. H.-Erich Wichmann.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rzehak, P., Meisinger, C., Woelke, G. et al. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol 22, 665–673 (2007). https://doi.org/10.1007/s10654-007-9167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-007-9167-5