Abstract
Objective
Prefrail and frail older adults are a heterogeneous population. The measurement of appendicular lean mass (ALM) may distinguish those at higher versus lower risk of poor outcomes. We examined the relationship between ALM and mortality among prefrail and frail older adults.
Design
This was a population-based cohort study.
Setting
The Third National Health and Nutrition Survey (NHANES III; 1988-1994).
Participants
Older adults (age ≥65 years) with pre-frailty or frailty defined using the Fried criteria.
Measurements
ALM was quantified using bioimpedance analysis. Multivariable-adjusted Cox regression analysis examined the relationship between ALM and mortality. Logistic regression analysis was used to determine if ALM added to age and sex improved the predictive discrimination of five-year and ten-year mortality.
Results
At baseline, the average age was 74.9 years, 66.7% were female, 86.3% and 13.7% were prefrail and frail, respectively. The mean ALM was 18.9 kg [standard deviation (SD): 5.5]. During a median 8.9 years of follow-up, 1,307 of 1,487 study participants died (87.9%). Higher ALM was associated with a lower risk of mortality. In a multivariable-adjusted regression model that accounted for demographic, behavioral, clinical, physical function, and frailty characteristics, each SD increase in ALM was associated with an 50% lower risk of mortality [Hazard Ratio: 0.50 (95% CI: 0.27-0.92); P=0.026]. The addition of ALM to age and sex improved the predictive discrimination of five-year (P=0.027) and ten-year (P=0.016) mortality.
Conclusion
ALM distinguishes the risk of mortality among prefrail and frail older adults. Additional research examining ALM as a therapeutic target is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The musculature of the arms and legs, known as appendicular lean mass (ALM), accounts for ≥75% of skeletal muscle in the body, and is required for ambulatory activity and physical functioning (1). Older adults lose approximately 0.1 kg (0.5%) of ALM annually after the age of 40-50 years (1-3). The progressive loss of ALM may be a key element in the development and progression of frailty (4, 5), a syndrome of poor global health that is highly prevalent among older adults that includes unintentional weight loss, impaired physical function, weakness, exhaustion, and low levels of physical activity (6). Prefrail and frail older adults are prone to experience disability, falls, hospitalization, and death (6). However, prefrail and frail older adults are a heterogeneous population with varying degrees of functional disability (7). The measurement of ALM among prefrail and frail older adults may distinguish those at highest risk of poor outcomes, including mortality. We tested the hypothesis that ALM is independently associated with mortality among a population based sample of prefrail and frail older adults aged ≥65 years.
Methods
Study Design and Participants
The Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III) was a stratified multistage study conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention, to provide health information on a nationally-representative sample of U.S. civilians (8). The NHANES III sample does not include persons residing in nursing homes, members of the armed forces, institutionalized persons, or US nationals living abroad. Participants provided written informed consent prior to completing any study-related activities. Participants in this analysis included adults of age ≥65 years.
Frailty Definition
We implemented a definition of frailty that has been operationalized previously in the NHANES III database (9). The five criteria for frailty included:
1. Low weight-for-height, defined using a body mass index (BMI) ≤18.5 kg/m2;
2. Slow walking speed, defined using the slowest quintile adjusted for sex, in a timed 2.4-meter walk;
3. Weakness, defined as having any level of difficulty or inability to lift or carry something as heavy as 4.5-kilograms;
4. Exhaustion, defined as having any level of difficulty or inability to walk from one room to another on the same floor;
5. Low levels of physical activity, defined as a self-report of being less active compared to men or women of a similar age.
Participants who met 1-2 or ≥3 of the 5 above-described criteria were classified as prefrail or frail, respectively (9).
Appendicular Lean Mass
ALM was quantified using bioimpedance analysis (BIA). BIA was assessed using a bioresistance body composition analyzer (Valhalla 1990B, Valhalla Medical, San Diego, California) (10). Whole-body BIA measurements were obtained between the right wrist and ankle while lying in the supine position (10, 11). All participants fasted for a minimum of six-hours. Body mass and height were measured with the participant dressed in an exam gown without shoes. ALM was calculated using a validated equation, where ALM (kg) equals: -6.296 + (Height2/Resistance × 0.227) + (Reactance × 0.072) + (Sex × 9.909) + (Weight × 0.072) + (Sex × Age × -0.098) + (Age × 0.054); where height is in centimeters, weight is in kilograms, sex is equal to 1 for males and 0 for females, and resistance and reactance are in ohms (12). ALM calculated using this equation accounts for 92.3% of the variability in ALM quantified using dual-energy x-ray absorptiometry among prefrail and frail older adults (r=0.961; R2=0.923).
Mortality Outcome
Vital status was identified using the National Death Index (NDI) database through December 31, 2011. Participants were linked to the NDI database using probabilistic matching that included 12 identifiers such as Social Security number, sex, and date of birth (13).
Covariates
Demographic information including age, sex, and race were reported using a standardized questionnaire (14). Behavioral and clinical information including smoking status, selfrated health, hospitalization, and falls were reported using a standardized questionnaire (14). Cognitive function was quantified using the short portable version of the Mini Mental Status Exam to form a score that ranges from 0 to 17, with higher scores indicating better cognition (15). The presence of comorbid health conditions was self-reported. Laboratory assay procedures have been described in detail (16, 17). Poor balance was defined as the inability to maintain a full tandem stand for 10 seconds (18). Gait speed was assessed using a four-meter walk, timed with a stopwatch.
Statistical Analysis
Cox proportional hazards regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (95% CI) between ALM and mortality. The assumption of proportional hazards was confirmed using log-log plots. Four regression models were specified to systematically understand the relationship between ALM and mortality after incrementally accounting for important covariates. To explore the discriminative capacity of ALM to prognosticate five- and tenyear year mortality, we calculated the area under the receiver operating characteristic (ROC) curve, known as the C-statistic, using logistic regression models. All analyses incorporated sample weights to account for nonresponse bias and multistage sampling probabilities (19). Stata/SE v.14.1 was used for all analyses.
Results
Cohort Characteristics
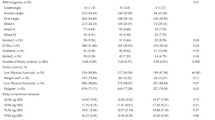
The mean age of study participants was 74.9 years and 66.7% were female (Table 1). The majority of study participants were prefrail (86.3%). Weakness was the most common individual frailty component (63.6%), followed by low physical activity (38.4%), slow walking (33.4%), exhaustion (17.1%), and low weight-for-height (6.4%). Participants reported a variety of comorbid health conditions (43.9% with ≥3 conditions). Participants had or were at risk for mobility disability, as reflected by a slow gait speed gait speed (76% <0.8 m/s), poor balance (42.3%), and recent falls (32.4%).
Appendicular Lean Mass and Mortality
Average ALM was 18.9 kg [standard deviation (SD): 5.5 kg]. During a median follow-up of 8.9 years [interquartile range: 4.6 to 14.6], we observed 1,307 deaths (87.9%). In multivariable-adjusted regression models, each SD increase in ALM was associated with a 50% lower risk of mortality [HR: 0.50 (95% CI: 0.27-0.92); P=0.026]. This relationship was robust to the inclusion of a variety of covariates (Figure). The relationship between ALM and mortality was not modified by sex (Pinteraction=0.73).
Discriminative Capacity of Appendicular Lean Mass
The addition to ALM to age and sex improved the predictive discrimination of five-year (c statistic 0.693 vs 0.677; P=0.027) and ten-year (c-statistic 0.731 vs 0.741; P=0.016) mortality.
Discussion
The principal finding of this study is that ALM is independently associated with mortality among a populationbased sample of prefrail and frail older adults aged ≥65 years. This relationship was consistent in analyses that accounted for other known prognostic factors such as age, sex, comorbid health conditions, and measures of physical functioning. These data indicate that ALM may serve as a sensitive objective measure that addresses the heterogeneity or health risk among prefrail and frail older adults.
Our findings suggest that ALM may have important implications for the longevity among prefrail and frail older adults. In addition to longevity, adequate ALM is necessary for ambulatory activity and physical functioning (6). Given the central role of muscle mass in the development and progression of frailty (4), improvement of ALM may serve as a potential therapeutic target to direct interventions that seek to improve the health outcomes of vulnerable older adults.
In a prior study, exercise improved muscle protein synthesis and ALM among obese frail older adults (20). Exercise may be a promising intervention to prevent the onset or delay the progression of frailty (21). Among 424 sedentary communitydwelling older adults, 12-months of physical activity reduced the risk of being considered frail (P=0.01), compared to a health education group that was not prescribed physical activity (22). Additional studies are needed to assess whether exercise may improve ALM and long term health outcomes among pre-frail and frail older adults (23).
The main strength of this study is the large sample size that, based on the sampling design, represents the US population of community-dwelling prefrail and frail older adults. Participants in our sample represented a spectrum of age (65-90 years) and ALM (10.6-34.4 kg). The cohort had sufficient length of follow-up to observe a high proportion of deaths, such that 87.9% of the cohort had died by the end of the follow-up period. The limitation to this study is that comorbid health conditions were self-reported. This likely underestimates the prevalence of comorbidities in this population which may attenuate the strength of the association between ALM and mortality.
In conclusion, our findings suggest that community-dwelling prefrail and frail older adults with a high ALM may be less likely to die compared to those with a low ALM. These data may help to identify which prefrail and frail older adults are at the highest risk for poor outcomes. Additional research examining the role of ALM in frailty is warranted.
Acknowledgements: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK105207) of the National Institutes of Health. The content does not necessarily represent the views of the National Institutes of Health.
Disclosures: The authors report no conflicts of interest.
Funding: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK105207) of the National Institutes of Health.
Ethical Standards: The study was approved by the committee on human research at the University of Pennsylvania. Written informed consent was obtained from all participants included in this study.
References
Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985). 1997;83:229–239.
Auyeung TW, Lee SWJ, Leung J, Kwok T, Woo J. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 communitydwelling older Chinese. Geriatrics & gerontology international. 2014;14:76–84.
Kitamura I, Koda M, Otsuka R, Ando F, Shimokata H. Six-year longitudinal changes in body composition of middle-aged and elderly Japanese: Age and sex differences in appendicular skeletal muscle mass. Geriatrics & gerontology international. 2014;14:354–361.
Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238.
Idoate F, Cadore EL, Casas-Herrero A, et al. Adipose tissue compartments, muscle mass, muscle fat infiltration, and coronary calcium in institutionalized frail nonagenarians. Eur Radiol. 2014:1–13.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:M146–M157.
Montero-Odasso M, Bergman H, Béland F, Sourial N, Fletcher JD, Dallaire L. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same? Arch Gerontol Geriatr. 2009;49:272–277.
Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;(32):1–407.
Brown JC, Harhay MO, Harhay MN. The Prognostic Importance of Frailty in Cancer Survivors. J Am Geriatr Soc. 2015;2015;63:2538–2543.
Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89:465–471.
Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–817.
Baar Hv, Hulshof P, Tieland M, de Groot C. Bio-impedance analysis for appendicular skeletal muscle mass assessment in (pre-) frail elderly people. Clinical Nutrition ESPEN. 2015.
Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–734.
National Center for Health Statistics. NHANES III Questionnaires, Datasets and Related Documentation. Available at: http://www.cdc.gov/nchs/nhanes/nh3data.htm.
Obisesan TO, Obisesan OA, Martins S, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–509.
National Center for Health Statistics. Laboratory Procedures Used for the Third National Health and Nutrition Exam Survey (NHANES III), 1988-1994. Available at: http://wonder.cdc.gov/wonder/sci_data/surveys/hanes/hanes3/type_txt/lab.asp.
Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem. 2005;51:450–452.
Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173.
Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity. 2011;19:312–318.
Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143.
Cesari M, Vellas B, Hsu FC, et al. A Physical Activity Intervention to Treat the Frailty Syndrome in Older Persons-Results From the LIFE-P Study. J Gerontol A Biol Sci Med Sci. 2014.
Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, J.C., Harhay, M.O. & Harhay, M.N. Appendicular lean mass and mortality among prefrail and frail older adults. J Nutr Health Aging 21, 342–345 (2017). https://doi.org/10.1007/s12603-016-0753-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-016-0753-7