Abstract
Objective
We aimed to investigate the association between metabolic syndrome (MS) and hearing impairment (HI) using nationally representative data from Korean adults.
Design, setting and participants
A total of 16,799 subjects (≥19 years old; 7,170 men and 9,629 women) who underwent pure tone audiometry testing were included in the analysis. Data were obtained from the fifth Korea National Health and Nutrition Examination Survey (2010–2012). Subjects were divided into two groups according to the presence of MS.
Results
Among the subjects with MS, 47% had HI. Logistic regression analysis revealed that MS was not an independent risk factor for HI, although increased fasting plasma glucose (OR 1·4, 95% CI: 1·1–1·8) was independently associated with HI. In addition, older age, male sex, very low body mass index (≤17·5 kg/m2), lower education level, smoking history, and occupational noise exposure were independently associated with HI. For low-frequency HI, independent risk factors included older age, lower educational level, lower economic status, and very low BMI (≤17·5 kg/m2). For high-frequency HI, independent risk factors included older age, male sex, lower educational level, lower economic status, increased blood pressure, lower high-density lipoprotein cholesterol, and smoking history.
Conclusions
MS itself was not an independent risk factor for HI, and, among the individual metabolic components, only increased fasting plasma glucose was independently associated with HI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MS) is a collection of metabolic risk factors that ultimately leads to atherosclerotic cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality (1). Although the worldwide prevalence of MS varies according to the regional and enrolled population, approximately one-fifth of the total American population is known to have MS (2). In Korea, the age-adjusted prevalence of MS had been increased from 24.9% in 1998 to 31.3% in 2007 (3). In general, MS is regarded as a state of chronic low-grade inflammation that is regulated by a combination of genetic and environmental factors, and it is accompanied by glucose intolerance, hypertension, visceral adiposity, atherogenic dyslipidemia, and a hypercoagulable state (4).
Hearing impairment (HI) is one of the most common chronic diseases in older adults after hypertension, arthritis (5). Risk factors for HI include: Aging, genetic predispositions, preterm birth, exposure to loud noise, ototoxic medications such as aspirin, loop diuretics, aminoglycosides and cisplatin, some illnesses such as local ear infections, chronic ear diseases, meningitis, and autoimmune diseases (6-11).
Among the individual metabolic risk factors that are related to HI, the influence of diabetes, hypertension, hyperlipidemia, obesity and high body mass index (BMI) on hearing have been thoroughly investigated by many researchers (12-15). However, to the authors’ best knowledge, the association between MS itself and HI has rarely been investigated.
Therefore, the aim of this study was to examine the association between MS and HI using nationally representative data from Korean adults.
Methods
Ethical consideration
This study was approved by the institutional review board of Eulji University (No. 2014-012-003).
Study population
The Korean National Health and Nutrition Examination Survey (KNHANES) is an annual national survey that has been conducted by the Korean Centers for Disease Control and Prevention since 1998. The KNHANES survey examines the health and nutritional status of a representative sample of the general Korean population. In this study, we analyzed data from the fifth KNHANES (2010–2012), which evaluated 25,534 subjects during the survey period. All participants provided their written informed consent before enrollment in the survey. For our analysis, data were excluded if the subject was <19 years old or if pure tone audiometry was not performed. After the exclusion of these subjects, data from 16,799 subjects were included in our analysis.
Definition of metabolic syndrome
Based on the modified NCEP-ATP III definition and the determinations of the Korean Society for the Study of Obesity, a subject was defined as having MS if he/she met three of the following five criteria: (1) a race-adjusted waist circumference that met the definition of abdominal obesity (men: ≥90 cm, women: ≥85 cm); (2) triglyceride levels of ≥150 mg/dL; (3) high-density lipoprotein (HDL) cholesterol levels of ≤40 mg/dL (men) or ≤50 mg/dL (women); (4) elevated blood pressure (≥130/85 mmHg) and/or antihypertensive drug treatment; and (5) elevated fasting blood glucose (FBG, ≥100 mg/dL) and/or drug treatment for elevated FBP (16, 17).
Sociodemographic data collection
All data regarding smoking, alcohol consumption, diet therapy, household income, education status, and occupational noise exposure were collected using a self-reported survey. Subjects with a current smoking habit or a history of smoking were classified as smokers, and lifetime non-smokers were classified as non-smokers. For alcohol consumption habits, lifetime non-drinkers or subjects who consumed <1 drink/month during the past year were classified as non-drinkers, while subjects who consumed >1 drink/month over the past year were classified as drinkers. For diet therapy, subjects were questioned regarding whether they had undergone diet therapy. Household income was classified into four groups: lower class, lower-middle class, upper-middle class, or upper class. Education status was classified into four groups: elementary school, middle school, high school, or university. For occupational noise exposure, subjects were asked if they had been exposed to loud noise at work for more than 3 months. Previous medically diagnosed histories of diabetes or hypertension were documented.
Body measurements
Blood pressure was measured three times, separated by intervals of 30 s, and the average of these measurements was adopted as the final blood pressure. Fasting plasma glucose (FPG), total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were tested after a minimum fast of 12 h. Height was measured using a SECA225 measuring device (SECA Deutschland, Hamburg, Germany), and weight was measured to one decimal point (0.1 kg) using a GL-6000-20 scale (CAS KOREA, Seoul, Korea). Waist circumference was measured using a tape measure (SECA200, SECA Deutschland) at the midpoint between the lowest rib and the iliac crest of the pelvis, after a modest expiration. BMI was calculated as weight divided by height squared (kg/m2).
Assessment of hearing
Ear, nose, and throat examinations were performed by otolaryngologists. Measurement of the air-conduction hearing threshold was conducted by well-trained examiners in a soundproof booth, using an SA 203 audiometer (Entomed, Malmö, Sweden), at the frequencies of 0.5, 1, 2, 3, 4, and 6 kHz (18). The arithmetic mean of the pure tone thresholds at 0.5, 1, 2, 3, 4, and 6 Hz was considered the mean hearing threshold. HI was diagnosed for subjects whose unilateral and/or bilateral mean hearing threshold exceeded 25 dB for these thresholds. The low-frequency hearing threshold was calculated as the average thresholds at 0.5, 1, and 2 kHz. The high-frequency hearing threshold was defined as the average thresholds at 3, 4, and 6 kHz. Low-frequency HI and high-frequency HI was diagnosed for subjects whose unilateral and/or bilateral average threshold exceeded 25 dB for the relevant thresholds.
Statistical analyses
All statistical analyses were performed using SPSS software (version 22.0, SPSS Inc., Chicago, IL), and the level of statistical significance was set at a p-value of <0.05. Complex-sample analyses were performed to account for the stratified multi-stage clustered probability design and weights in KNHANES. General linear regression analysis was performed to compare the differences between continuous variables. Chisquared analysis was performed to compare the differences between nominal variables. Multivariate logistic regression analysis was performed to evaluate the associations between HI and the variables that were of interest.
Results
Population characteristics
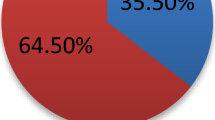
We included a total of 16,799 subjects (age, ≥19 years old) who had undergone pure tone audiometry during the fifth KNHANES (2010–2012). The study population consisted of 7,170 men (42.7%) and 9,629 women (57.3%), with a mean age of 50 years (standard deviation: 16.45 years, range: 19–97 years). Among these subjects, 4,161 subjects (24.8%) had MS. Subject characteristics are listed in Table 1, and the weighed characteristics of subjects with MS are listed in Table 2. The weighted prevalence of MS among Korean adults was 22.3%, and the prevalence of HI was 31.7%. Among the subjects who had MS, 46.8% also had HI, and there was a significant difference in the prevalence of HI when subjects were compared according to MS status (p < 0.001).
Weighted results of the self-report questionnaires regarding hearing status
Subjects with MS reported significantly more subjective hearing discomfort that the subjects who did not have MS (p < 0.001) (Table 3). Regarding the use of hearing aids or cochlear implants, subjects with MS tended to use these hearing amplifiers more often than the subjects who did not have MS. Although the prevalence of tinnitus was not significantly different when subjects were stratified according MS status, subjects with MS reported that they experienced significantly more lifestyle discomforts (p < 0.001).
Pure tone audiometry results according to MS status
The weighted mean hearing thresholds for subjects with MS was 24.9 dB in the right ear and 25.6 dB in the left ear, and these were significantly higher than those for the subjects who did not have MS (right ear: 17.0 dB, left ear: 17.4 dB; both, p < 0.001) (Figure 1). When the thresholds were analyzed according to frequency, significantly higher hearing thresholds were observed for both ears among subjects with MS for all tested frequencies (p < 0.001) (Figure 2).
Association between HI and MS or individual metabolic components
Bivariate analysis and multivariate logistic regression analysis was performed to examine the association between HI and MS or the individual metabolic risk factors (Table 4). Bivariate analysis revealed that all of the tested variables were significantly associated with HI (p < 0.001), with the exception of diet therapy (p = 0.256). However, after adjusting for age, sex, BMI, economic status, education level, a history of smoking, alcohol consumption, diet therapy, and occupational noise exposure, the multivariate logistic regression analysis revealed that MS (odds ratio [OR]: 0.8, 95% confidence interval [CI]: 0.5–1.2) itself was not an independent risk factor for HI. Among the individual metabolic components, only increased FBG (OR: 1.4, 95% CI: 1.1–1.8) was independently associated with HI. In addition, older age, male sex, very low BMI (≤17.5 kg/m2), lower education level, a history of smoking, and occupational noise exposure was independently associated with HI (Table 4).
Regarding the affected frequencies, older age, lower educational level, lower economic status, and very low BMI (≤17.5 kg/m2) were significantly associated with low-frequency HI. Similarly, older age, low educational level, and lower economic status were significantly associated with high-frequency HI. In addition, male sex, elevated blood pressure or antihypertensive treatment, lower HDL-C, and a history of smoking were independent risk factors for high-frequency HI (Table 5).
Discussion
In this study of data from a large population-based survey, we found that MS itself was not an independent risk factor for HI, and, among the individual metabolic components, only increased FPG was independently associated with HI. Although not presented as outcomes in this study, we also found that the number of included independent metabolic risk factors, or combination of the specific individual metabolic risk factors that were used to diagnose MS, were not associated with prevalence of HI. These findings indicate that MS alone, as diagnosed using the typical criteria, did not account for all of HI that we observed. This result is consistent with the findings of Chang et al. that have reported an insignificant relationship between MS and age-related HI (ARHI) (19). All of these suggest that the impact of MS itself on hearing may be lower less than expected. Rather, clinician should pay special attention to the glycemic control in MS patients for prevention of HI. reassurance to MS patients who are supersensitive to their medical conditions can also be achieved by explaining our results in clinical practice.
Smoking and diabetes have been reported to be associated with elevated hearing thresholds at frequencies of 0.5–8 Hz, according to data from the National Health and Nutrition Examination Survey (NHANES) in the US (13). Similarly, a recent meta-analysis has demonstrated that HI was 2.1-fold more prevalent in patients with diabetes than in patients without diabetes, and that result was independent of the subjects age (20). Furthermore, levels of hemoglobin A1C and creatinine have been reported to be associated with the occurrence or progressive deterioration of HI in patients with diabetes (21, 22). The cause for this association may be related to changes in anatomic structures, such as those induced by microangiopathy, degeneration of the stria vascularis, or loss of cochlear outer hair cells regardless of treatment with insulin or oral hypoglycemic drugs (23).
Although one previous study has reported that the prevalence of low-frequency and high-frequency HI was increased among patients with diabetes (24), we did not observe an association between frequency-specific HI and diabetes in this study. Instead, high-frequency HI was associated with elevated blood pressure and/or antihypertensive treatment, as well as lower HDL-C, while low-frequency HI was not associated with MS or any of the individual metabolic components.
The association between hypertension and HI remains controversial. In our study, hypertension was not an independent risk factor for HI, and similar results have been reported using data from NHANES (24). In contrast, other studies with smaller sample sizes have often reported positive results. For example, one study of 154 pairs cases (45–64 years old) found that there was a significant association between hypertension and an increased hearing threshold, and the authors insisted that hypertension may be one of the risk factors that are responsible for accelerating degeneration in ARHI (25). In addition, the influence of hypertension on hearing may vary according to sex, as other studies have demonstrated that an association exists between high systolic blood pressure and low-frequency hearing loss among elderly women (26).
Unlike LDL-C, high HDL-C is a protective factor for cardiovascular disease (27), and low HDL-C has been reported to increase the risk of low/mid-frequency HI by 2·2-fold (24). From an anatomic perspective, high cholesterol affects both the inner ear blood supply and also the lateral wall stiffness of the cochlear outer hair cells (28, 29). Whereas, others reported that the association between lipid level and hearing loss was so small in a cross-sectional study and this association was no longer significant in longitudinal analysis (30). Given that there was no significant association between HDL-C levels and HI in our study, except for a small relationship between high-frequency HI and low HDL-C levels, we assume that our results partially support the absence of a relevant relationship.
Among the individual metabolic components, abdominal obesity has been reported to be an independent risk factor for ARHI, independent of BMI (31). Regarding sex-specific analysis, waist circumference was associated with poor central auditory function among older male subjects, and an association between visceral adipose tissue and HI has been reported among women who are ≥40 years old (32). However, our findings do not support a significant association between abdominal obesity and HI; therefore, we assume that hearing is likely not affected by abdominal obesity.
Finally, we found that the very low BMI (≤17.5 kg/m2) increased the risk of HI by 3.5-fold and the risk of low-frequency HI by 4.1-fold. Similarly, very low BMI was associated with a high risk of death among Asian subjects (33). In contrast, a European multi-center study has demonstrated that high BMI was related to HI, and a recent Danish study has indicated that high BMI and low-frequency HI may be associated (15, 34). We assume that the differences in these associations may be caused by ethnicity-related differences in subject characteristics.
This study contains several limitations. First, we could not differentiate between type 1 and type 2 diabetes. Although both types of diabetes are reported to be associated with HI in previous studies, the effects of diabetes on hearing loss might be different according to type. Second, we classified smoking status as either smoker or lifetime non-smoker, although a dose-dependent effect for smoking has been reported in a previous study (15). Third, we did not exclude subjects with congenital hearing loss or conductive hearing loss in this study, therefore the mixed nature of hearing loss might have affected our findings.
In conclusion, MS itself was not an independent risk factor for HI, and, among the individual metabolic components, only increased FPG was independently associated with HI. In addition, older age, male sex, very low BMI (≤17.5 kg/m2), low socioeconomic status, a history of smoking, and occupational noise exposure were independently associated with HI.
Acknowledgements: This paper was supported by Eulji University in 2015.
Conflict of interest: No conflict of interests.
References
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112(17): 2735–2752.
Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62(8): 697–703.
Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care 2011;34(6); 1323–1328.
Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;943162. doi: 10.1155/2014/943162.
Walling AD, Dickson GM. Hearing loss in older adults. Am Fam Physician 2012;85(12):1150–1156.
Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res 2013;303:30–38.
Du W, Guo Y, Wang C, Liu X. A systematic review and meta-analysis of common mutations of SLC26A4 gene in Asian populations. Int J Pediatr Otorhinolaryngol 2013;77(10): 1670–1676.
Alves C, Oliveira CS. Hearing loss among patients with Turner’s syndrome: literature review. Braz J Otorhinolaryngol 2014;80(3): 257–263.
Zimmerman E, Lahav A. Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J Perinatol 2013(1); 33:3–8.
Sliwinska-Kowalska M, Davis A. Noise-induced hearing loss. Noise Health 2012;14(61): 274–280.
Mijovic T, Zeitouni A, Colmegna I. Autoimmune sensorineural hearing loss: the otology-rheumatology interface. Rheumatology (Oxford) 2013;52(5): 780–789.
Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008;149(1): 1–10.
Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol 2009;30(2): 139–145.
Axelsson A, Lindgren F. Is there a relationship between hypercholesterolaemia and noise-induced hearing loss? Acta Otolaryngol 1985;100(5-6): 379–386.
Fransen E, Topsakal V, Hendrickx JJ, Van Laer L, Huyghe JR, Van Eyken E et al. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol 2008;9(3): 264–276.
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/ American Heart Association conference on scientific issues related to definition. Circulation 2004;109(3): 433–438.
Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75(1): 72–80.
Kang JW, Choi HS, Kim K, Choi JY. Dietary vitamin intake correlates with hearing thresholds in the older population: the Korean National Health and Nutrition Examination Survey. Am J Clin Nutr 2014;99(6): 1407–1413.
Chang NC, Chien CY, Hsieh MH, Lin WY, Ho KY. The association of insulin resistance and metabolic syndrome with age-related hearing loss. J Diabetes Metab 2014; doi: 10.4172/2155-6156.1000440.
Horikawa C, Kodama S, Tanaka S, Fujihara K, Hirasawa R, Yachi Y et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013;98(1): 51–58.
Lerman-Garber I, Cuevas-Ramos D, Valdés S, Enríquez L, Lobato M, Osornio M et al. Sensorineural hearing loss—a common finding in early-onset type 2 diabetes mellitus. Endocr Pract 2012;18(4): 549–557.
Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol 2003;24(3): 382–386.
Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg 2006;132(9): 934–938.
Bainbridge KE, Hoffman HJ, Cowie CC. Risk factors for hearing impairment among U.S. adults with diabetes: National Health and Nutrition Examination Survey 1999–2004. Diabetes Care 2011;34(7): 1540–1545.
de Moraes Marchiori LL, de Almeida Rego Filho E, Matsuo T. Hypertension as a factor associated with hearing loss. Braz J Otorhinolaryngol 2006;72(4): 533–540.
Rosenhall U, Sundh V. Age-related hearing loss and blood pressure. Noise Health 2006;8(31): 88–94.
Tran-Dinh A, Diallo D, Delbosc S, Varela-Perez LM, Dang QB, Lapergue B et al. HDL and endothelial protection. Br J Pharmacol 2013;169(3): 493–511.
Ciccone MM, Cortese F, Pinto M, Di Teo C, Fornarelli F, Gesualdo M et al. Endothelial function and cardiovascular risk in patients with idiopathic sudden sensorineural hearing loss. Atherosclerosis 2012;225(2): 511–516.
Nguyen TV, Brownell WE. Contribution of membrane cholesterol to outer hair cell lateral wall stiffness. Otolaryngol Head Neck Surg 1998;119(1): 14–20.
Simpson AN, Matthews LJ, Dubno JR. Lipid and C-reactive protein levels as risk factors for hearing loss in older adults. Otolaryngol Head Neck Surg 2013;148(4): 664–670.
Hwang JH, Chen JC, Yang WS, Liu TC. Waist circumference is associated with pitch pattern sequence score in older male adults. Int J Audiol 2012;51(12): 920–925.
Kim TS, Park SW, Kim do Y, Kim EB, Chung JW, So HS. Visceral adipose tissue is significantly associated with hearing thresholds in adult women. Clin Endocrinol (Oxf) 2014;80(3): 368–375.
Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011;364(8): 719–729.
Frederiksen TW, Ramlau-Hansen CH, Stokholm ZA, Brødsgaard Grynderup M, Hansen ÅM, Lund SP et al. Atherogenic risk factors and hearing thresholds. Audiol Neurootol 2014;19(5): 310–318.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.Y., Choi, Y.J., Choi, H.J. et al. Metabolic syndrome is not an independent risk factor for hearing impairment. J Nutr Health Aging 20, 816–824 (2016). https://doi.org/10.1007/s12603-015-0647-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-015-0647-0