Abstract
Giardiasis is a re-emerging infectious disease with outbreaks reported globally specially in children and malnourished individuals leading to malabsorption, growth retardation, and severe diarrhea. Thus, in the present study, prophylactic administration of synbiotic as the functional food was used to assess its antigiardial potential in malnourished murine giardiasis. Interestingly, prior administration of synbiotic (Lactobacillus casei + inulin) even to malnourished-Giardia-infected mice led to increased body mass, small intestine mass, lactobacilli counts, and reduced severity of giardiasis as evident by decreased cyst and trophozoite counts. Synbiotic therapy further boosted the innate and acquired immune response resulting into increase in nitric oxide, antigiardial secretory IgA and IgG antibody levels along with IL-6 and IL-10 cytokines, and decreased levels of inflammatory TNF-α cytokine in both serum and intestinal fluid in malnourished-synbiotic-Giardia-infected mice compared with malnourished-Giardia-infected mice. More specifically, histopathological and scanning electron microscopy analysis of the small intestine also confirmed the modulatory potentials of synbiotic in malnourished-synbiotic-Giardia mice which had less cellular and mucosal damage compared with severely damaged, mummified, and blunted villi in malnourished-Giardia-infected mice. Taken together, this is the first experimental study to report that prior supplementation of synbiotic restored the gut morphology and improved the immune status of the malnourished-Giardia-infected mice, and could be considered as the prophylactic adjunct therapy for malnourished individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardiasis is an acute infectious gastrointestinal disease affecting children and adults worldwide. It is caused by Giardia intestinalis, an intestinal eukaryotic, flagellated protist and is much more frequent in developing countries with incidence rate of 30% than in developed countries like USA where incidence rate is 3–7%, but outbreaks of disease in nurseries and day care centers are common [1, 2]. Giardia infection can lead to acute diarrhea with abdominal discomfort and malabsorption or be asymptomatic and at times becomes chronic giardiasis [3]. School children, malnourished individuals, common variable immunodeficiency (CVID) patients, hypogammaglobulineamic patients, homosexuals, and people with blood group-A are highly susceptible to giardiasis [4, 5]. In addition, malnutrition, the biggest problem faced by the human kind particularly in underdeveloped and developing countries, also enhances the susceptibility and severity of giardiasis due to altered anthropometry and gut morphology leading to malabsorption, maldigestion, diarrhea, and growth retardation mainly in young children less than 5 years of age [6,7,8,9].
Antiprotozoal antibiotics such as metronidazole, tinidazole, quinacrine, furazolidone, and nitazoxanide are commonly used to treat giardiasis but due to recurrence of the disease and adverse effects (metallic taste, headache, nausea, vomiting) along with emergence of antibiotic resistance, interests of scientists have shifted towards alternative biointerventions such as functional foods and phytotherapy for the prevention and management of giardiasis [9,10,11]. Functional food can be defined as the nonnutritive ingredients that provide benefits to maintain health in human beings [12]. Moreover, functional foods form a part of our normal daily diet and are composed of naturally occurring components which improve host immunity, prevent diseases, control physical and psychic behavior, and slow down the aging process [13, 14]. Functional foods not only include nutrients but also probiotics, prebiotics, and synbiotics. Probiotics are defined as live microorganisms which, when administered in adequate amounts, confer a health benefit on the host [15]. A prebiotic is defined as “a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improves host health” [16]. Synbiotic is a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract, by selectively stimulating the growth of one or a limited number of health promoting bacteria, thereby improving host welfare [17].
In our earlier studies, we have observed that prior administration of both probiotic or prebiotic alone to malnourished mice modulated giardiasis but in combination as the synbiotic (L. casei and inulin) has not been investigated and rose our interest [7,8,9]. Moreover, synbiotic has been found to be an effective prophylactic agent in various diseases and infections such as colorectal cancer, Crohn’s disease, type II diabetes, cardio vascular diseases, and Toxoplasma gondii infection [17,18,19]. Therefore, the present study was designed to delineate the prophylactic potential of synbiotic in malnourished murine giardiasis.
Materials and Methods
Chemicals
De Man Rogosa Sharpe (MRS) broth, prebiotic inulin were purchased from Hi-Media Laboratory Pvt. Ltd. (Mumbai, India), cytokine (TNF-α, IL-6, IL-10) ELISA kits were procured from Krishgen Bio-Systems, India, and Antimouse IgA and IgG antibodies were procured from Gentex, India.
Animals
LACA mice (20–25 g) were procured from the Central Animal House, Panjab University, Chandigarh, India. The animals were housed under the standard condition of light and dark cycle and were provided standard pellet diet (Hindustan Lever Products Limited, Kolkata, India). However, malnourished animals were fed 1% protein diet (Ashirwad Private Limited, Kharar, Punjab, India) and water ad libitum. Before supplementation to animals, water and feed were monitored for any bacterial or parasitic contamination by Gram’s and Lugol’s iodine staining technique as per Tiwari et al. 2009 [20]. Only parasite-free animals were employed to carry out experiment. Care and use of animals was in accordance with the guidelines of institutional ethical committee (PU/IAEC/14/128).
Induction of Malnutrition
Mice were fed with 1% protein diet for 15–20 days, after that, those mice which had reduced about 35–55% of their original body mass were marked as malnourished [8].
Parasite
Giardia intestinalis (Portland Strain I) was grown in TYI-S-33 medium supplemented with antibiotic solution (streptomycin and penicillin), pH 7, then sterilized with a 0.22-μm Seitz filter. The trophozoites were grown at 37 °C for 48–72 h, harvested by chilling in ice for 15 min, followed by centrifugation at 200g for 15 min, resuspended in phosphate buffered saline (0.15M PBS pH 7.2) at a concentration of 5 × 106 trophozoites /0.1 mL [21].
Probiotic
Probiotic Lactobacillus casei MTCC 1423 was procured from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India and was grown in MRS broth at 37 °C for 18 h, centrifuged at 1200g for 10 min at 4 °C, and washed, and cells were resuspended in PBS to a final concentration of 109 CFU/0.1 mL [22].
Prebiotic
Prebiotic inulin was dissolved in distilled water (20 mg/mL) and 100 μL was given orally to mice as a single dose via canula [23].
Synbiotic
Probiotic in combination with inulin formed the synbiotic (109 L. casei CFU/0.1 mL + 2 mg/0.1 mL inulin) [23].
Groups of Animals
Forty-two animals were divided into seven groups comprising of six animals in each group [9], group I, control; group II, malnourished; group III, Giardia-infected; group IV, malnourished-Giardia-infected; group V, malnourished-synbiotic; group VI, malnourished-synbiotic-Giardia: These mice were fed synbiotic orally with canula daily for 1 week prior to Giardia infection; group VII, malnourished-Giardia-synbiotic: These mice were fed synbiotic orally via canula simultaneously with Giardia infection. However, feeding of synbiotic was carried out daily till end of the experiment in animals belonging to groups V, VI, and VII (Fig. 1).
Follow Up of the Animals
After respective treatment of the animals belonging to various groups, the body mass, cyst, and lactobacilli count were analyzed. On day 9 post infection (PI), mice were sacrificed by retro-orbital plexus bleeding route for the analysis of small intestine mass, trophozoite count, nitric oxide (NO) levels, antigiardial IgA and IgG antibody, cytokine levels (TNF-α, IL-10, IL-6), and vis-a-vis histopathological and surface alterations of the small intestine.
Determination of Body Mass
The body mass of mice was recorded on every alternate day using an ordinary balance scale (SD- 300, SD Fine Chemicals Ltd., Chandigarh India) [8].
Enumeration of Giardia Cysts
After respective treatments, cyst count was performed on every alternate day, i.e., 1, 3, 5, 7, and 9 post infection (PI). Briefly 100 mg of the freshly passed fecal sample was mixed thoroughly with 1 mL formal saline (10%) using pestle and mortar, stained with Lugol’s iodine, and was counted in a hemocytometer [21].
Lactobacilli Enumeration
To confirm whether the lactobacilli were able to survive the harsh conditions of gastrointestinal tract (GIT) vis-à-vis affect giardiasis, the lactobacilli count was performed on every 3rd day, i.e., 0, 3, 6, and 9 PI, respectively. Freshly voided fecal material was homogenized, serially diluted, and plated on MRS agar followed by incubation at 37 °C for 48 h [21].
Determination of Small Intestine Mass, Collection of Serum, and Intestinal Fluid and Giardia Trophozoites
Mice were sacrificed on day 9 PI via retro-orbital plexus bleeding route and the whole small intestine was removed and weighed on digital scale (Sartorius AG-BT-224S). The intestinal fluid was obtained by flushing the small intestine with 1 mL of chilled normal saline (0.85% NaCl) in a sterile tube. Both serum and intestinal fluid were stored at − 70 °C until further analysis. The trophozoites were enumerated from the proximal portion of the small intestine which was removed and placed in 5 mL of ice-chilled isotonic saline solution and minced. After 15–20 min, the trophozoites were counted using a hemocytometer [21].
Estimation of Nitric Oxide
The amount of nitric oxide was measured both in serum and intestinal fluid of all mice according to Griess reaction [24]. Briefly, 100 μL of intestinal fluid or serum was mixed with 400 μL of distilled water and 500 μL of Griess reagent was added. The mixture was then incubated at 37 °C for 10 min and optical density was measured at 548 nm. Nitric oxide was quantitated using NaNO2 as a standard and results were expressed in μmoles of NaNO2.
Monitoring of Giardia-Specific Antibodies
Enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of antigiardial antibodies in both serum and intestinal fluid as per Goyal and Shukla 2013 [25]. Briefly, microtiter plate (96 wells) was coated with Giardia antigen (1 μg/mL) with overnight incubation at 4 °C and washed with PBS containing 0.05% Tween-20. Both serum and intestinal fluid from each mouse were diluted serially in ELISA buffer containing 0.5% BSA (bovine serum albumin); 100 μL of diluted samples (serum and intestinal fluid) was added to each well coated with antigen for 2 h at 37 °C. The plate was washed and 100 μL of ELISA buffer containing suitably diluted concentration of horseradish peroxidase conjugated rabbit antimouse IgA and IgG was added to each well and incubated at 37 °C for 1 h. Finally, after washing, 100 μL of substrate (0.05% OPD in 0.1 M citrate buffer and 0.01% H2O2) was added and the reaction was stopped with 25 μL of 4N H2SO4 after 30 min. Optical density was read at 490 nm and results were expressed as the highest dilution of optical density showing the maximum antigiardial antibody levels.
Cytokine Estimation
The levels of anti-inflammatory cytokines (IL-6, IL-10) and pro-inflammatory cytokine (TNF-α) were quantified in both serum and intestinal fluid using ELISA-based kits as per manufacturer’s instructions. Briefly, 100 μL of each standard and sample was added to wells of ELISA plate, incubated overnight at 4 °C, and washed thoroughly, and biotinylated antibody (100 μL) was added to each well, incubated at 37 °C for 1 h, and washed thoroughly followed by the addition of prepared streptavidin solution (100 μL). The plate was incubated at 37 °C for 45 min, then, tetramethylbenzidine (100 μL) was added and incubated in the dark at 37 °C for 30 min. Finally, 50 μL of stop solution (0.2 M sulfuric acid) was added and optical density was read at 450 nm. Results were expressed as the concentration of cytokines per mL of fluids (pg/mL) using the standard cytokines [25].
Histopathological Analysis
The proximal jejunum was removed after sacrificing the mice and fixed in 10% buffered formalin for 24 h, dehydrated in ascending concentration of ethyl alcohol, rinsed in xylene, and mounted in molten DPX at 58–62 °C. Slices of 4–5 μm thickness were stained with hematoxylin and eosin (H&E) and were examined under light microscopy [25].
Scanning Electron Microscopy
The upper part of small intestine was removed, washed with PBS, stapled on cardboard, and fixed in 4% glutaraldehyde for 1 h. After that, samples were dehydrated in different grades of alcohol (50, 70, 80, 90, 100, and 100%) for 10, 15, 15, 20, and 30 min, respectively. The samples were desiccated, mounted on aluminum stubs, coated with gold palladium at a thickness of 20.0 Å, and examined by scanning electron microscopy (SEM) (JEOL, JEM 160 model) [21].
Statistical Analysis
Results were expressed as mean and ± standard deviation (SD). The inter group variation was assessed by one-way analysis of variance (ANOVA) followed by post hoc test comparison Bonferroni and statistical significance at p < 0.05 was calculated.
Results
Body and Small Intestine Mass
Body mass of mice belonging to all malnourished groups (II, IV, V, VI, VII) was significantly (p < 0.05) lower from beginning of the experiment and remained low at each point of observation till end compared with Giardia-infected (group III)/control (group I) mice (Fig. 2). It was interesting to observe though synbiotic supplementation to malnourished mice either prior or simultaneously with Giardia-infection led to significant (p < 0.05) increase in body mass of malnourished-synbiotic-Giardia (group VI) and malnourished-Giardia-synbiotic (group VII) compared with malnourished-Giardia-infected (group IV) mice/ malnourished (group II) mice yet was less than Giardia- infected (group III)/ control (group I) mice (Fig. 2). Further, we observed that mass of the small intestine increased significantly (p < 0.05) in malnourished-synbiotic-Giardia (group VI) compared with counter controls (group II, IV, VII) but Giardia-infected/control mice had higher small intestinal mass (Fig. 3).
Cyst, Trophozoite, and Lactobacilli Count
Oral inoculation of Giardia trophozoite led to establishment of Giardia infection as was evident by the presence of both cyst and trophozoite in mice belonging to malnourished-Giardia-infected (group IV), malnourished-synbiotic-Giardia (group VI), and Giardia-infected (group III; Figs. 4 and 5). Interestingly, supplementation of synbiotic either prior or simultaneously even to malnourished Giardia-infected mice (groups VI, VII) significantly (p < 0.05) reduced the cyst and trophozoite count compared with counter control groups (III, IV) of mice (Figs. 4, 5, and 6). More specifically, the lactobacilli counts in feces of mice belonging to malnourished-synbiotic (group V), malnourished-synbiotic-Giardia (group VI), and malnourished-Giardia-synbiotic (Group VII) were significantly (p < 0.05) higher till end of experiment compared with counter control groups (II, III, IV) of mice (Fig. 6).
Innate, Humoral, and Cell-Mediated Immune Response
It was interesting to find that administration of synbiotic even to malnourished mice led to significantly (p < 0.05) increased levels of nitric oxide, antigiardial IgA and IgG antibodies and anti-inflammatory cytokines IL-6 and 10, and decreased levels of pro-inflammatory cytokine TNF-α in malnourished-synbiotic-Giardia (group VI) or malnourished-Giardia-synbiotic (group VII) but was significantly (p < 0.05) high compared with counter control groups (II, III) of mice (Tables 1 and 2).
Histopathological Observations
Histologically, the small intestine of malnourished (group II) mice had mummified, disrupted damaged crypts ad villi, and depleted mucosal coat compared with healthy well-structured crypts and mucosal layer in control (group I) mice (Fig. 7a and b). However, the small intestine of Giardia-infected (group III) mice had hypertrophy of the villi, absence of goblet cells, and increased lymphonuclear infiltration in the lamina propria while malnourished-Giardia-infected (group IV) mice showed atrophy, extensive blunting of villi, crypts, mild ileitis, and less number goblet cells (Fig. 7c and d). Interestingly, it was observed that the small intestine of mice belonging to malnourished-synbiotic-Giardia (group VI) or malnourished-Giardia-synbiotic (group VII) had disorganized, disoriented, disrupted, and inflamed microvilli/ crypts but somewhat better mucosal layer that was comparable with the mucosal coat of malnourished-synbiotic (group V) mice having swollen villi and lymphocytic infiltration (Fig. 7e–g).
Photomicrograph of the small intestine of mouse on day 9 post inoculation showing: a normal morphology of crypts, villi, and healthy mucosal coat in control; b disrupted crypts and villi along with the unhealthy and damaged microvilli and mucosal coat in malnourished; c hypertrophy of the villi and severely damaged, dissolved villi tips along with heavy lymphocytic infiltration, and absence of goblet cells in Giardia-infected; d altered disorganized microvilli, crypts, mild ileitis, and less number of goblet cells in malnourished-Giardia-infected; e lymphocyte infiltration in the lamina propria and swelling of villi with normal mucosal coat in malnourished-synbiotic; f accumulation of cellular exudates with lymphocyte infiltration and disoriented villi in malnourished-synbiotic-Giardia-infected; g normal mucosal coat with dissolving villi and disrupted epithelium in malnourished-Giardia-synbiotic (H&E stain 100×; 40 μm)
Scanning Electron Microscopy
Scanning electron microscopy showed that the surface of the small intestine of malnourished mice (group II) was mummified, blunted, and disoriented microvilli along with its reduced length compared with normal well-organized gut architecture of control (group I) mice (Fig. 8a and b). However, Giardia-infected (group III) mice had damaged and disrupted microvilli with deposition of cellular exudates compared with severely damaged and disrupted microvilli along with deposition of cellular exudates in malnourished-Giardia-infected (group IV) mice (Fig. 8c and d). It was observed that supplementation of synbiotic even to malnourished mice improved the architecture of the small intestine but had blunted microvilli (Fig. 8e). Interestingly, it was found that supplementation of synbiotic to malnourished mice (group V) either prior or simultaneously to Giardia infection (groups VI, VII) showed improved villi morphology but malnourished-synbiotic-Giardia-infected mice (group VI) had better arrangement and microvilli morphology than malnourished-Giardia-synbiotic (group VII) mice (Fig. 8f and g).
Scanning electron micrograph of the small intestine of mouse on day 9 post inoculation showing: a normal well-organized, properly distributed microvilli in control (100 μm); b mummified, disrupted, blunted microvilli with reduced length of microvilli in malnourished (100 μm); c damaged and disrupted microvilli with deposition of cellular exudates and ileitis in Giardia-infected (100 μm); d damaged and disrupted microvilli along with deposition of cellular exudates in malnourished-Giardia-infected (100 μm); e blunted microvilli with improved morphology (300 μm); f improved morphology and orientation of microvilli with the deposition of cellular exudates in malnourished-synbiotic-Giardia-infected (300 μm); g damaged, disoriented microvilli morphology in malnourished-Giardia-synbiotic (300 μm)
Discussion
The emerging resistance to antiprotozoal therapy against giardiasis and the increasing global inclination towards the usage of natural products has diverted the interest of scientific community towards the use of functional foods as an alternate or microbial interference therapy for various gastrointestinal diseases [26, 27]. Probiotics, prebiotics, and synbiotics are the various potential candidates for functional foods and the efficacy of synbiotic against diarrheal diseases has been very well supported by good number of studies [28, 29]. These studies have reported that synbiotic mixture (Lactobacillus species and Fructooligosaccharides) ameliorated acute diarrhea by reducing frequency, duration, and consistency of stool in hospitalized children. Recently in 2016, a randomized clinical trial conducted on 200 children suffering from dysentery also reported that synbiotic, as an adjuvant therapy to the standard treatment of dysentery, led to considerable reduction in the extent of dysentery and weight loss [30]. Since information pertaining to the use of synbiotic in giardiasis is not available and formed the basis of the present study to monitor the efficacy of synbiotic in malnourished Giardia-infected mice. Furthermore, in our earlier studies, we have observed that giardiasis is more severe and prolonged in malnourished mice due to severely altered anthropometry, biochemical, and histological parameters [7] but prior or simultaneous supplementation of either probiotic or prebiotic alone to malnourished-Giardia-infected mice led to increased body mass, reduced giardiasis both in terms of cyst and trophozoite counts due to increased lactobacilli count, improved gut morphology, and immune status [8, 9, 31].
It is very well documented that probiotic modulates both the gut microbiome and immunoresponse but their effect is species and strain specific [25, 32]. Additionally, prebiotics, the food for probiotic, have also been implicated both experimentally and clinically in various intervention trials due to their ability to reduce the production of potentially toxic metabolites mainly by suppressing the specific enzyme activities (β-glucuronidase, β-glucosidase) in colon and may induce immunomodulation by increasing the production of IL-10 and interferon-gamma from Peyer’s patches [23, 33,34,35,36]. Moreover, several studies have also reported that the supplementation of prebiotics in diet improved gut microbiome especially bifidobacteria and lactobacilli and have been beneficial in managing diarrhea [34, 36, 37].
More specifically, in the present study, we have observed that prior or simultaneous administration of synbiotic to malnourished mice ameliorated gut morphology and immune response by modulating both gut microbiome and immune response that reduced the severity of Giardia infection in malnourished-Giardia- infected mice and corroborates with earlier studies [7,8,9, 38, 39].
Based on the present and our earlier observations, it can be postulated that synbiotic provides better environment for the survival of good gut microbiome due to access of prebiotic inulin, that could be fermented, and thereby augmenting the beneficial bacteria. Moreover, due to presence of prebiotic, the number of lactic acid bacteria increased that competed with Giardia, for the attachment with enterocytes and nutrients, resulting into the elimination of Giardia trophozoites. In addition to competitive inhibition, the attachment of probiotics to enterocytes may have increased the synthesis of NO, enhanced mucosal immunity in terms of increased production of secretary IgA and systemic IgG, and cytokines that may have inhibited the growth and replication of Giardia trophozoites leading to reduced gut inflammation. Moreover, in our earlier study, we have observed that prebiotic administration to mice led to increased lactobacilli counts and the production of short-chain fatty acids that may have further aided into the clearance of Giardia trophozoites along with restoration of gut architecture.
In a nut shell, it can be stated that prophylactic administration of synbiotic has modulated malnourished murine giardiasis. However, randomized clinical trials are recommended to validate its use in human giardiasis due to difference in human and murine gut microbiome.
References
Auerbach PS (2012) Wilderness medicine, 6th edn. Elsevier, Philadelphia, p 2277
Munoz GJ, Aldasoro E, Requena A, Comin AM, Pinazo MJ (2013) Refractory giardiasis in Spanish travelers. Travel Med Infect Dis 11(2):126–129. https://doi.org/10.1016/j.tmaid.2012.10.004
Saghaug CS, Sørnes S, Peirasmaki D, Svärd S, Langeland N, Hanevik K (2016) Human memory CD4+ T cell immune responses against Giardia lamblia. Clin Vaccine Immunol 23(1):11–18. https://doi.org/10.1128/CVI.00419-15
Lindquist KR, Palm D, Svard SG (2006) Giardia immunity—an update. Trend Parasitol 22(1):26–31. https://doi.org/10.1016/j.pt.2005.11.005
Ratanapo S, Mungthin M, Soontrapa S, Faithed C, Siripattanapipong S, Rangsin R, Naaglor T, Piyaraj P, Taamasri P, Leelayoova S (2008) Multiple modes of transmission of giardiasis in primary schoolchildren of a rural community, Thailand. Am J Trop Med Hyg 78(4):611–615
Leitch GJ, Udezulu IA, He Q, Visvesvara GS (1993) Effect of protein malnutrition on experimental giardiasis in Mongolian gerbil. Scand J Gastroenterol 28(10):885–893. https://doi.org/10.3109/00365529309103130
Shukla G, Sidhu RK (2011a) Effect of Giardia duodenalis in protein malnourished and renourished mice. Cent Eur J Med 6:762–769
Shukla G, Sidhu RK (2011b) Lactobacillus casei as a probiotic in malnourished Giardia lamblia-infected mice: a biochemical and histopathological study. Can J Microbiol 57(2):127–135. https://doi.org/10.1139/W10-110
Shukla G, Bhatia R, Sharma A (2016) Prebiotic inulin supplementation modulates the immune response and restores gut morphology in Giardia duodenalis infected malnourished mice. Parasitol Res 115(11):4189–4198. https://doi.org/10.1007/s00436-016-5196-x
Neiva VA, Maria NS, Ribeiro FRF, Maria SS et al (2014) Plant species used in giardiasis treatment: ethnopharmacology and in vitro evaluation of anti-Giardia activity. Rev Bras Farmacogn 24(2):215–224. https://doi.org/10.1016/j.bjp.2014.04.004
Rodríguez CJL, Rufino GY, Ponce MM, Delgado G (2015) In vitro activity of 'Mexican Arnica' Heterotheca inuloides Cass natural products and some derivatives against Giardia intestinalis. Parasitology 142(04):576–584. https://doi.org/10.1017/S0031182014001619
Hasler CM (2002) Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. J Nutr 132:3772–3781
International Life Sciences Institute Europe (1999) FUFOSE: scientific concepts of functional foods in Europe. Br J Nutr 81:1S–27S
Varela SL, Gross MG, Marcos A (2002) Functional foods and the immune system: a review. Eur J Clin Nutr 3:S29–S33
FAO/WHO. Expert Consultation Group (2007) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. WHO, Geneva
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Cláudia MR, Veruska MC, Maria IF (2011) Effects of synbiotic-based Bifidobacterium animalis in female rats experimentally infected with Toxoplasma gondii. Comp Immunol Microbiol Infect Dis 34:111–114
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5(4):1417–1435. https://doi.org/10.3390/nu5041417
Verma A, Shukla G (2014) Synbiotic (Lactobacillus rhamnosus + Lactobacillus acidophilus + inulin) attenuates oxidative stress and colonic damage in 1, 2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague–Dawley rats: a long-term study. Eur J Cancer Prev 23(6):550–559. https://doi.org/10.1097/CEJ.0000000000000054
Tiwari RP, Hoondal GS, Tewari R (2009) Laboratory techniques in microbiology and biotechnology. Abhishek Publications, Chandigarh
Shukla G, Devi P, Sehgal R (2008) Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci 53(10):2671–2679. https://doi.org/10.1007/s10620-007-0197-3
Shukla G, Sharma G, Goyal N (2010) Probiotic characterization of Lactobacilli and yeast strains isolated from whey beverage and therapeutic potential of Lactobacillus yoghurt in murine giardiasis. Am J Biomed Sci 3:248–261
Verma A, Shukla G (2013) Administration of prebiotic inulin suppresses 1, 2 dimethylhydrazine dihydrochloride induced procarcinogenic biomarkers fecal enzymes and preneoplastic lesions in early colon carcinogenesis in Sprague Dawley rats. J Funct Foods 5(2):991–996. https://doi.org/10.1016/j.jff.2013.02.006
Green LC, Wagner DA, Glogowski J, Shipper PL, Wishnok J, Rannerbaum SR (1982) Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Anal Biochem 126:121–158
Goyal N, Shukla G (2013) Probiotic Lactobacillus rhamnosus GG modulates the mucosal immune response in Giardia intestinalis-infected BALB/c mice. Dig Dis Sci 58(5):1218–1225. https://doi.org/10.1007/s10620-012-2503-y
Gardner TB, Hill DR (2001) Treatment of giardiasis. Clin Microbiol Rev 14(1):114–128. https://doi.org/10.1128/CMR.14.1.114-128.2001
Yuan H, Ma Q, Ye L, Piao G (2016) The traditional medicine and modern medicine from natural products. Molecules 21(5). https://doi.org/10.3390/molecules21050559
Dinleyici EC, Dalgic N, Guven S, Ozen M, Kara A, Arica V, Metin-Timur O, Sancar M, Kurugol Z, Tanir G, Ozturk D, Aydogdu S, Tutanc M, Eren M, Vandenplas Y (2013) The effect of a multispecies synbiotic mixture on the duration of diarrhea and length of hospital stay in children with acute diarrhea in Turkey: single blinded randomized study. Eur J Pediatr 172(4):459–464. https://doi.org/10.1007/s00431-012-1903-5
Ahmed RR, Ghafoor MA, Kazi Y, Bai C, Khan N (2014) Role of synbiotic (combination of pre and probiotic) in the management and prevention of acute watery diarrhoea. World Appl Sci J 32:226–230
Kahbazi M, Ebrahimi M, Zarinfar N, Arjomandzadegan M, Fereydouni T, Karimi F, Najmi AR (2016) Efficacy of synbiotics for treatment of bacillary dysentery in children: a double-blind, randomized, placebo-controlled study. Adv Med 2016:1–6. https://doi.org/10.1155/2016/3194010
Shukla G, Sidhu RK, Verma A (2012) Restoration of anthropometric, biochemical and histopathological alterations by Lactobacillus casei supplementation in Giardia intestinalis infected renourished BALB/c mice. Antonie Van Leeuwenhoek 102:161–172
Marchesi JR, Adams DH, Fava F (2015) The gut microbiota and host health: a new clinical frontier. Gut 65(2):330–339. https://doi.org/10.1136/gutjnl-2015-309990
Roller M, Rechkemmer G, Watzl B (2004) Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr 134(1):153–156
Meyer D, Stasse-Wolthuis M (2009) The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 63(11):1277–1289. https://doi.org/10.1038/ejcn.2009.64
Rao VA (2001) The prebiotic properties of oligofructose at low intake levels. Nutr Res 21(6):843–848. https://doi.org/10.1016/S0271-5317(01)00284-6
Tuohy KM, Finlay RK, Wynne AG, Gibson GR (2001) A human volunteer study on the prebiotic effects of HP-inulin—faecal bacteria enumerated using fluorescent in situ hybridization. Anaerobe 7(3):113–118. https://doi.org/10.1006/anae.2001.0368
Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K (2005) Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr 40(1):36–42. https://doi.org/10.1097/00005176-200501000-00007
Jayawardena P (2014) Underlying causes of child and maternal malnutrition in the estate sector of Sri Lanka. J S Asian Stud 02:241–255
Hesham MS, Mekhali A, Azlu M, Ner U (2005) Giardiasis as a preindicator of child malnutrition in Orang Asli children in Malaysia. Trans R Soc Trop Med Hyg 99:686–691
Acknowledgements
Authors greatly acknowledge the help provided by Mr. Mohan Singh Bhandari, Senior Technician, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, for maintaining the Giardia culture.
Funding
Financial assistance provided by the Department of Science and Technology-Purse, New Delhi, India is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Shukla, G., Sharma, A., Bhatia, R. et al. Prophylactic Potential of Synbiotic (Lactobacillus casei and Inulin) in Malnourished Murine Giardiasis: an Immunological and Ultrastructural Study. Probiotics & Antimicro. Prot. 11, 165–174 (2019). https://doi.org/10.1007/s12602-017-9368-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9368-5


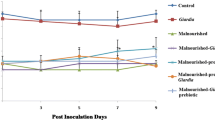




 Control,
Control,  malnourished,
malnourished,  Giardia,
Giardia,  malnourished-Giardia,
malnourished-Giardia,  malnourished-synbiotic,
malnourished-synbiotic,  malnourished-synbiotic-Giardia,
malnourished-synbiotic-Giardia,  malnourished-Giardia-synbiotic]
malnourished-Giardia-synbiotic]


 Giardia,
Giardia,  malnourished-Giardia,
malnourished-Giardia,  malnourished-synbiotic-Giardia,
malnourished-synbiotic-Giardia,  malnourished-Giardia-synbiotic]
malnourished-Giardia-synbiotic]
 Control,
Control,  malnourished,
malnourished,  Giardia,
Giardia,  malnourished-Giardia,
malnourished-Giardia,  malnourished-synbiotic,
malnourished-synbiotic,  malnourished-synbiotic-Giardia,
malnourished-synbiotic-Giardia,  malnourished-Giardia-synbiotic]
malnourished-Giardia-synbiotic]
