Abstract
Probiotics have been successfully used for the treatment of acute diarrhea in children and this effect depends on the strains and dose. The aim of this study was to assess the effect of a synbiotic mixture on the duration of diarrhea and the length of hospital stay in children with acute watery diarrhea. This is a prospective randomized, multicenter single blinded clinical trial in hospitalized children with acute watery diarrhea. All children were treated with conventional hydration therapy with or without a daily dose of a synbiotic (2.5 × 109 CFU live bacteria including Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Enterococcus faecium, and 625 mg fructooligosaccharide) for 5 days. The primary endpoint was duration of diarrhea and duration of hospitalization was the secondary endpoint. Among 209 eligible children, 113 received the synbiotic mixture and 96 served as a control. The duration of diarrhea was significantly shorter (∼36 h) in children receiving the synbiotic group than the controls (77.9 ± 30.5 vs. 114.6 ± 37.4 h, p < 0.0001). The duration of hospitalization was shorter in children receiving the synbiotic group (4.94 ± 1.7 vs. 5.77 ± 1.97 days, p = 0.002). The effect of synbiotic mixture on diarrhea started after 24th hours and stool frequency significantly decreased after 24th and 48th hours. The percentage of diarrhea-free children is significantly higher in synbiotic group at 48th and 72nd hours of synbiotic group. In conclusion, this study showed a reduction in diarrhea duration by approximately 36 h and a reduction in the duration of hospitalization with approximately 1 day in children with acute diarrhea with this synbiotic mixture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diarrhea is defined as a change in bowel movements with an increase in water content, volume, and usually frequency of stools and is mainly due to an infectious cause. Despite improvements in public health and economic wealth, acute diarrhea continues to be a leading cause of morbidity, hospitalization, and mortality worldwide [8, 14]. Among children living in developed countries, diarrheal illnesses are usually caused by viruses and may result in hospitalization and increased health care costs [5, 14]. Measures to prevent diarrhea include breastfeeding, hand washing, careful personal/general hygiene, clean food/water, and vaccination for enteric diseases such as rotavirus [11].
The main method of therapy for all individuals with dehydration caused by diarrhea is oral or parental rehydration, which consists of fluid and electrolyte replacement [5, 8, 11, 14]. But the latter does not substantially shorten the frequency/duration of diarrhea and has not been found to reduce stool volume, prompting a growing interest in adjunctive treatments [17]. Probiotics have been proposed as a complementary therapy in the treatment of acute diarrhea [6, 7, 10, 12, 17, 18]. A large number of randomized controlled trials showed that some probiotic strains exert antidiarrheal effects, particularly in children [10]. The most widely evaluated outcomes are duration and severity of diarrhea and duration of hospitalization [10]. Current evidence also indicates that probiotic effects are strain-specific. Lactobacillus GG, Saccharomyces boulardii, and Lactobacillus reuteri are the best studied strains [6, 7, 10, 12].
In May 2008, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society of Pediatric Infectious Diseases Expert Working Group stated that only probiotic strains with proven clinical efficacy and in appropriate dosage can be recommended as an adjunct for the management in children with acute gastroenteritis to rehydration therapy [11]. Probiotics have been successfully used for the treatment of acute gastroenteritis in children and adults. However, more research is needed to guide the use of particular probiotic regimens and strains and as there is still no evidence of efficacy for many preparations. The objective of this study was to assess the efficacy and safety of a synbiotic mixture including Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Enterococcus faecium, and fructooligosaccharides as an adjunct to rehydration therapy in the treatment of children hospitalized because of acute watery diarrhea.
Methods
This was a multicenter, randomized, single blind, parallel group, controlled, hospital-based clinical trial in children of both sexes, aged between 3 and 120 months. Acute diarrhea was defined as the presence of three or more liquid or loose stools, as defined by Bristol criteria ≥type 6 per day [16]. Inclusion criteria were: an episode of mild to moderate acute diarrhea (>4 (semi)watery stools/day according to Bristol criteria (Bristol criteria ≥6)) of likely infectious origin in infants and children since at least lasting more than 12 h and less than 72 h, requiring hospitalization [16]. We enrolled children with clinical signs of mild to moderate dehydration (prolonged capillary refill time, abnormal skin turgor, and percentage loss of body weight). Dehydration was evaluated on clinical grounds and estimated weight loss [16]. Patients had to be mild and moderately dehydrated. Subjects with clinical features of hypovolemic shock and/or necessitating admission at the intensive care unit were excluded. Other exclusion criteria were the use of antibiotics or probiotics 1 month before admission, severe malnutrition, vaccination with rotavirus vaccine, and severity of chronic underlying disease including immunocompromised conditions.
The principal investigator (ECD) site did not enroll children and was blind to the treatment of the patients and their outcomes. Children received conventional therapy (oral or intravenous rehydration) with or without a daily dose of the synbiotic (NBL Probiotic Gold). The primary endpoint was the duration of diarrhea (in hours). Secondary outcome measures were duration of hospitalization (days), diarrhea at the 3rd day of intervention, mean frequency of the daily stool, and diarrhea at the end of therapy (5th day). Adverse events were also recorded.
Rehydration and electrolyte replacement were done using oral rehydration salts (ORS) (glucose 20 g; sodium 90 mmol/L; potassium 20 mmol/L; bicarbonate 30 mmol/L). NBL Probiotic Gold® (Nobel, Turkey) includes 2.5 × 109 CFU live bacteria per single sachet including L. acidophilus, L. rhamnosus, B. bifidum, B. longum, E. faecium, 625 mg fructooligosaccharide, and vitamins A, B1, B2, B6, E, and C. On admission, children were examined clinically, and the weight, fever, and degree of dehydration were recorded. All children were randomly assigned according to a computer-generated randomization list. One group received ORS and/or intravenous therapy (control group), and the second group received daily synbiotic sachet (synbiotic group) in addition to ORS and/or intravenous therapy for 5 days.
The frequency and consistency of the stools were recorded. The duration of diarrhea was defined as the time in hours from admission until cessation of diarrhea which was defined as the first normal stool according to Bristol score (a score <5 is described as normalization of stool). Other recorded parameters were the incidence on ongoing diarrhea after 3 days of treatment (proportion of patients in each study group) and length of hospitalization (time in days from admission until discharge from hospital).
Statistical analysis
The sample size needed was calculated based on the mean duration of diarrhea and standard deviation (SD) from previous similar studies. With the assumption of mean difference on duration of diarrhea for 1 day (24 h) between the treatment and control group, we calculated that a sample of 64 children for each group would be required for the study to have 80 % power with a significance level = 0.05 and sigma = 2 (two-tailed test). To investigate the subgroup analysis, we doubled the sample size. Statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Variables were tested for normal distribution and compared using the independent t test, Mann–Whitney U test, and χ 2 or Fisher's exact tests, as appropriate. Statistical significance was set at p < 0.05. The local ethical committee approved the study and written informed consent was obtained from the parents of the children.
Results
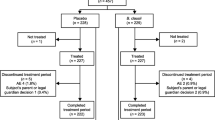
We enrolled 256 patients: 15 children from the synbiotic group and 32 children from the control group were excluded because of antibiotic prescription (post-randomization), parental refusal to continue the study, detection of underlying disease during hospitalization, and lack of data after hospital discharge (lack of parental compliance). In total, data from 209 children could be evaluated: 113 in the synbiotic and 96 in the control group (Fig. 1). All recorded are normally distributed except the age of enrolled children. Gender and age distribution were similar between the synbiotic and control group (p = 0.051 and p = 0.711). Mean duration of diarrhea before the intervention was similar between the synbiotic and control groups (p > 0.05; Table 1). The clinical characteristics and severity of gastroenteritis did not differ between treatment and control group. Mean number of stool frequency during the 24 h prior to admission was 6.94 ± 3.6 day−1 in the synbiotic group and was 6.78 ± 1.8 day−1 in control group (p = 0.699). The duration of diarrhea was significantly reduced in the synbiotic group when compared to the control group (mean ± SD) (77.9 ± 30.5 vs. 114.6 ± 37.4 h, p < 0.0001) (Table 1). Mean length of hospital stay was shorter in the synbiotic group than the control group (4.94 ± 1.7 vs. 5.77 ± 1.97 days, p = 0.002) (Table 1 and Fig. 2).
The effect (diarrhea-free percentage of children) of the synbiotic mixture was best observed at 48 and 72 h of the synbiotic intervention (Table 2). At 72nd hours of the study, 45.2 % of the children receiving synbiotic still had watery diarrhea while this was the case in 85.5 % of children in the control group (RR 0.53; 95 % CI, 0.42–0.66; p < 0.0001).
The mean frequency of daily stools during the 5 days of intervention is summarized in Table 3. Mean frequency of daily stools per day was significantly lower at 24 and 48 h with the synbiotic intervention compared to the control group (p < 0.001 for both). At 72, 96, and 120 h, the mean stool frequency per day was similar between both groups. No adverse effects (rash, drug-related fever or nausea, etc.) related to the synbiotic use were noted.
Discussion
Reduction of the duration of diarrhea and shortening of the hospital stay are important aims in the treatment of acute infectious diarrhea from medical, social, and economical perspectives. ORS is the mainstay treatment of acute diarrhea for dehydration [11]. However, fluid and electrolyte replacement does not substantially shorten the frequency/duration of diarrhea [17]. In this study, the control group received only rehydration while the intervention received rehydration and synbiotic intervention once daily. The synbiotic mixture includes L. acidophilus, L. rhamnosus, B. bifidum, B. longum, E. faecium, and fructooligosaccharides (FOS). The synbiotic was shown to be effective and safe as an adjunct to ORS in the treatment of acute infectious diarrhea in children, reducing both the duration of diarrhea as well as the length of hospitalization.
Current evidence indicates that some probiotic strains reduce the duration and severity of diarrhea and duration of hospitalization. However, these effects are strain-specific and product-specific. Regarding the treatment of acute diarrhea in children, few agents, including Lactobacillus GG, S. boulardii, and L. reuteri, have been shown to be effective in the treatment of acute diarrhea [7, 10, 12]. Recently, probiotic mixtures with different amounts and strains are commercialized. Different probiotic mixtures or synbiotics have been evaluated and showed contradictory results. Up to now, few studies evaluated the efficacy of synbiotics, a mixture of probiotics and prebiotics in the treatment of infectious diarrhea. Berni-Canani et al. [2] compared different probiotic products in a randomized controlled trial in children with acute diarrhea and showed that the median duration of diarrhea was shorter (∼37 h, similar with our study) with a probiotic mixture of Lactobacillus delbrueckii var bulgaricus, Streptoccoccus thermophilus, L. acidophilus, and B. bifidum. Chen et al. [4] showed that with a mixture of Bacillus mesentericus, Enterococcus faecalis, and Clostridium butyricum, the mean duration of diarrhea was about 26 h shorter than placebo and also hospital stay was shorter. Grandy et al. [9] also showed 24 h of reduction in duration of diarrhea in children receiving probiotic mixture including L. acidophilus, L. rhamnosus, B. longum, and S. boulardii. Vandenplas and colleagues [16] performed randomized, prospective placebo-controlled parallel clinical trial in children with acute diarrhea using the synbiotic food supplement (Probiotical®: S. thermophilus, L. rhamnosus, L. acidophilus, Bifidobacterium lactis, Bifidobacterium infantis, and fructooligosaccharides). They also found that the median duration of diarrhea was 1 day shorter in the synbiotic group, resulting in a decreased prescription of additional medication. In this study, we evaluated the effect of a synbiotic mixture (NBL Probiotic Gold®) including L. acidophilus, L. rhamnosus, B. bifidum, B. longum, and E. faecium. We used single sachet per day including 2.5 × 109 CFU live bacteria. This is the first study with this synbiotic mixture (a sachet contains also FOS). Our study has some limitations. First one, our study is an allocated randomized multicenter prospective study, but not a placebo-controlled study. Also we did not perform stool cultures. Stool cultures were not performed, as is the case in many studies with probiotics [16]. According to the evidence-based guidelines of the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society of Infectious Disease, it is not recommended [11]. At the time of enrollment, a high number of children were excluded, especially in the control group, which was mainly based on parental reasons. Nevertheless, we enrolled more than the required 64 children in each group. Our results are in line with a recent Cochrane analysis including 56 trials in children [1]. The mean duration of diarrhea was shortened in synbiotic group with approximately 36 h. The effect started at 24th since the stool frequency was significantly decreased at the 24th and 48th hours after starting the study. More than 50 % of the children receiving synbiotic had normal stool consistency (diarrhea-free) at 72 h of intervention while this was the case in only 15 % of in control group.
The Cochrane review concluded that specific probiotics can reduce the duration of diarrhea with about 24 h and that stool frequency decreases on the 2nd day, as in our study [1]. Mean length of hospital stay is shorter (approximately 20 h) in synbiotic group than the control group. Studies using different probiotics showed also a 1-day reduction of hospitalization. One-day reduction in the duration of diarrhea and/or hospitalization is of potential social and economical benefits [6].
In our study period, this synbiotic formulation remained safe and no adverse effects were reported. To the best of our knowledge, there are no published reports about this synbiotic formulation on acute infectious diarrhea in children and adults. This synbiotic formulation has been developed with dual-coated technology. Another synbiotic formulation with similar dual-coated technology (same formulation) showed that dual-coated probiotics have stronger probiotic effects when compared to uncoated formulation [3]. The potential role of dual-coated technology in clinical settings, which is not evaluated in our study, should be evaluated with further research. These synbiotic products contain prebiotic oligosaccharides as well as 625 mg FOS. Nondigestible carbohydrates were shown to not reduce the duration of diarrhea, although a synbiotic may confer additional benefits over a probiotic by increasing bifidobacteria levels [13, 15, 16]. The addition of a prebiotic may induce a theoretical benefit to normalize more rapidly the gastrointestinal flora composition of the host [16].
Our study showed that this mixture reduced the duration of diarrhea (approximately 36 h) and reduced the duration of hospitalization (approximately 20 h) in children with acute diarrhea. These children were more likely to be diarrhea-free at the first 48 h of synbiotic intervention and passed significantly fewer stools. This probiotic mixture may be used in children with acute diarrhea and mild to moderate dehydration.
References
Allen SJ, Martinez EG, Gregorio GV, Dans LF (2010) Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 10:CD003048
Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, Albano F, Passariello A, De Marco G, Manguso F, Guarino A (2007) Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 335:340
Cha MJ, Chung MJ, Kim JE, Lee KO, Ha NJ (2011) Comparison of dual coated (Duolac) and uncoated lactic acid bacteria from potential probiotics. Biotechnol Biotechnol 25:2489–2493
Chen CC, Kong MS, Lai MW, Chao HC, Chang KW, Chen SY, Huang YC, Chiu CH, Li WC, Lin PY, Chen CJ, Li TY (2010) Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr Infect Dis J 29:135–138
Cheng AC, McDonald JR, Thielman NM (2005) Infectious diarrhea in developed and developing countries. J Clin Gastroenterol 39:757–773
Dinleyici EC, Eren M, Ozen M, Yargic ZA, Vandenplas Y (2012) Effectiveness and safety of Saccharomyces boulardii for acute infectious diarrhea. Expert Opin Biol Ther 12:395–410
Floch MH, Walker WA, Madsen K et al (2011) Recommendations for probiotic use—2011 update. J Clin Gastroenterol 45 Suppl:S168–S171
Gadewar S, Fasano A (2005) Current concepts in the evaluation, diagnosis and management of acute infectious diarrhea. Curr Opin Pharmacol 5:559–565
Grandy G, Medina M, Soria R, Terán CG, Araya M (2010) Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis 10:253
Guandalini S (2011) Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 45(Suppl):S149–S153
Guarino A, Albano F, Ashkenazi S et al (2008) European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Paediatr Gastroenterol Nutr 46(Suppl 2):S81–S122
Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, Krabshuis J, Lemair T, Kaufmann P, de Paula JA, Fedorak R, Shanahan F, Sanders ME, Szajewska H, Ramakrishna BS, Karakan T, Kim N, Review Team; Invited outside experts (2012) World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics. J Clin Gastroenterol 46:468–481
Hoekstra JH, Szajewska H, Zikri MA, Micetic-Turk D, Weizman Z, Papadopoulou A, Guarino A, Dias JA, Oostvogels B (2004) Oral rehydration solution containing a mixture of non-digestible carbohydrates in the treatment of acute diarrhea: a multicenter randomized placebo controlled study on behalf of the ESPGHAN working group on intestinal infections. J Pediatr Gastroenterol Nutr 39:239–245
O'Ryan M, Lucero Y, O'Ryan-Soriano MA, Ashkenazi S (2010) An update on management of severe acute infectious gastroenteritis in children. Expert Rev Anti Infect Ther 8:671–682
Passariello A, Terrin G, De Marco G, Cecere G, Ruotolo S, Marino A, Cosenza L, Tardi M, Nocerino R, Berni Canani R (2011) Efficacy of a new hypotonic oral rehydration solution containing zinc and prebiotics in the treatment of childhood acute diarrhea: a randomized controlled trial. J Pediatr 158:288–289
Vandenplas Y, De Hert SG, PROBIOTICAL-study group (2011) Randomised clinical trial: the synbiotic food supplement Probiotical vs. placebo for acute gastroenteritis in children. Aliment Pharmacol Ther 34(8):862–867
Vandenplas Y, Salvatore S, Vieira M, Devreker T, Hauser B (2007) Probiotics in infectious diarrhoea in children: are they indicated? Eur J Pediatr 166:1211–1218
Vandenplas Y, Veereman-Wauters G, De Greef E et al (2011) Probiotics and prebiotics in prevention and treatment of diseases in infants and children. J Pediatr (Rio J) 87:292–300
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinleyici, E.C., Dalgic, N., Guven, S. et al. The effect of a multispecies synbiotic mixture on the duration of diarrhea and length of hospital stay in children with acute diarrhea in Turkey: Single blinded randomized study. Eur J Pediatr 172, 459–464 (2013). https://doi.org/10.1007/s00431-012-1903-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-012-1903-5