Abstract
Although several studies have evaluated the effect of synbiotic intake on metabolic profiles in patients with diabetes, findings are inconsistent. This systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to summarize the evidence on the effect of synbiotic intake on metabolic profiles in patients with diabetes. The PubMed, EMBASE, Web of Science, and Cochrane Library databases were systematically searched. All RCTs published up to 12 November 2016 were included. Two review authors independently assessed study eligibility, extracted data, and evaluated risk of bias of included studies. Heterogeneity was measured with a Q test and with I 2 statistics. Data were pooled by using the fix or random-effect model based on the heterogeneity test results and expressed as standardized mean difference (SMD) with 95% confidence interval (CI). A total of seven randomized controlled trials were included. Synbiotic consumption significantly changed glucose metabolism, including fasting plasma glucose (FPG) (SMD = −0.29; 95% CI, −0.47, −0.10), insulin concentrations (SMD = −0.84; 95% CI, −1.61, −0.06), homeostasis model assessment of insulin resistance (HOMA-IR) (SMD = −0.80; 95% CI, −1.58, −0.03), homeostatic model assessment-B cell function (HOMA-B) (SMD = −0.36; 95% CI, −0.71, −0.01), quantitative insulin sensitivity check index (QUICKI) (SMD = 0.46; 95% CI, 0.09, 0.82), and significantly improved lipid profiles, such as triglycerides (SMD = −0.36; 95% CI, −0.55, −0.17), very low density lipoprotein-cholesterol (SMD = −0.31; 95% CI, −0.55, −0.08), and total cholesterol (SMD = −0.32; 95% CI, −0.67, −0.03), but had no effect on low density lipoprotein-cholesterol (SMD = −0.07; 95% CI, −0.58, 0.43) and high density lipoprotein-cholesterol concentrations (SMD = −0.25; 95% CI, −0.81, 0.31). Synbiotic may result in an improvement in FPG, insulin, HOMA-IR, HOMA-B, QUICKI, triglycerides, and total cholesterol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired glucose metabolism, insulin resistance, and dyslipidemia are causally related to a greater risk of several chronic disorders, including diabetes, obesity, fatty liver, and cardiovascular diseases (CVDs) [1]. Blood glucose and lipid profiles can be controlled by proper eating pattern to prevent or control diabetes or related disorders [2]. In addition, existing evidence suggests that supplements such as omega-3 fatty acids [3], vitamin D [4], and dairy products [5] can improve glycemic control and lipid profiles or reduce risk of diabetes and CVD.
Probiotic and synbiotic are suggested to manage metabolic profiles of patients suffering from diseases related to metabolic syndrome. Synbiotics refer to nutritional supplements that are combining probiotics and prebiotics in a form of synergism [6]. Few studies have evaluated the effects of synbiotic-containing products on glucose metabolism and lipid profiles among patients with type 2 diabetes mellitus (T2DM) [7] and pregnant women [8]; however, findings were inconsistent. Such controversial findings complicate approaches to and conclusions about synbiotic use. In a meta-analysis by Beserra et al. [9], synbiotic supplementation among overweight or obese adults resulted in reductions in plasma fasting insulin and triglyceride fractions, while prebiotic supplementation resulted in reduction of triglycerides, plasma total cholesterol, and low density lipoprotein (LDL)-cholesterol levels and increased high density lipoprotein (HDL)-cholesterol level. In another study by Ruan et al. [10], reduced fasting glucose, insulin concentrations, and homeostasis model assessment of insulin resistance (HOMA-IR) were observed among percipients who consumed probiotic supplement compared with controls. Synbiotics are being used to modulate gut microbiota with favorable benefits for glucose homeostasis parameters and lipid profiles through mechanisms such as the production of short-chain fatty acid (SCFA), carbon disulfide, and methyl acetate [11] and decreased expression of inflammation-relevant genes [12], energy harvest, storage and expenditure from diet, satiety hormone balance, regulation of lipid synthesis, and improvement of markers of insulin metabolism and modulating the immune function [13].
Numerous randomized controlled trials (RCTs) have been conducted to determine whether synbiotic supplementation has a causal effect on glucose metabolism and lipid profiles. This study aimed to systematically review the current evidence on the effect of synbiotic supplementation on glucose metabolism and lipid profiles in RCTs among patients with diabetes and to summarize the available findings in a meta-analysis, if possible.
Methods
Search Strategy
Relevant studies were systematically searched from online databases PubMed, EMBASE, Web of Science, and Cochrane Library databases up to 12 November 2016. The search was conducted based on PICOS elements (Table 1). Search terms included patients [“diabetes” OR “T2DM” OR “gestational diabetes mellitus (GDM)”], intervention (“synbiotic” OR “symbiotic” AND “supplementation” OR “intake”), and outcomes [“fasting plasma glucose (FPG)” OR “insulin” OR “homeostatic model assessment-B cell function (HOMA-B)” OR “homeostatic model assessment-B cell function (HOMA-B)” OR “total-cholesterol” OR “triglycerides” OR“LDL-cholesterol” OR “HDL-cholesterol” OR “VLDL-cholesterol” OR “quantitative insulin sensitivity check index (QUICKI)”]. Search was conducted by two independent researchers. References cited in the selected studies were manually searched for additional relevant articles. Additionally, the relevant research centers and experts of the field were contacted to find unpublished studies. Our search was restricted to studies published in the English language.
Selection Criteria
The eligibility criteria were human RCTs, patients with T2DM or GDM, and administration of synbiotic or symbiotic supplements. Studies that did not reported mean changes of glucose metabolism and lipid profiles, along with standard deviation (SD) for the intervention and control groups, the abstracts of seminars without full text, case reports, and studies that did not obtain the minimum required score of quality assessment process were excluded.
Quality Assessment
Data extraction and study quality assessment were conducted by two independent reviewers (ZA and MA), according to Cochrane Collaboration risk of bias tool. The scale includes three domains related to quality of clinical trials: (1) random sequence generation description (0 = no description, 1 = inadequate description, 2 = adequate description), (2) blinding process (2 = double blinding with adequate description, 1 = double blinding with inadequate description, 0 = wrong usage of double blinding), and (3) withdrawal of patients (1 = the number and reasons of patients withdrawal described, 0 = otherwise). In the event of disagreement, resolved by discussion until consensus was reached.
Statistical Methods
RevMan software (Cochrane Review Manager, version 5.2) and STATA version 12.0 (Stata Corp., College Station, TX) were used for data analyses. Heterogeneity was evaluated through the Cochran (Q) and I-squared tests (I 2). Given the existing heterogeneity between studies, when I 2 exceeds 50% or P < 0.05, the random-effect model was used; otherwise, the fixed-effect model was applied. Inverse variance method and Cohen statistics were used for estimation of standardized mean difference (SMD) and 95% CI for verifying the outcome behavior of each study group (intervention/control). Sensitivity analyses also undertook in the trials one by one to evaluate the reliability of the pooled mean difference. In addition, the Cochrane Collaboration risk of bias tool was used to assess the methodological quality of the RCTs. Potential publication bias was assessed through visual inspection of funnel plots and quantitatively assessed using Egger’s tests.
Results
Search Results and Trial Flow
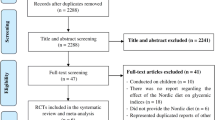
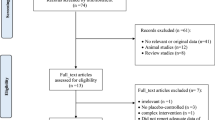
A total of 1328 studies were identified through the database search. Of these, 302 duplicate articles, 261 not randomized controlled trials, and 4 review articles were excluded. After reading titles and abstracts, 761 articles were excluded and 23 full text articles were assessed for eligibility. One article was included form the references cited in the selected studies. The remaining 23 articles were retrieved for further review, and 9 were deemed relevant. Of these, we excluded 14 articles that examined non-diabetic patients (n = 3), did not presented required data for meta-analyses (n = 11), and did not administrated symbiotic (n = 2). Finally, seven studies were found to be appropriate for in this meta-analysis [8, 14,15,16,17,18,19] (Fig. 1).
Characteristics of Included Studies
Totally, seven studies with 482 participants were included in the final meta-analysis. Six studies were double blind, and one study was single blind [17]. Four studies used parallel design, and two used crossover design [15, 16]. The intervention duration varied from 6 to 12 weeks. Six studies have investigated the effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with T2DM and one study in patients with GDM [14]. Six studies have reported changes in FPG, triglycerides, total cholesterol, and HDL-cholesterol, and five studies have reported changes in insulin concentrations, HOMA-IR, and LDL-cholesterol, and four studies have reported changes in HOMA-B, QUICKI, and very low density lipoprotein (VLDL)-cholesterol levels. The synbiotic species and used dosage were varied between studies. Five studies have used combination of more than two strains, whereas two studies have used a single species of probiotics [15, 16]. Total daily dose of probiotic intake was varied from 106 colony-forming units (CFU) to 108 CFU, except for one study that has used 1500 mg probiotic capsule twice daily. Participants of three studies in the intervention group consumed synbiotic capsules, and those in the control group consumed placebo capsules. Participants of four studies in the intervention group consumed synbiotic food, and those in the control group consumed control food. The characteristics of included studies are presented in Table 2. The methodological quality based on authors’ judgments about each risk of bias item for each included study is shown in Fig. 2.
Pooled Effects of Synbiotic on Glucose Metabolism
Figure 3 shows the forest plots for effect of synbiotic on glucose metabolism parameters. We observed that synbiotic consumption significantly improved glucose metabolism, such as FPG (SMD −0.29; 95% CI, −0.47, −0.10), insulin concentrations (SMD = −0.84; 95% CI, −1.61, −0.06; I 2 = 92.6%, P < 0.001), HOMA-IR (SMD = −0.80; 95% CI, −1.58, −0.03; I 2 = 92.6%, P < 0.001), HOMA-B (SMD = −0.36; 95% CI, −0.71, −0.01; I 2 = 53.0%, P = 0.094), and QUICKI (SMD = 0.46; 95% CI, 0.09, 0.82; I 2 = 55.7%, P = 0.08). Evidence of inter-study heterogeneity was observed across studies on glucose metabolism parameters; therefore, the random-effect model was used. Sensitivity analysis was performed by removing the trials one by one to evaluate the reliability of the pooled standardize mean difference; expect Asemi et al. [15] study, results remained consistent after removing the trials for insulin and HOMA-IR.
Pooled Effects of Synbiotic on Lipid Profiles
Similar results were observed for lipid profiles. The effect of synbiotic supplementation on triglycerides and VLDL-cholesterol levels was examined in seven RCTs; significant decrease in SMD −0.32 (95% CI, −0.67, −0.03), −0.36 (95% CI, −0.55, −0.17), and −0.31 (95% CI, −0.55, −0.08) was observed between intervention and placebo groups for any of the lipid profiles, respectively (Table 3). Due to heterogeneity of studies, the results of total cholesterol studies were combined by using random-effect model (I 2 = 66.4%, P = 0.011). Synbiotic consumption significantly decreased triglycerides (SMD = −0.36; 95% CI, −0.55, −0.17), VLDL-cholesterol (SMD = −0.31; 95% CI, −0.55, −0.08), and total cholesterol (SMD = −0.32; 95% CI, −0.67, −0.03), but had no significant effect on LDL-cholesterol (SMD = −0.07; 95% CI, −0.58, 0.43) and HDL-cholesterol concentrations (SMD = −0.25; 95% CI, −0.81, 0.31) (Fig. 4). Sensitivity analysis showed that removing studies with high heterogeneity in lipid profiles did not change the pooled effect.
a–e Meta-analysis lipid profiles’ standardized mean difference estimates for a total cholesterol, b for triglycerides, c for LDL-cholesterol, d for HDL-cholesterol, and e for VLDL-cholesterol in synbiotic and placebo groups (CI = 95%). FPG fasting plasma glucose, HOMA-IR homeostasis model assessment of insulin resistance, HOMA-B homeostatic model assessment-B cell function, QUICKI quantitative insulin sensitivity check index
Meta-Regression and Subgroup Analyses
Subgroup analyses for all metabolic profiles were done based on bacteria strain (Figs. 3 and 4). The univariate meta-regression analyses based on bacteria strain, time of intervention, and the number of bacteria did not show any statistically significant subgroup-effect interactions on lipid profiles (P ≥ 0.05 for all comparisons). However, among glucose metabolism, parameters as FPG based on time of intervention and insulin by bacteria strain and the number of bacteria had statistically significant subgroup-effect interactions (P < 0.05) (Table 4).
Publication Bias
The Egger’s regression was performed to detect potential publication bias. Egger’s regression indicated no significant publication bias for all indices (Β = 1.67, P = 0.605).
Discussion
To our knowledge, this is the first meta-analysis of RCTs that examined the effect of synbiotic supplementation on glucose metabolism and lipid profiles among patients with diabetes. We show that synbiotic supplementation may result in an improvement in FPG, insulin, HOMA-IR, HOMA-B, QUICKI, triglycerides, total cholesterol, and VLDL-cholesterol levels, but did not affect LDL-cholesterol and HDL-cholesterol levels in patients with diabetes.
The hypothesis that probiotics and synbiotics may be involved in the maintenance of healthy gut microbiota and the management of glucose metabolism and lipid profiles has received much attention. In a study by Gomes et al. [20], the ratio of bacteroidetes species in T2DM was correlated positively with fasting plasma glucose. The alterations in gut microbiota have recently been reported in subjects with T2DM, and this may be reversible with probiotic intake [21]. The results of our meta-analysis revealed that synbiotic supplementation significantly reduced FPG, insulin levels, HOMA-IR, and HOMA-B and increased QUICKI score in patients with diabetes. In a recent meta-analysis by Kasinska et al. [22], a significant effect of probiotic supplementation on reducing HbA1c levels and HOMA-IR was observed; however, there was no effect on FPG and insulin concentrations. In another meta-analysis study by Beserra et al. [9], synbiotic intake in adults with overweight or obesity significantly decreased insulin and triglyceride levels and prebiotic supplementation decreased total cholesterol and LDL-cholesterol values in overall analysis, and decreased triglycerides and increased HDL-cholesterol values in patients with diabetes. In addition, a meta-analysis by Samah et al. [23] showed that FGD was significantly lower following consumption of probiotic supplements. The findings of our meta-analysis are in agreement with the previous review, suggesting that a combination of probiotic species in synbiotic supplements is more effective than single-species probiotics [24].
An interesting observation in the current meta-analysis was that synbiotic supplementation was associated with improvement in HOMA-IR among participants with impaired glucose tolerance and insulin resistance at baseline, a common feature in T2DM patients. Insulin resistance is pathogenic for several prevalent disorders such as T2DM, CVD, polycystic ovary syndrome, non-alcoholic fatty liver disease, and several cancers [25]. Accurate mechanism of synbiotic function on glucose metabolism is unclear. The glucose-lowering effects of synbiotics may be related to the reduction in oxidative stress activities [26]. Previous studies have shown that specific strains of lactic acid bacteria have antioxidant properties [27, 28]. For instance, Yadav et al. [29] revealed that probiotic dahi-supplemented diet, a fermented milk containing Lactobacillus acidophilus and Lactobacillus casei, delayed the progression of glucose intolerance, hyperglycemia, and hyperinsulinemia via decreased oxidative stress in animal models. In addition, synbiotics may have antidiabetic effects through modulating immune responses and systemic low-grade inflammation, in particular by reducing inflammatory cytokines [30] and suppressing the nuclear factor kappa light chain enhancer of activated B cell (NF-κB) pathway [31]. Furthermore, probiotic and inulin intake may improve insulin resistance through upregulation in the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) gene [32, 33]. Wang et al. [34] found that L. casei significantly increased the expression of PPAR-γ gene in a rat model of acute liver failure induced by lipopolysaccharide and d-galactosamine for 30 days. Downregulation in the expression of hepatic genes involved in lipogenesis and fatty acid elongation/desaturation by inulin may also result in improvement in markers of insulin metabolism [35].
We found that synbiotic supplementation in patients with diabetes significantly reduced triglycerides, total cholesterol, and VLDL-cholesterol levels, but did not alter LDL-cholesterol and HDL-cholesterol levels. Although several meta-analyses studies have demonstrated that probiotic supplementation is effective for the improvement of hyperlipidemia, the characteristics of subjects who consume probiotics with the most beneficial effects remained unclear. In a meta-analysis of 30 RCTs with 1624 participants by Cho et al. [36], probiotic supplementation resulted in statistically significant decreases in total cholesterol and LDL-cholesterol compared to control subjects by 7.8 and 7.3 mg/dL, respectively, but did not affect HDL-cholesterol or triglyceride concentrations. In another meta-analysis study of 33 RCTs by Shimizu et al. [37], probiotic interventions, including fermented milk products and probiotics, led to significant changes in total cholesterol and LDL-cholesterol levels; however, it did not influence HDL-cholesterol and triglyceride levels. However, Agerholm-Larsen et al. [38] revealed that the duration of probiotic consumption had no significant effect on total cholesterol and LDL-cholesterol reduction using regression analysis. This difference in findings with other studies was likely due to a short duration (4–8 weeks) of supplementation in trial conducted by Agerholm-Larsen et al. Long-term (>4 weeks) synbiotic supplementation was more effective in reduction of lipid profiles, which could be useful in reduced risk of CVD. Synbiotic intake may decrease triglycerides and VLDL-cholesterol values through lipolysis of triglycerides and transform triglyceride-rich particles into small [39], suppressing the NF-κB pathway [31], and gut microbiota-SCFA-hormone axis [40]. Several possible mechanisms proposed for the removal of cholesterol from media, such as assimilation of cholesterol during growth by L. acidophilus [41], binding of cholesterol to the cellular surface, disruption of cholesterol micelles [42], and deconjugation of bile salt and bile salt hydrolase activity [41]. The meta-analysis findings regarding potential benefit of probiotics to manage or prevent metabolic disorders are influenced by differences in the study design, inclusion criteria of the studies, and method for data analysis. One consideration is that the studies were conducted in many different geographic areas; therefore, the metabolic modifier factors, such as diet, lifestyle, and genetic background, are different. For example, the effectiveness of probiotic intervention could depend on the patient’s intestinal microbiome, and this is highly regulated by the factors, including age, diet, lifestyle, and genetics [43]. Of these, diet is easiest to modify and presents the simplest route for therapeutic intervention. In a study by Wu et al. [44], it observed that microbiome composition changed detectably within 24 h of initiating a high-fat/low-fiber or low-fat/high-fiber diet but that enterotype identity remained stable during the 10-day study. Therefore, alternative enterotype states are related to long-term diet [44]. In addition, functional immaturity of the immune system and intestinal epithelium can affect the aberrant intestinal colonization pattern occurring in preterm neonates [45]. Drugs, especially chronic medication, can exert a strong impact on intestinal microbiota [46]. A misbalance of this intestinal microbial community can act as an important source of infection, or inflammation, and can be involved, as well, in gastrointestinal diseases and other extra-intestinal disorders. Probiotic intervention might also be more effective when provided with a prebiotic in a synbiotic combination, but the effectiveness of this intervention could depend dramatically on diet or genetics. We have previously shown that consumption of the synbiotic bread compared with probiotic bread and placebo among diabetic patients had more beneficial effects on insulin metabolism [19]. Prior studies have reported that inclusion of prebiotic substances like inulin can stimulate the growth and/or metabolic activity of the selected bacterial groups including bifidobacteria or lactobacilli and might increase production of SCFA in the colon [47, 48]. Different probiotics might be more beneficial when considering what the endogenous microbiome is. The effectiveness of different types of probiotics or prebiotics can be different in diverse populations, as the endogenous microbiome is affected by the person’s diet or genetic background. For instance, the consumption of probiotic containing L. acidophilus and Bifidobacterium lactis in patients with T2DM improved fasting blood glucose and antioxidant status [26], whereas probiotic supplementation containing L. acidophilus and B. lactis in overweight men and women did not affect glycemic control [49]. Therefore, a specific type of probiotic might not be useful or effective for all.
Synbiotic supplementation may result in an improvement in FPG, insulin, HOMA-IR, HOMA-B, QUICKI, triglycerides, total cholesterol, and VLDL-cholesterol levels, but did not affect LDL-cholesterol and HDL-cholesterol levels in patients with diabetes. Additional prospective studies regarding the effect of synbiotic intake on glucose homeostasis parameters and lipid profiles in patients with diabetes are necessary.
References
McGillicuddy FC, Roche HM (2012) Nutritional status, genetic susceptibility, and insulin resistance—important precedents to atherosclerosis. Mol Nutr Food Res 56:1173–1184. doi:10.1002/mnfr.201100785
Schubert CM, Rogers NL, Remsberg KE, Sun SS, Chumlea WC, Demerath EW, Czerwinski SA, Towne B, Siervogel RM (2006) Lipids, lipoproteins, lifestyle, adiposity and fat-free mass during middle age: the Fels Longitudinal Study. Int J Obes 30:251–260
Moosheer SM, Waldschutz W, Itariu BK, Brath H, Stulnig TM (2014) A protein-enriched low glycemic index diet with omega-3 polyunsaturated fatty acid supplementation exerts beneficial effects on metabolic control in type 2 diabetes. Prim Care Diabetes 8:308–314. doi:10.1016/j.pcd.2014.02.004
Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A (2013) Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr 98:1425–1432. doi:10.3945/ajcn.113.072785
Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y, Li Q (2013) Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One 8:e73965. doi:10.1371/journal.pone.0073965
de Vrese M, Schrezenmeir J (2008) Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 111:1–66. doi:10.1007/10_2008_097
Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A (2016) Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int J Mol Sci :17(6). doi:10.3390/ijms17060928.
Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, Asemi Z (2014) Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 49:695–701. doi:10.1007/s11745-014-3901-z
Beserra BT, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MG, Trindade EB (2015) A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr 34:845–858. doi:10.1016/j.clnu.2014.10.004
Ruan Y, Sun J, He J, Chen F, Chen R, Chen H (2015) Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PLoS One 10:e0132121. doi:10.1371/journal.pone.0132121
Vitali B, Ndagijimana M, Maccaferri S, Biagi E, Guerzoni ME, Brigidi P (2012) An in vitro evaluation of the effect of probiotics and prebiotics on the metabolic profile of human microbiota. Anaerobe 18:386–391. doi:10.1016/j.anaerobe.2012.04.014
Voltolini C, Battersby S, Etherington SL, Petraglia F, Norman JE, Jabbour HN (2012) A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 153:395–403. doi:10.1210/en.2011-1457
Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104(Suppl 2):S1–S6. doi:10.1017/S0007114510003363
Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z (2016) The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr 116:1394–1401. doi:10.1017/S0007114516003457
Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A (2014) Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 33:198–203. doi:10.1016/j.clnu.2013.05.015
Asemi Z, Alizadeh SA, Ahmad K, Goli M, Esmaillzadeh A (2016) Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 35:819–825. doi:10.1016/j.clnu.2015.07.009
Moroti C, Souza Magri LF, de Rezende CM, Cavallini DC, Sivieri K (2012) Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 11:29. doi:10.1186/1476-511X-11-29
Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, Taheri S, Asemi Z (2017) A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 125:21–27. doi:10.1055/s-0042-105441
Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z (2014) Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab 65:34–41. doi:10.1159/000365153
Gomes AC, Bueno AA, de Souza RG, Mota JF (2014) Gut microbiota, probiotics and diabetes. Nutr J 13:60. doi:10.1186/1475-2891-13-60
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5:e9085. doi:10.1371/journal.pone.0009085
Kasinska MA, Drzewoski J (2015) Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn 125:803–813. doi:10.20452/pamw.3156
Samah S, Ramasamy K, Lim SM, Neoh CF (2016) Probiotics for the management of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 118:172–182. doi:10.1016/j.diabres.2016.06.014
Chapman CM, Gibson GR, Rowland I (2011) Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 50:1–17. doi:10.1007/s00394-010-0166-z
Prudente S, Trischitta V (2015) The TRIB3 Q84R polymorphism, insulin resistance and related metabolic alterations. Biochem Soc Trans 43:1108–1111. doi:10.1042/BST20150115
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28:539–543. doi:10.1016/j.nut.2011.08.013
Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97:809–817. doi:10.1007/s00253-012-4241-7
Uskova MA, Kravchenko LV (2009) Antioxidant properties of lactic acid bacteria—probiotic and yogurt strains. Vopr Pitan 78:18–23
Yadav H, Jain S, Sinha PR (2007) Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23:62–68
de Moreno de Leblanc A, Perdigon G (2010) The application of probiotic fermented milks in cancer and intestinal inflammation. Proc Nutr Soc 69:421–428. doi:10.1017/S002966511000159X
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025
Deepak V, Ram Kumar Pandian S, Sivasubramaniam SD, Nellaiah H, Sundar K (2016) Optimization of anticancer exopolysaccharide production from probiotic Lactobacillus acidophilus by response surface methodology. Prep Biochem Biotechnol 46:288–297. doi:10.1080/10826068.2015.1031386
Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, Deldicque L, Bindels LB, Pachikian BD, Sohet FM, Mignolet E, Francaux M, Larondelle Y, Delzenne NM (2011) Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 22:712–722. doi:10.1016/j.jnutbio.2010.05.009
Wang Y, Xie J, Li Y, Dong S, Liu H, Chen J, Wang Y, Zhao S, Zhang Y, Zhang H (2016) Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur J Nutr 55:821–831. doi:10.1007/s00394-015-0904-3
Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S (2015) Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem 26:929–937. doi:10.1016/j.jnutbio.2015.03.010
Cho YA, Kim J (2015) Effect of probiotics on blood lipid concentrations: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 94:e1714. doi:10.1097/MD.0000000000001714
Shimizu M, Hashiguchi M, Shiga T, Tamura HO, Mochizuki M (2015) Meta-analysis: effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS One 10:e0139795. doi:10.1371/journal.pone.0139795
Agerholm-Larsen L, Bell ML, Grunwald GK, Astrup A (2000) The effect of a probiotic milk product on plasma cholesterol: a meta-analysis of short-term intervention studies. Eur J Clin Nutr 54:856–860
Matsuoka H, Miura A, Hori K (2009) Symbiotic effects of a lipase-secreting bacterium, Burkholderia arboris SL1B1, and a glycerol-assimilating yeast, Candida cylindracea SL1B2, on triacylglycerol degradation. J Biosci Bioeng 107:401–408. doi:10.1016/j.jbiosc.2008
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG (2013) Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 288:25088–25097. doi:10.1074/jbc.M113.452516
Klaver FA, van der Meer R (1993) The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl Environ Microbiol 59:1120–1124
Lye H-S, Rahmat-Ali GR, Liong M-T (2010) Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J 20:169–175. doi:10.1016/j.idairyj.2009.10.003
Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE (2006) Metagenomic analysis of the human distal gut microbiome. Science 312:1355–1359
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi:10.1126/science.1208344
Arboleya S, Ang L, Margolles A, Yiyuan L, Dongya Z, Liang X, Solís G, Fernández N, de Los Reyes-Gavilán CG, Gueimonde M (2012) Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe 18:378–380. doi:10.1016/j.anaerobe.2012.04.013
Perez-Cobas AE, Artacho A, Knecht H, Ferrús ML, Friedrichs A, Ott SJ, Moya A, Latorre A, Gosalbes MJ (2013) Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS One 8:e80201. doi:10.1371/journal.pone.0080201
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, Prince RL (2014) The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr 68:447–452. doi:10.1038/ejcn.2013.294
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
For Studies with Human Subjects
All procedures in selected papers followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Funding
The current study was founded by a grant from the Vice Chancellor for Research, KUMS, in Iran.
Rights and permissions
About this article
Cite this article
Tabrizi, R., Moosazadeh, M., Lankarani, K.B. et al. The Effects of Synbiotic Supplementation on Glucose Metabolism and Lipid Profiles in Patients with Diabetes: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Probiotics & Antimicro. Prot. 10, 329–342 (2018). https://doi.org/10.1007/s12602-017-9299-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9299-1