Abstract
Previous study showed that dietary Bacillus licheniformis (B. licheniformis) administration contributes to the improvement of laying performance and egg quality in laying hens. In this study, we aimed to further evaluate its underlying mechanisms. Three hundred sixty Hy-Line Variety W-36 hens (28 weeks of age) were randomized into four groups, each group with six replications (n = 15). The control group received the basal diet and the treatment groups received the same basal diets supplemented with 0.01, 0.03, and 0.06% B. licheniformis powder (2 × 1010 cfu/g) for an 8-week trial. The results demonstrate that B. licheniformis significantly enhance the intestinal barrier functions via decreasing gut permeability, promoting mucin-2 transcription, and regulating inflammatory cytokines. The systemic immunity of layers in B. licheniformis treatment groups is improved through modulating the specific and non-specific immunity. In addition, gene expressions of hormone receptors, including estrogen receptor α, estrogen receptor β, and follicle-stimulating hormone receptor, are also regulated by B. licheniformis. Meanwhile, compared with the control, B. licheniformis significantly increase gonadotropin-releasing hormone level, but markedly reduce ghrelin and inhibin secretions. Overall, our data suggest that dietary inclusion of B. licheniformis can improve the intestinal barrier function and systemic immunity and regulate reproductive hormone secretions, which contribute to better laying performance and egg quality of hens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inclusion of probiotics in diets for layers is preferred to replace antibiotics [1] and improve growth performance, feed conversion efficiency, and egg quality [2–4]. It is reported that probiotics have beneficial impacts on the poultry performance by synthesizing vitamins [5], inducing the digestive enzyme [6], utilizing undigestible carbohydrates [7], stimulating lactic acid, and releasing bacteriocins [8]. Several probiotics, such as Lactobacillus, Streptococcus, Saccharomyces, Aspergillus, and Bacillus, have been selected and applied in poultry production [9]. However, Bacillus, the spore-forming bacteria, are ideally suited as feed additives, because they have higher resistance to harsh environments [10] and have the ability to produce a variety of enzymes including protease, amylase, and lipase [11]. Bacillus licheniformis, which has been broadly applied in livestock and aquaculture as growth promoter and competitive exclusion agent [12, 13], has demonstrated a positive effect in aiding nutrient digestion and absorption in the host’s body [13, 14], inhibiting the growth and reproduction of pathogens by producing antimicrobial active substances and reacting with oxygen that retards the growth and reproduction of pathogens [15].
In our previous study, we found that dietary B. licheniformis supplementation effectively improves laying performance and egg quality by increasing eggshell thickness and strength in a dose-dependent manner in laying hens via decreasing the stress response, up-regulating the growth hormones, and improving intestinal mucosal structure [16]. In recent years, it has been reported that probiotics also benefit animals by enhancing intestinal barrier function and positively modulating the immune system [17]. However, to our knowledge, little research has been conducted on related mechanisms of B. licheniformis in improving laying performance and egg quality in layers in the respect of immunity and gut health. The objective of this study is to further explore the effects of dietary B. licheniformis supplementation on laying hens and its molecular mechanisms in terms of gut barrier, the systemic immunity, and hormone gene expression.
Materials and Methods
Birds and Management
The experiment was carried out in accordance with the Chinese guidelines for animal welfare and approved by the Animal Welfare Committee of Animal Science College, Zhejiang University. The birds and management is referred to Lei et al. [16]. Briefly, a total of 360 Hy-Line Variety W-36 hens, 28 weeks of age, were randomly divided into four groups, each group with six replications and each replication with 15 hens.
Bacterial Strain and Diet
B. licheniformis strain was obtained from China General Microbiological Culture Collection Center (CGMCC1.3448) and prepared by Microbiology and Genetic Engineering Laboratory, Institute of Feed Sciences, Zhejiang University, China. B. licheniformis was cultured in Luria-Bertani media, kept at 37 °C for 24 h, and shaken at 180 r/min. Bacteria suspensions were centrifuged to obtain pure bacterial cells at 5000 g for 10 min at 4 °C. Subsequently, bacteria were washed twice with sterile 0.85% (8.5 g/L) sodium chloride solution. The culture purity and identification were constantly checked by the spreading plate method [18]. B. licheniformis powders (2 × 1010 cfu/g) were added to the basal diet at levels of 0.01, 0.03, and 0.06% to form the three types of treatment diets. Starch was used to dilute B. licheniformis, and the same amount of starch was also added to each group to compensate for the difference in nutrient composition of the diets. B. licheniformis powders were stored at room temperature. The composition and nutrition of the basal experimental diet can be found in our previous study [16]. Diets were stored in a dry and well-ventilated storeroom.
Sample Collection
At the end of the experiment, no hens died. Birds were fasted for 12 h [19, 20] and blood samples of 12 hens (two birds per replicate) were drawn from the axillary vein into vacuum tubes (5 mL) containing coagulant. After centrifugation for 10 min at 4 °C (3000×g, Centrifuge 5804R, Eppendorf, Germany), pure serum samples were obtained. The ovaries and mid-jejunum segments were carefully dissected and rinsed with sterilized saline. Jejunal mucosa was gently scraped off. All the samples were placed in liquid nitrogen immediately and stored at −70 °C till further analysis.
RNA Extraction and RT-qPCR
The RNA extraction and RT-qPCR were referred to previous research [21]. Briefly, total RNA was extracted using RNAiso Plus method (TaKaRa, Dalian, China). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using M-MLV reverse transcriptase (TaKaRa, Dalian, China). Transcriptional changes were then identified by quantitative PCR, which was performed using the Premix Ex TaqTM with SYBR Green (TaKaRa, Dalian, China) and the ABI 7500 Fast Real-Time PCR system (Applied Biosystemics, Carlsbad, CA, USA). The thermocycle protocol lasted for 30 s at 95 °C, followed by 40 cycles of 5-s denaturation at 95 °C, 34-s annealing/extension at 60 °C, and then a final melting curve analysis to monitor purity of the PCR product. Primer sequences are presented in Table 1. The 2−∆∆Ct method was used to estimate mRNA abundance. ∆C t is C t, target−C t, reference and ∆∆C t is ∆C t, treatment−∆C t, control [21]. Relative gene expression levels were normalized to those of the eukaryotic reference gene β-actin.
ELISA
Levels of immunoglobulin A (IgA), immunoglobulin G (IgG), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-10, and tumor necrosis factor α (TNF-α) were quantified using a sandwich ELISA kit (Komabiotech Ltd., Seoul, Korea) according to instructions. Briefly, serum samples were pipetted into wells coated with antibodies specific for IgA, IgG, IL-1β, IL-2, IL-4, IL-6, IL-10, and TNF-α. After incubation, biotinylated monoclonal secondary antibiotics were added, followed by streptavidin-peroxidase. After incubation and washing, the bound cytokines were visualized by developing the peroxidase reaction through the addition of H2O2 and the absorbency of each well was determined by SpectraMax M5 (MD, USA) [22].
Statistical Analysis
Data were statistically analyzed by one-way ANOVA procedure of SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). When significant differences were found (P < 0.05), Tukey’s test was further performed. Statements of significance were based on P < 0.05. The data were expressed as the means ± SEM.
Results
The Transcript Level of Genes Related to Intestinal Physical Barrier Function
We previously demonstrated that B. licheniformis lead to the improvement of intestinal mucosa structure [16]. In the presence of an intact epithelial cell layer, the paracellular pathway between cells must be sealed. This function is achieved by physical barrier, especially by tight junctions [23]. Besides, mucin 2 (MUC2) is the most abundant mucin, which creates the first line of defense against microbial encroachment [24]. Hence, here, we focused on the transcriptions of genes encoding tight junctions and mucin (MUC) in jejunal mucosa to further explore the physical barriers of layers. Figure 1a shows that B. licheniformis at three dosages all significantly increase claudin 1 (CLDN-1) gene expressions, while only 0.03% B. licheniformis up-regulate the claudin 2 (CLDN-2) levels. Although 0.01 and 0.03% B. licheniformis have no obvious effects on zonula occludens 1 (ZO-1) and occluding 1 (OCLIN-1), 0.06% B. licheniformis markedly elevate their expressions. In addition, B. licheniformis at three dosages dramatically up-regulate MUC2 expressions as well (Fig. 1b).
Effects of B. licheniformis supplementation on physical barrier in jejunum of laying hens. Total RNA was extracted and the expressions of a cldn-1, cldn-2, ZO-1, and OCLN-1 and b were measured by real-time PCR. Data are expressed as mean ± SEM from three independent experiments. Different letters indicate values significantly different (P < 0.05) among the groups
The Transcript Level of Genes Related to the Intestinal Immunological Barrier Function
Compared to the control group, the IL-6 and TNF-α transcriptions in jejunal mucosa of 0.01, 0.03, and 0.06% B. licheniformis groups show no significant changes. While, IL-1β expression in 0.03% B. licheniformis group is significantly decreased and this is reversed to normal when the B. licheniformis dosage reaches to 0.06% (Fig. 2a). Moreover, IL-10 and IL-4 mRNA expressions of all the B. licheniformis treatments are also elevated, but 0.01% B. licheniformis do not alter the IL-4 transcript level significantly (Fig. 2b).
Effects of B. licheniformis supplementation on immunological barrier in jejunum of laying hens. a Pro-inflammatory factor mRNA expressions. Total RNA was extracted and the expressions of IL-6, IL-1β, and TNF-α were measured by real-time PCR. b Anti-inflammatory factor mRNA expressions. Total RNA was extracted and the expressions of IL-10 and IL-4 were measured by real-time PCR. Data are expressed as mean ± SEM from three independent experiments. Different letters indicate values significantly different (P < 0.05) among the groups
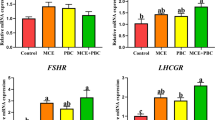
The Transcript Level of Genes Encoding Hormones and Hormone Receptors
Our previous findings indicate that B. licheniformis at three dosages can significantly enhance levels of estradiol (E2) and follicle-stimulating hormone (FSH) [16]. We therefore investigated the mRNA expression of genes encoding estrogen receptor (ESR) and FSH receptor (FSHR) to further verify the impact of B. licheniformis on hormone secretions. Figure 3a shows that 0.01 and 0.03% B. licheniformis dramatically up-regulate ESRα and ESRβ expressions respectively, while 0.06% B. licheniformis decrease FSHR transcript levels markedly. Furthermore, B. licheniformis of three concentrations significantly increase gonadotropin-releasing hormone (GnRH) expressions and decrease inhibin (INH) transcriptions compared with the control group, while 0.03 and 0.06% B. licheniformis down-regulate Ghrelin levels significantly in a dose-dependent manner (Fig. 3b).
Effects of B. licheniformis supplementation on mRNA expressions of genes encoding hormone receptors (a) and hormones (b) in ovary of laying hens. Total RNA was extracted and the expressions of ESRα, ESRβ, FSHR, GnRH, Ghrelin, and INH were measured by real-time PCR. Data are expressed as mean ± SEM from three independent experiments. Different letters indicate values significantly different (P < 0.05) among the groups
The Systemic Immunity
The effects of B. licheniformis on immunoglobulin and cytokine levels in serum are also evaluated. Results reveal that compared to the control group, 0.01% B. licheniformis have no obvious impact on IgA and IgG levels. However, 0.01% B. licheniformis significantly decrease IL-1β, IL-6, and TNF-α levels but increase IL-4 secretions. Further, 0.03% B. licheniformis administration significantly elevates IgG and IL-4 concentrations, but drops the secretions of IL-1β and IL-6. As the dose of B. licheniformis increases to 0.06%, IL-1β and IL-6 levels are significantly down-regulated, while IL-4 concentration is enhanced (Table 2). But no significant differences of IgA and IgG are found in the 0.06% B. licheniformis group.
Discussion
There is growing evidence that proves that the consumption of probiotics can improve the egg production and egg quality of laying hens [25–27]. In our previous research, B. licheniformis administration is able to enhance the laying performance and up-regulate the secretions of hormones and antioxidases [16]. Here, results reinforce the published data on gene level and provide novel evidence for the multifaceted mode of B. licheniformis on the egg production and egg quality, including the intestinal barrier and immune functions.
Intestinal epithelia form a functional barrier that separates the intestine from the outside world, and this requires the formation of tight junctions that allow cells to adhere tightly to each other and control the intestinal permeability [28]. Tight junctions are composed of numerous structural and functional proteins, such as CLDN, ZO, and OCLN. It has long been reported that probiotics can play a role in enhancing intestinal barrier functions. VSL#3 probiotics decrease colonic epithelial permeability by increasing expressions of ZO-1 and OCLN in rats [29] and Escherichia coli Nissle 1917 also inhibit gut leakage by enhancing ZO-1 expression in mice [30]. Moreover, Streptococcus thermophilus and Lactobacillus acidophilus can augment the OCLN level to avoid E. coli invasion in intestinal epithelial cells [31]. In the present study, we got similar results. The transcript levels of all the tested tight junctions are up-regulated by B. licheniformis and this effect is more obvious when B. licheniformis is at a higher dosage. Besides, the highly glycosylated mucins secreted by goblet cells also create an important defense line against microbial encroachment [24]. Among them, MUC2, the most abundant mucin, plays an essential part in the organization of the intestinal mucous layers at the epithelial surface of the intestine [32]. Recent report has demonstrated that VSL#3 administration enhances MUC2 secretion and gene expression in rat colonic loops effectively [33]. Our results also reveal that B. licheniformis treatment dramatically increases jejunal MUC2 gene expression in a dose-dependent manner, implying the inhibition of epithelial cell adherence for enteric pathogens. It is known that compromised intestinal epithelial integrity may facilitate the invasion of endotoxins from gut microbes, resulting in a local imbalance of anti- and pro-inflammatory molecules in the intestines [25]. Thus, the improved gut physical barrier induced by B. licheniformis raises the possibility that it may also participate in enhancing the intestinal immunological barrier function. According to Shimazu et al. [34] and Perdigon et al. [35], probiotics down-regulate gene expressions of pro-inflammatory cytokines in porcine intestinal epithelial cells and stimulate the gut immune cells to release IL-4 and IL-10. Our findings are consistent with these findings in that we demonstrate B. licheniformis also significantly decrease the expression of pro-inflammatory cytokines IL-1β and TNF-α but increase the transcriptions of anti-inflammatory cytokines IL-4 and IL-10. The main function of anti-inflammatory cytokines is to limit and ultimately terminate inflammatory responses [36]. Over-production of pro-inflammatory cytokines has an adverse effect on intestinal mucosal integrity [37]. Recent studies have indicated that most pro-inflammatory cytokines induce a pathologic opening of the intestinal physical barrier and increase intestinal epithelial permeability [38]. Since it is suggested that intestinal damage may lead to the decreased egg production and quality in chickens [39], we speculate that the enhancement of intestinal, physical, immunological barrier functions caused by B. licheniformis can lead to the increase of intestinal health of laying hens, contributing to the improvement of laying performance and egg quality.
Faults in establishing intestinal immunity can lead to disease, inducing local and often also systemic inflammation [40]. Therefore, the enhanced gut immunological barrier inspired us to determine the systemic immunity. Evidence suggest that one of the putative effects of probiotics is the alteration of immune function [41, 42], and in recent decades, probiotics have been used in animals and humans to modulate the humoral immunity in order to enhance the disease resistance capacity [42, 43]. Lactobacilli treatment leads to higher antibody production in chickens [44, 45]. Multi-strain probiotics and yeast can increase egg production and egg quality while increasing the antibody titer in serum as well [46]. Our results demonstrate that B. licheniformis supplementation significantly increases IgG level in serum, but no significant differences are found for IgA concentration. Further, Klasing reported that excessive amounts of cytokines may decrease feed intake and increase energy expenditure, thereby reducing the performance of livestock [47]. Here, we find that levels of pro-inflammatory cytokines IL-1β and IL-6 are significantly reduced in all the B. licheniformis treatment groups. However, TNF-α concentrations are only decreased with 0.01% B. licheniformis treatment but returned to normal when the levels of B. licheniformis in the diet increase to 0.03 and 0.06%. On the contrary, the levels of anti-inflammatory cytokine IL-4 are markedly induced in all the probiotic groups, although IL-10 is only slightly enhanced when the concentration of B. licheniformis reaches to 0.06%. As it has been demonstrated that immunity changes are critical to the laying performance of hens [48, 49], we summarize that B. licheniformis can enhance the laying performance of layers by regulating immune function.
In birds, the onset of breeding involves the activation of various hormones. Estrogens play a fundamental role in the regulation of female sexual differentiation and reproduction. ESRs show an appropriate expression profile in the developing embryo [50]. The present study demonstrates that B. licheniformis increase ESRα and ESRβ transcript levels, but this effect is not observed in the 0.06% B. licheniformis group. FSH is a pituitary glycoprotein hormone, which can stimulate and regulate ovarian follicular development and egg production in chicken. FSH signal transduction is mediated by the FSHR [51]. In this study, 0.01 and 0.03% B. licheniformis have no obvious influence on FSHR transcriptions, but 0.06% B. licheniformis significantly down-regulate FSHR gene expressions. Although the FSHR expressions are reduced, FSH levels are much higher with B. licheniformis treatment [16]. Taken together, B. licheniformis can improve egg production and quality via increasing E2, FSH concentrations [16], and ESR expressions.
Besides the hormone receptors, we also detected the mRNA expressions of hormone genes GnRH, Ghrelin, and INH. Present findings suggest that GnRH gene expressions enhanced with B. licheniformis treatments, but Ghrelin and INH transcriptions decreased. The secretions of GnRH from the hypothalamus stimulate the release of luteinizing hormone (LH) and FSH, which in turn activates gonadal development and release of sex steroids, including E2 and testosterone [52] to regulate reproductive functions [53]. Ghrelin is a gut-brain peptide that functions in the regulation of growth hormone release and food intake [54]. Reports have demonstrated that Ghrelin production can inhibit the secretion of estrogen and GnRH [55, 56]. INH is a dimeric glycoprotein and is important for regulation of dominant follicle development [57]. The deletion of the α-subunit gene of INH markedly elevates serum concentrations of FSH and causes gonadal tumors in immature mice [57–60]. Therefore, the altered expressions of genes encoding GnRH, Ghrelin, and INH caused by B. licheniformis are important for the improvement of reproductive performance.
In summary, present experiments indicate that dietary B. licheniformis administration improves egg production and egg quality in laying hens via mechanisms as follows: (1) enhancing jejunal physical and immunological barriers, (2) regulating systemic immunity, (3) promoting gene expression of ESR and (4) positively modulating expression of genes encoding the hormones that are related to ovulation.
References
Khan SH, Atif M, Mukhtar N, Rehman A, Fareed G (2011) Effects of supplementation of multi-enzyme and multi-species probiotic on production performance, egg quality, cholesterol level and immune systemic in laying hens. J Appl Anim Res 39:386–398
Nahashon SN, Nakaue HS, Mirosh LW (1994a) Production variables and nutrient retention in single comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult Sci 73:1699–1711
Nahashon SN, Nakaue HS, Snyder SP, Mirosh LW (1994b) Performance of single comb White Leghorn layers fed corn-soybean meal and barley-corn-soybean meal diets supplemented with a direct-fed microbial. Poult Sci 73:1712–1723
Jin LZ, Ho YW, Abdullah N, Jalaludin S (1997) Probiotics in poultry: modes of action. World Poultry Sci J 53:351–368
Coates ME, Fuller R (1977) The genotobiotic animal in the study of gut microbiology. Microbial ecology of the gut. Academic Press, London, pp 311–346
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–215
Salianeha N, Shirzadb MR, Seifi S (2011) Performance and antibody response of broiler chickens fed diets containing probiotic and prebiotic. J Appl Anim Res 39:65–67
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl):396S–402S
Tannock GW (2001) Molecular assessment of intestinal microflora. Am J Clin Nutr 73:410S–414S
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Hagedorn SR, Bradley G, Chapman PJ (1985) Glutathione-independent isomerization of maleylpyruvate by Bacillus megaterium and other gram-positive bacteria. J Bacteriol 163:640–647
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220
Liu X, Yan H, Lv L, Xu Q, Yin C, Zhang K, Wang P, Hu J (2012) Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas J Anim Sci 25:682–689
Rozs M, Manczinger L, Vagvolgyi C, Kevei F (2001) Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol Lett 205:221–224
Kim Y, Cho JY, Kuk JH, Moon JH, Cho JI, Kim YC, Park KH (2004) Identification and antimicrobial activity of phenylacetic acid produced by Bacillus licheniformis isolated from fermented soybean, Chungkook-Jang. Curr Microbiol 48:312–317
Lei K, Li YL, Yu DY, Rajput IR, Li WF (2013) Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci 92:2389–2395
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius E (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15:443–452
Zuberbuehler CA, Messikommer RE, Arnold MM, Forrer RS, Wenk C (2006) Effects of selenium depletion and selenium repletion by choice feeding on selenium status of young and old laying hens. Physiol Behav 87:430–440
Cui X, Li Y, Liu R, Zheng M, Li Q, Wen J (2016) Follicle-stimulating hormone increases the intramuscular fat content and expression of lipid biosynthesis genes in chicken breast muscle. J Zhejiang Univ Sci B 17:303
Lei K, Li YL, Wang Y, Wen J, Wu HZ, Yu DY, Li WF (2015) Effect of dietary supplementation of Bacillus subtilis B10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J Zhejiang Univ Sci B 16:487–495
Hata H, Sakaguchi N, Yoshitomi H et al (2004) Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell–mediated spontaneous autoimmune arthritis in mice. J Clin Invest 114:582–588
Anderson JM, Van Italie CM, Fanning AS (2004) Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16:140–145
Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153
Deng W, Dong XF, Tong JM, Zhang Q (2012) The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci 91:575–582
Yousefi M, Karkoodi K (2007) Effect of probiotic Thepax® and Saccharomyces cerevisiae supplementation on performance and egg quality of laying hens. Int J Poult Sci 6:52–54
Yörük MA, Gül M, Hayirli A, Macit M (2004) The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens. Poult Sci 83:84–88
Zihni C, Balda MS, Matter K (2014) Signaling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci 127:3401–3413
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M (2009) Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296:G1140–G1149
Ukena SN, Singh A, Dringenberg U et al (2007) Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2:e1308
Resta-Lenert S, Barrett KE (2003) Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988–997
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069
Caballero-Franco C, Keller K, De Simone C, Chadee K (2007) The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292:G315–G322
Shimazu T, Villena J, Tohno M, Fujie H, Hosoya S, Shimosato T et al (2012) Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the toll-like receptor signaling pathway. Infect Immun 80:276–288
Perdigon G, Maldonado Galdeano C, Valdez JC, Medici M (2002) Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56(Suppl 4):S21–S26
Liu YL, Huang JJ, Hou YQ et al (2008) Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr 100:552–560
Huang Y, Li YL, Huang Q et al (2012) Effect of orally administered Enterococcus faecium EF1 on intestinal cytokines and chemokines production of suckling piglets. Pak Vet J 32:81–84
Al-Sadi R, Boivin M, Ma T (2009) Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14:2765–2778
Smit HF, Dwars RM, Davelaar FG, Wijtten GA (1998) Observations on the influence of intestinal spirochaetosis in broiler breeders on the performance of their progeny and on egg production. Avian pathol: journal of the WVPA 27:133–141
Izcue A, Coombes JL, Powrie F (2006) Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev 212:256–271
Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S (2001) Probiotics: effects on immunity. Am J Clin Nutr 73:444S–450S
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44:26–46
Benyacoub J, Czarnecki-Maulden GL, Cavadini C, Sauthier T, Anderson RE, Schiffrin EJ, von der Weid T (2003) Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J Nutr 133:1158–1162
Koenen ME, Kramer J, van der Hulst R, Heres L, Jeurissen SH, Boersma WJ (2004) Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br Poult Sci 45:355–366
Panda AK, Rama Rao SS, Raju VLN, Sharma SS (2008) Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J Sci Food Agr 88:43–47
Asli MM, Hosseini SA, Lotfollahian H, Shariatmadari F (2007) Effect of probiotics, yeast, vitamin E and vitamin C supplements on performance and immune response of laying hen during high environmental temperature. IntJ Poultry Sci 6:895–900
Klasing KC (1988) Nutritional aspects of leukocytic cytokines. J Nutr 118:1436–1446
van Eck JH (1983) Effects of experimental infection of fowl with EDS’76 virus, infectious bronchitis virus and/or fowl adenovirus on laying performance. Vet Q 5:11–25
Hampson DJ, McLaren AJ (1999) Experimental infection of laying hens with Serpulina intermedia causes reduced egg production and increased faecal water content. Avian Pathol 28:113–117
Smith CA, Andrews JE, Sinclair AH (1997) Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes. J Steroid Biochem Mol Biol 60:295–302
Zhao LH, Chen JL, Xu H, Liu JW, Xu RF (2010) Cloning and expression of FSHb gene and the effect of FSHβ on the mRNA levels of FSHR in the local chicken. Asian Austral J Anim 23:292–301
Wang XJ, Li Y, Song QQ, Guo YY, Jiao HC, Song ZG, Lin H (2013) Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. J Lipid Res 54:1860–1876
Ikemoto T, Park MK (2007) Comparative analysis of the pituitary and ovarian GnRH systemics in the leopard gecko: signaling crosstalk between multiple receptor subtypes in ovarian follicles. J Mol Endocrinol 38:289–304
Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S (2001) Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281:1220–1225
Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L (2004) Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362:103–107
Fernandez-Fernandez R, Tena-Sempere M, Roa J, Castellano JM, Navarro VM, Aguilar E, Pinilla L (2007) Direct stimulatory effect of ghrelin on pituitary release of LH through a nitric oxide-dependent mechanism that is modulated by estrogen. Reproduction 133:1223–1232
Jimenez-Krassel F, Winn ME, Burns D, Ireland JL, Ireland JJ (2003) Evidence for a negative intrafollicular role for inhibin in regulation of estradiol production by granulosa cells. Endocrinology 144:1876–1886
Matzuk MM, Finegold MJ, Su J-GJ, Hsueh AJW, Bradley A (1992) Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319
Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A (1994) Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A 91:8817–8821
Kumar TR, Wang Y, Matzuk MM (1996) Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology 137:4210–4216
Acknowledgements
This study was supported by The National 863 Project (Grant No. 013AA102803D) and the Key Science and Technology Program of Zhejiang Province, China (Grant No. 2006C12086).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal manipulations were conducted in accordance with the guidelines for animal welfare and approved by the Animal Welfare Committee of Animal Science College, Zhejiang University.
Rights and permissions
About this article
Cite this article
Wang, Y., Du, W., Lei, K. et al. Effects of Dietary Bacillus licheniformis on Gut Physical Barrier, Immunity, and Reproductive Hormones of Laying Hens. Probiotics & Antimicro. Prot. 9, 292–299 (2017). https://doi.org/10.1007/s12602-017-9252-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9252-3