Abstract
The main drawback of current antibiotic therapies is the emergence and rapid increase in antibiotic resistance. Nocardiae are aerobic, Gram-positive, catalase-positive, non-motile actinomycetes. Nocardia brasiliensis was reported as antibiotic producer. The purpose of the study was to determine antibacterial activity of N. brasiliensis PTCC 1422 against isolated Enterobacteriaceae from urinary tract infections (UTIs). The common bacteria from UTIs were isolated from hospital samples. Antimicrobial susceptibility test was performed for the isolated pathogens using Kirby–Bauer disk diffusion method according to clinical and Laboratory Standards Institute guideline. Antagonistic activity of N. brasiliensis PTCC 1422 was examined with well diffusion methods. Supernatant of N. brasiliensis PTCC 1422 by submerged culture was analyzed with gas chromatography–mass spectrometry. Isolated strains included Escherichia coli, Klebsiella pneumoniae, Serratia marcescens and Proteus mirabilis. The most common pathogen isolated was E. coli (72.5 %). Bacterial isolates revealed the presence of high levels of antimicrobial resistances to ceftriaxone and low levels of resistance to cephalexin. Supernatant of N. brasiliensis PTCC 1422 showed antibacterial activity against all of the isolated microorganisms in well diffusion method. The antibiotic resistance among the uropathogens is an evolving process, so a routine surveillance to monitor the etiologic agents of UTI and the resistance pattern should be carried out timely to choose the most effective empirical treatment by the physicians. Our present investigation indicates that the substances present in the N. brasiliensis PTCC 1422 could be used to inhibit the growth of human pathogen. Antibacterial resistance among bacterial uropathogen is an evolving process. Therefore, in the field on the need of re-evaluation of empirical treatment of UTIs, our present. The study has demonstrated that N. brasiliensis PTCC 1422 has a high potential for the treatment of UTIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nocardia spp., strictly aerobic actinomycetes, are Gram-positive, weakly acid-fast and dichotomous branching bacilli. They are essentially soil saprophytes worldwide and involve in the decomposition of plant material. They are not normal flora in humans or animals [18]. Urinary tract infections (UTIs) are one of the most prevalent bacterial infections. Worldwide, about 150 million people are diagnosed with UTIs each year. The most common infection caused by opportunistic Enterobacteriaceae is a urinary tract infection (UTI). Enterobacteriaceae are Gram-negative, facultatively anaerobic rods belonging to the γ-class of proteobacteria. The improper and uncontrolled use of many antibiotics resulted in the occurrence of antimicrobial resistance, which becomes a major health problem worldwide. Multiple drug resistance has significantly increased in recent years [2, 14, 16]. Multiple antimicrobial resistances among Gram-negative organisms have been a long-term and well-recognized problem with urinary tract infections. GC-MS is used for the identification and quantification of bioactive compounds of N. brasiliensis [8, 12].
Experimental designs are excellent techniques for the optimization of culture conditions to achieve optimal production [1, 3]. One of the most promising sources of bioactive compounds is N. brasiliensis, which was reported as an antibiotic producer [6, 9, 11]. This study was designed with the aim of screening of antibacterial compounds production by N. brasiliensis PTCC 1422 against isolated Enterobacteriaceae from UTIs.
Materials and Methods
Samples (urines) were collected from UTI patients of North Hospital of Iran. Preliminary isolation and identification were based on the microscopic, cultural characteristics and other standard biochemical analysis [2, 16]. Bacterial strains isolates were cultured on nutrient agar and incubated at 30 °C. The strains were carried out for Gram staining and shape under light microscope. Various biochemical tests were performed for the identification of isolated bacteria according to Bergey’s Manual of Systematic Bacteriology.
The most prevalent organisms were chosen and subjected to antimicrobial sensitivity test. The AST for each isolate was carried out on Muller–Hinton agar by Kirby–Bauer disk diffusion technique. The microorganism suspensions used for inoculation were prepared at 108 CFU (colony-forming units)/ml by diluting fresh cultures at McFarland 0.5. The several antibiotics (Himedia Co.) were used for the antibiotic sensitivity test. Standardization of the technique controls variation in results, and interpretation is based on comparison of inhibition zones with published criteria for zone diameters [13, 14].
N. brasiliensis PTCC 1422 was grown in 50 ml of starch casein broth by submerged culture containing in 250-ml flasks, incubated at 28 °C in a shaker (150 rpm) for 7 days and centrifuged at 4000 rpm for 10 min, and the clear supernatant broth samples were tested for their antagonistic activity against the isolated pathogens by agar well diffusion method [15, 20]. Wells of 6 mm diameter were prepared in the nutrient agar plates. Isolated pathogenic bacteria were swabbed onto the nutrient agar surface [10]. The wells were filled with the 70 μl of culture supernatant, and the diameter of inhibition zones was measured after incubation for 24 h at 37 °C.
GC-MS Analysis
In this technique, the carrier gas was helium (99.999 %), linear velocity was 30 cm/s, and temperature of the split–splitless inlet was 220 °C (splitless time −1.0 min). Oven temperature program was as follows: The initial temperature of 60 °C was kept for 2 min and then raised 8 °C/min to 220 °C (kept for 4 min). The sample was ionized by electron ionization (EI) at an energy of 70 eV and mass spectrometry (quadrupole) ion range m/z = 35/500 atomic mass unit (amu) (quant. ions 95, 107, 108, 195, 197, 212, 112, 125 and 149, or ions for selected ion monitoring). The temperature of the interface was 200 °C [7].
Results
The microorganisms were confirmed as Escherichia coli (72.5 %), Klebsiella pneumoniae (3.2 %), Serratia marcescens (0.8 %) and Staphylococcus aureus (14.1 %) (Fig. 1). By standard confirmatory tests, all the isolates were found to be Gram-negative. According to Bergey’s Manual of Determinative Bacteriology and the Laboratory Manual for Identification of Bacteria, the isolates were identified. The four isolates obtained were subjected to study morphological and biochemical characteristics (Table 1).
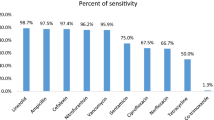
Antibiotic resistance pattern revealed that these bacteria were highly resistance to ceftriaxone and showed the lowest percentage of resistance to cephalexin. High rate of multiple drug resistance was recorded among all isolates. The resistance rates of bacteria isolated from UTIs against different antibiotics are presented in Table 2.
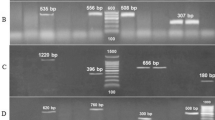
N. brasiliensis PTCC 1422 was able to produce antibacterial supernatant against Enterobacteriaceae organisms. The formation of inhibition zone around the pathogenic strains was due to the production of secondary metabolites by N. brasiliensis PTCC 1422 (Fig. 3). Frequency of antimicrobial activity of N. brasiliensis against isolated bacteria from UTIs is shown in Fig. 2.
The highest concentrations of determined compounds in real-level samples of N. brasiliensis by GC-MS method are presented in Table 3. Phthalic acid as highest concentration of supernatant of N. brasiliensis was determined.
Discussion
The increasing rate of UTIs pathogens resistance to commonly used antibiotics, coherent prescription and use of antibiotics is advocated. With the increasing use of antibiotics, the serious problem of antibiotic resistance is gradually increasing [4, 17]. Thus, the result of the present investigation revealed that N. brasiliensis PTCC 1422 is the potent source of novel antibiotics and was found to be of potential antagonistic against test organisms which can control variety of pathogenic organisms. The GC-MS method for the determination of bioactive compounds in N. brasiliensis, which was optimized in this study, can be useful in the routine analysis in laboratories. The isolates showed their resistance to different antibiotics. This widespread resistance could be attributed to excessive or indiscriminate use of antibiotics. N. brasiliensis PTCC 1422 exhibited high antibacterial activity against Enterobacteriaceae including E. coli (48 %), K. pneumonia (36.3 %), P. mirabilis (24.1 %) and S. marcescens (33.3 %) in well diffusion method, and the inhibition zones were in the range of 7–19 mm. Similar study indicated the antagonistic activity of actinomycetes isolates such as Nocardia against human pathogen (S. aureus, Proteus vulgaris, P. aeroginosa, E. coli, B. subtilis, B. megaterium, K. pneumoniae, C. albicans, A. niger, S. cervisiae), and the inhibition zones were in the range of 7–20 mm [5, 15, 19]. Thus, in our present investigation, the result indicates that the substances present in the N. brasiliensis PTCC 1422 could be used to inhibit the growth of human pathogen. The supernatant of N. brasiliensis PTCC 1422 was analyzed by GC-MS, and phthalic acid was most fraction. The chemical analysis compounds can find places in the database for the development of antimicrobial substances. This study has demonstrated that N. brasiliensis PTCC 1422 has a high potential for the treatment of UTIs.
Conclusion
Antibacterial resistance among bacterial uropathogen is an evolving process. Therefore, in the field on the need of re-evaluation of empirical treatment of UTIs, our present investigation indicates that the substances present in the N. brasiliensis PTCC 1422 could be used to inhibit the growth of human pathogen. The study has demonstrated that N. brasiliensis PTCC 1422 has a high potential for the treatment of urinary tract infections (UTIs).
References
Annadurai G (2000) Design of optimum response surface experiments for adsorption of direct dye on chitosan. Bioprocess Eng 23(5):451–455
Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, Raz R (2004) Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis 23(3):163–167
El-Helow E, Sabry S, Amer R (2000) Cadmium biosorption by a cadmium resistant strain of Bacillus thuringiensis: regulation and optimization of cell surface affinity for metal cations. Biometals 13(4):273–280
Karatzas KA, Webber MA, Jorgensen F, Woodward MJ, Piddock LJ, Humphrey TJ (2007) Prolonged treatment of Salmonella enterica serovar Typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J Antimicrob Chemother 60(5):947–955
Khucharoenphaisan K, Sripairoj N, Sinma K (2012) Isolation and identification of actinomycetes from termite’s gut against human pathogen. Asian J Anim Vet Adv 7:68–73
Komatsu K, Tsuda M, Shiro M, Tanaka Y, Mikami Y, Kobayashi JI (2004) Brasilicardins B–D, new tricyclic terpernoids from actinomycete Nocardia brasiliensis. Bioorg Med Chem 12(21):5545–5551
Ligor M, Buszewski B (2006) An investigation of the formation of taste and odour contaminants in surface water using the headspace SPME-GC/MS method. Pol J Environ Stud 15(3):429–435
Maharjan S, Koju D, Lee HC, Yoo JC, Sohng JK (2012) Metabolic engineering of Nocardia sp. CS682 for enhanced production of nargenicin A1. Appl Biochem Biotechnol 166(3):805–817
Mikami Y, Yazawa K, Nemoto A, Komaki H, Tanaka Y, Graefe U (1999) Production of erythromycin E by pathogenic Nocardia brasiliensis. J Antibiot 52(2):201–202
Mitra A, Santra SC, Mukherjee J (2008) Distribution of actinomycetes, their antagonistic behaviour and the physico-chemical characteristics of the world’s largest tidal mangrove forest. Appl Microbiol Biotechnol 80(4):685–695
Mukai A, Fukai T, Matsumoto Y, Ishikawa J, Hoshino Y, Yazawa K, Mikami Y (2006) Transvalencin Z, a new antimicrobial compound with salicylic acid residue from Nocardia transvalensis IFM 10065. J Antibiot 59(6):366–369
Noor N, Ajaz M, Rasool SA, Pirzada ZA (2004) Urinary tract infections associated with multidrug resistant enteric bacilli: characterization and genetical studies. Pak J Pharm Sci 17(2):115–123
Pitout JD (2010) Infections with extended-spectrum β-lactamase-producing Enterobacteriaceae. Drugs 70(3):313–333
Pitout JD, Nordmann P, Laupland KB, Poirel L (2005) Emergence of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) in the community. J Antimicrob Chemother 56(1):52–59
Rakshanya U, Shenpagam H, Devi DK (2011) Antagonistic activity of actinomycetes isolates against human pathogen. J. Microbiol Biotech Res 1(2):74–79
Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP et al (2008) Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch Intern Med 168(17):1897–1902
Singh N, Rai V (2013) In vitro antimycotic activity of a new isolate Streptomyces fradiae MTCC 11051 against the multi-drug resistant pathogenic fungi. J Pharm Res 7(4):331–336
Srifuengfung S, Poonwan N, Tribuddharat C, Chokephaibulkit K (2007) Prevalence of nocardia species isolated from patients with respiratory tract infections at Siriraj Hospital, Thailand. J Infect Dis Antimicrob Agents 24:1–6
Verma VC, Gond SK, Kumar A, Mishra A, Kharwar RN, Gange AC (2009) Endophytic actinomycetes from Azadirachta indica A. Juss.: isolation, diversity, and anti-microbial activity. Microb Ecol 57(4):749–756
Vimal V, Rajan BM, Kannabiran K (2009) Antimicrobial activity of marine actinomycete, Nocardiopsis sp. VITSVK 5 (FJ973467). Asian J Med Sci 1(2):57–63
Acknowledgments
This study was supported by Department of Microbiology, Faculty of Sciences, Lahijan Branch, Islamic Azad University, Lahijan, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hossnieh Kafshdar Jalali, Abdolreza Salamatzadeh, Arezou Kafshdar Jalali, Hamed Haddad Kashani, Salman Ahmadi Asbchin and Khosro Issazadeh declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jalali, H.K., Salamatzadeh, A., Jalali, A.K. et al. Antagonistic Activity of Nocardia brasiliensis PTCC 1422 Against Isolated Enterobacteriaceae from Urinary Tract Infections. Probiotics & Antimicro. Prot. 8, 41–45 (2016). https://doi.org/10.1007/s12602-016-9207-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9207-0