Abstract
Enhanced production of heterologously expressed plantaricin (plnE) from Escherichia coli BL21 (DE3) was achieved from a small- to large-scale batch culture. Starting from a 15-ml shake-flask culture grown in Luria–Bertani (LB) broth, the protein expression could be scaled up using 50 ml, 100 ml, 1 l, and 2 l batch culture. Using similar condition, plantaricin E (PlnE) was successfully expressed in a 30-l stirred fermenter. The protein was expressed as TRX-(His)6-fusion protein and separated by Ni2+ affinity chromatography. Growth in two complex media, LB and Terrific broth (TB), was optimized and compared for the production of PlnE, which was higher in LB in comparison with that of TB. In the fermenter, 140 and 180 mg of PlnE could be produced from 12 l of culture volume at 30 and 25 °C, respectively. The yield of heterologously purified PlnE was found to be 1.2–1.5 %, which was much higher in comparison with the plantaricins produced from the native strain of Lactobacillus plantarum (0.3–0.7 %). Overproduction of PlnE with the help of heterologous expression can overcome the constraint of the low yield from producer strain and provides an easy and low-cost strategy for large-scale production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriocins of lactic acid bacteria (LAB) are defined as ribosomally synthesized proteins or protein complexes usually antagonistic to genetically closely related organisms [1]. In the last decade, some workers have demonstrated that several of these bacteriocins are also active against certain pathogenic Gram-negative bacteria [2–4]. Bacteriocins have not only been recommended for food biopreservation to control food spoilage microorganisms, but they have also been considered as therapeutic molecules for medical and veterinary use, which can inhibit the growth of pathogenic microorganisms [5, 6]. Their narrow inhibitory spectrum can be beneficial as they would inhibit certain pathogens without affecting the commensal microbiota. This is in contrast to the indiscriminate killing of microorganisms by conventional antibiotics that can disrupt the ecological balance of the indigenous microbiota, often resulting in negative clinical consequences [7, 8]. Moreover, resistance to several of these antibiotics is emerging fast but resistance to bacteriocins is fairly uncommon [9].
Lactobacillus plantarum is a very versatile species producing many different types of plantaricins. Different bacteriocinogenic strains of L. plantarum have potent antimicrobial activity and also play an essential role in natural biopreservation of the food products [10, 11]. Several plantaricins, such as plantaricin EF, JK, MG, UG1, 423, S, W, and LR/14, have been reported in the literature, having strong antimicrobial activity against a large number of species [4, 12–15].
Large quantities of highly pure protein are a prerequisite for various researches, involving high-throughput screening, structure–function analysis and any further application. Plantaricins, like other bacteriocins, can be purified by standard methods of three, four, or more steps, but peptide recovery is generally low [16]. We have already shown that the constraint of low yield can be overcome by expressing these peptides in a heterologous system of Escherichia coli [15]. The interest in these plantaricins has emanated from the fact that they exhibit significant antimicrobial activity against a broad range of bacteria that include food spoilage organisms, such as Listeria and several other Gram-positive organisms [15].
In this study, expression of plnE in E. coli BL21 (DE3) was monitored from small-scale shake-flask condition to large-scale batch culture. A comparison of growth profile and protein production of PlnE was done using two different complex media, Luria–Bertani (LB) and Terrific broth (TB), and its production was studied in a fermenter (30 l), using optimized culture conditions.
Materials and Methods
Growth and Culture Conditions
Plantaricin gene (plnE) was amplified from soil metagenome and cloned in pET32a(+) vector (Novagen, USA) using E. coli XL1 blue competent cells [15]. Ampicillin (50 µg/ml) was used as selection marker. The strain was maintained in glycerol stock (−80 °C). For protein expression, it was freshly transformed in E. coli BL21 (DE3) and the strain harbouring recombinant plasmid was grown in LB (Himedia, India) at 37 °C, 200 rpm in a Controlled Environment Shaker Incubator (Kuhner, Biogentek, Switzerland). In one experiment, comparative analysis of growth and protein expression was carried out, wherein the strain was grown in LB and TB [24 g Tryptone (Himedia, India), 12 g yeast extract (Himedia, India), 9.4 g potassium diphosphate (Himedia, India), 2.2 g potassium monophosphate (Himedia, India), and 4 ml glycerol (Sigma-Aldrich, USA), per litre, pH 7.0]. In the next case, large-scale production of plantaricin was carried out in industrial-level fermenter.
Expression Profile of Protein in Batch Cultivation
Primary culture was raised from freshly transformed colony and grown in LB overnight at 37 °C at 200 rpm, in the presence of 50 µg/ml of ampicillin (Sigma-Aldrich, USA). Subculturing with 1 % inoculums was done at 37 °C, 200 rpm, and as OD600 reached 0.6, cultures were induced by addition of 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, USA). This was further grown for additional 4 h at 30 and 37 °C, and 5 h at 16 and 25 °C. Initially, 15 ml of culture was checked for protein expression. For protein expression, cells were harvested by centrifugation (Sigma, USA) at 10,000g for 10 min and processed as described later. Gradual scaling up was done using 50 ml, 100 ml, 1 l, and 2 l cultures under shake-flask optimized growth conditions, and the level of protein expression was monitored.
For comparative analysis of growth and protein expression, recombinant E. coli strain expressing plnE was grown in LB and TB. Primary culture (initial OD600 ~ 0.01) was raised in LB in the presence of 50 µg/ml of ampicillin, overnight at 37 °C, 200 rpm. Secondary culture was raised in 100 ml in LB and TB at 37 °C, 200 rpm. After 2 h, the temperature was lowered to 22 °C for 30 min and 0.5 mM of IPTG induction was given. Uninduced control cells in both LB and TB were grown parallely. The growth of all the samples was measured spectrophotometrically (Bio-Rad, USA) (OD600) at an interval of 2 h. After a further growth of 5 and 9 h post-induction, cells were harvested by centrifugation at 10,000g for 10 min, and total proteins were isolated as described later. An aliquot from both the induced and uninduced samples from the two media was serially diluted and plated on LB agar plate, and the number of colonies was compared in terms of CFU/ml. Survivability of recombinant strains was checked by patching some 100 colonies (from both LB and TB) randomly on LB agar containing ampicillin (50 µg/ml).
Large-Scale Production in Fermenter
In the present investigation, plantaricin (PlnE) production was scaled up in a 30-L stirred bioreactor [Scigenics (India) Pvt. Ltd]. Twelve litres of LB medium (pH 7.0) prepared and sterilized in situ was inoculated with 1.2 % inoculum raised as overnight growth in LB. The impeller was maintained at 200 rpm, and compressed air was sparged into the medium at a constant rate of 0.5 vvm. The fermentation parameters, such as temperature, pH, dissolved oxygen, and airflow, were continuously monitored. The experiment was carried out at two particular temperatures, 30 and 25 °C, respectively. When OD600 reached 0.4, both the cultures were induced with IPTG (0.5 mM). During the fermenter run, the foaming was controlled by using appropriate amount of silicon oil as anti-foaming agent. Samples (10 ml) were withdrawn periodically at 1-, 2-, and 3-h interval post-induction, and OD600 was checked accordingly. The withdrawn samples were centrifuged at 10,000g, 10 min, and the cell pellet was processed for protein purification.
Protein Purification
Purification of (His)6-TRX-PlnE from E. coli BL21 (DE3) was carried out with the help of Ni–NTA affinity chromatography [15]. Briefly, cell pellet obtained was suspended in lysis buffer containing 20 mM Tris, 50 mM NaCl, and 10 mM imidazole (pH 8.0). The resuspended cell was disrupted by sonication for 3 min, using 30 % amplitude (Sonics, USA), at room temperature (25 °C), and the samples were kept on ice. Supernatant as cell-free extract was collected by centrifugation (8000g, 10 min, 4 °C) and was used as soluble fraction. This was loaded onto Ni–NTA column (Qiagen, Germany), and purification of protein was done using Ni–NTA affinity chromatography.
The bound PlnE protein was eluted in different imidazole concentrations of 100, 200, 300, and 500 mM. Eluted fractions were resolved for expression of recombinant PlnE from small scale to bioreactor using 15 % SDS-PAGE following the protocol described by [17]. The gel was stained with Coomassie Brilliant Blue. The purified protein was quantified following BCA method and by absorbance at 280 nm (Bio-Rad, USA).
Protein Quantification
The concentration of purified PlnE was determined by BCA method using bicinchoninic acid kit for protein determination (BCA1-1KT; Sigma-Aldrich, USA).
Results
In small-scale (15 ml) shake-flask cultures of E. coli BL21 (DE3) raised in LB, first the recombinant PlnE was expressed at four different growth temperatures, (16, 25, 30, and 37 °C) (Fig. 1). The TRX (His)6-PlnE could be recovered in soluble fraction, under all of these conditions. After enterokinase treatment, both TRX and histidine tags were removed and cleaved PlnE was obtained (Fig. 2).
SDS-PAGE analysis showing expression of Pln-fusion protein in E. coli. PlnE: induced sample at 16 °C (lane 1), uninduced sample at 16 °C (lane 2), molecular mass marker, Sigma (lane 3), induction at 25 °C (lane 4), uninduced sample at 25 °C (lane 5), induction at 30 °C (lane 6), uninduced sample at 30 °C (lane 7), induction at 37 °C (lane 8), uninduced sample at 37 °C (lane 9)
Small-scale test expression is widely used as a predictive tool to determine the conditions required to produce soluble protein and to establish the optimal scale for the large-scale growth. Since maximum expression was observed when IPTG induction was given at 25 °C, further scaling up of plantaricin production was done from 100 ml to 2 l of batch culture at this temperature. When 100 ml batch culture was raised, the eluted fused protein was quantified to be in 1.0 mg and from 2 l of batch culture, the yield of fused PlnE was found to be 21 mg.
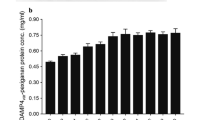
The effect of growth medium on protein yield was quantified and compared using LB and TB, as described in ‘Materials and Methods’. In TB, no expression of protein was observed when cultures were grown above 25 °C. With lowering of temperature (22 °C), expression of PlnE was observed in TB-grown cells, but to a lesser level in comparison with LB. After a brief acclimatization (30 min) to lower temperature, both the cultures were induced with IPTG (0.5 mM). At this stage of induction in LB and TB, the OD600 of cultures was 0.6 and 0.4, respectively. The growth was monitored turbidimetrically for 12 h. As shown in Fig. 3a, initially the growth was higher in LB, but subsequently, the growth of the induced samples was almost similar in the two media. In comparison, however, uninduced TB-grown cells maintained higher growth profile compared to the cells grown in LB (Fig. 3b). The viable cell count (CFU/ml) determined by plating 5- and 9-h induced cells also revealed the similar results.
Both the uninduced and induced cells, grown LB and TB, were harvested by centrifugation and processed for protein isolation as described in Materials and Methods. After 9-h induction, total protein in both types of cell was estimated to be ~25 mg/ml. Of these, the proportion of TRX-PlnE(His)6 protein was quantified to be 1.2 and 0.76 mg/ml, respectively.
As the yield of recombinant PlnE protein was higher in LB, large-scale production was carried out in a stirred bioreactor in a culture volume of 12 l. When grown at 30 and 25 °C, the growth of cells (OD600) was found to be 0.4 after 2 and 3 h, respectively. After IPTG induction at 30 °C, OD600 was found to be 0.6, 0.75 and 0.9 at 1, 2, and 3 h, respectively. When grown at 25 °C, the OD600 after 1-, 2-, 3-, and 4-h interval was 0.56, 0.7, 0.85, and 1.0, respectively. After 3-h induction, the yield of purified plantaricin E protein in 12 L of LB was 140 and 180 mg from 30 and 25 °C, respectively. All the randomly chosen recombinant strains were found to survive in presence of ampicillin. The entire work has been explained schematically in Table 1.
Discussion
Several studies have been performed on heterologous expression to improve the yield of bacteriocins and other antimicrobial compounds [18, 19]. In this study, it has been clearly demonstrated that the production of recombinant plantaricin can be achieved from a heterologous host, E. coli. Since the previous work published by our group showed that the recombinant plantaricins were obtained in active and soluble form and had strong antimicrobial activity [4, 15], it was therefore logical to produce them in large-scale batch cultures to increase their production. Further, one of the long-sought goals in recombinant protein production is to achieve a high-level expression of cloned genes. As shown by this study, the yield (in terms of mg/l) was maintained in low as well as high production culture volume and the feasibility of the elevated levels of desired protein production in optimized media has been established.
Growth and medium compositions are the most important parameters which influence the level of accumulation of a protein [20]. In uninduced condition, the growth of recombinant plnE strain was higher in TB in comparison with LB, but in induced condition, it followed a more or less similar pattern. It was also observed that the cell density of recombinant BL21 cells growing in TB was very high at 37 °C, and there was no or little expression of PlnE. The lowering of temperature led to some protein expression. In case of LB also, the expression of PlnE was more in 25 °C in comparison with 37 °C. Similar result was observed when the culture was raised in large-scale fermenter. Though E. coli optimal growth temperature is 37 °C, lower temperatures have been recommended to limit the aggregation of recombinant proteins so as to bring them in soluble state [20, 21]. Generally, expression at low temperature condition leads to increased stability and correct folding patterns, not allowing the hydrophobic interaction to occur as inclusion body formation is temperature dependent. Any expression associated with toxic phenotype observed at 37 °C incubation conditions gets suppressed at low temperature [22]. Moreover, slower rates of protein production allow newly transcribed recombinant proteins to fold properly [23]. The higher cell density observed in uninduced recombinant strain carrying PlnE, grown in TB, is a common observation as recombinant protein production imposes a significant stress in the organism.
The optimization of cell growth conditions and media thus seems to be target protein dependent, and there does not seem to be any empirical rules reported to date on this aspect. It has been amply demonstrated that production of these bacteriocins/antimicrobial peptides is used as a strategy to gain a competitive advantage by producer organism. What is not known is whether LAB produce them in different ecological niches under most growing conditions. In E. coli, for example, colicin production is favoured under nutrient-poor conditions, because there seems to be a trade-off between the cost and benefit of production of antimicrobial compounds [24, 25]. Interestingly, our results show that the same rule may apply to a foreign protein expressed in E. coli. However, there was no plasmid loss as the presentative population from both the culture media was able to survive on ampicillin, used as plasmid marker, indicating the high stability of recombinant plasmids.
Further to our study on enhancing the level of PlnE in increasing production volume, yield enhancement could be established in a stirred bioreactor. In order to be commercially viable, any cultivation method has to meet a number of criteria, which include a high volumetric productivity, a high final concentration, stability, and reproducibility of the process and the applicability of low-cost, high soluble substrates. Notwithstanding their usefulness in laboratory environments, batch cultures are not always suitable for large-scale production. Since, in batch cultures, the growth factors cannot be controlled, growth rapidly becomes limited by reduced oxygen and other metabolic by products [26]. In our case, at 30 °C, production of soluble and active PlnE obtained in batch culture was more or less similar than that of the culture raised in fermenter. Production of PlnE was much higher when the strain was grown in 25 °C than in 30 °C.
The yield of purified PlnE was found to be 1.2–1.5 % at 30 and 25 °C, respectively, which was much higher in comparison with the plantaricins produced from the culture filtrate of native strain of L. plantarum (0.3–0.7 %) [27]. Thus, the present work explains the feasibility of cost-effective active plantaricin production from shake flask to the industrial level of a fermenter.
Summary
This study was successfully accomplished in two steps: first, optimization of media by comparing growth profile in two enriched media; second, production of plantaricin E in milligram quantities in fermenter. The protein was present in the soluble form and found to be highly stable, irrespective of the culture condition, which may serve an economic and low-cost strategy for the biotechnological large-scale production of plantaricin for various applications.
References
Nes IF, Johnsborg O (2004) Exploration of antimicrobial potential in LAB by genomics. Curr Opin Biotechnol 15:100–104
Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS (2006) Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother 50:3111–3116
Svetoch EA, Eruslanov BV, Levchuk VP, Perelygin VV, Mitsevich EV, Mitsevich IP, Stepanshin J, Dyatlov I, Seal BS, Stern NJ (2011) Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl Environ Microbiol 77:2749–2754
Pal G, Srivastava S (2014) Inhibitory effect of plantaricin peptides (Pln E/F and J/K) against Escherichia coli. World J Microbiol Biotechnol 30:2829–2837
Montalban-Lopez M, Sanchez-Hidalgo M, Valdiva E, Martinez-Bueno M, Maqueda M (2011) Are bacteriocins underexploited? Novel applications for old antimicrobials. Curr Pharmacol Biotechnol 12:1205–1220
Lohans CT, Vederas JC (2012) Development of class IIa bacteriocins as therapeutic agents. Int J Microbiol. doi:10.1155/2012/386410
Eckert R, Qi F, Yarbrough DK, He J, Anderson MH, Shi W (2006) Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob Agents Chemother 50:1480–1488
Rea MC, Sit CS, Claytona E, O’Connor PM, Whittal RM, Zheng J, Vederas JC, Ross RP, Hill C, Thuricin CD (2010) A post translationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci USA 18:9352–9357
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Todorov SD (2009) Bacteriocins from Lactobacillus plantarum—production, genetic organization and mode of action. Braz J Microbiol 40:209–221
Dalie DKD, Deschamps AM, Richard-Forget F (2010) Lactic acid bacteria-potential for mould growth and mycotoxins: a review. Food Control 21:370–380
Fimland N, Rogne P, Fimland G, Nissen-Meyer J, Kristiansen PE (2008) Three dimensional structure of the two peptides that constitute the two-peptide bacteriocin plantaricin EF. BBA-Proteins and Proteomics 1784:1711–1719
Nissen-Meyer J, Oppegård C, Rogne P, Haugen HS, Kristiansen PE (2010) Structure and mode-of-action of two-peptide (class IIb) bacteriocins. Probiotics Antimicrob Proteins 2:52–60
Siezen RJ, van Hylckama Vlieg JET (2011) Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact 201110(Suppl 1):S3
Pal G, Srivastava S (2013) Cloning and heterologous expression of plnE, -F, -J, and -K genes derived from soil metagenome and purification of active plantaricin peptides. Appl Microbiol Biotechnol 98:1441–1447
Sarika AR, Lipton AP, Aishwary MS (2012) Comparative assessment of bacteriocin production in free and immobilized Lactobacillus plantarum MTCC B1746 and Lactococcus lactis MTCC B440. J Appl Sci Res 8:2197–2202
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Basanta A, Herranz C, Gutierrez J, Criado R, Hernandez PE, Cintas LM (2010) Use of the yeast Pichia pastoris as an expression host for secretion of enterocin L50, a leaderless two-peptide (L50A and L50B) bacteriocin from Enterococcus faecium L50. Appl Environ Microbiol 76:3314–3324
Borrero J, Kunze G, Jiménez JJ, Böer E, Gútiez L, Herranz C, Cintas LM, Hernández EP (2012) Cloning, production, and functional expression of the bacteriocin enterocin A, produced by Enterococcus faecium T136, by the yeasts, Pichia pastoris, Kluyveromyes lactis, Hansenula polymorpha and Arxula adeninivorans. Appl Environ Microbiol 78:5956–5961
Sivashanmugam A, Murray V, Cui C, Zhang Y, Wang J, Li Q (2009) Practical protocols for production of very high yields of recombinant proteins using Escherichia coli. Protein Sci 18:936–948
Tripathi NK, Shrivastva A, Biswa KC, Rao PVL (2007) Optimization of culture medium for production of recombinant dengue protein in Escherechia coli. Ind Biotechnol 46:105–113
Sahdev S, Khattar SK, Saini KS (2008) Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Mol Cell Biochem 307:249–264
Vera A, Gonzalez-Montalban N, Aris A, Villaverde A (2007) The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol Bioeng 96:1101–1106
Riley MA, Gordon DM (1999) The ecological role of bacteriocins in bacterial competition. Trends Microbiol 7:129–133
Eijsink VGH, Axelsson L, Diep DB, Havarstein LS, Holo H, Nes IF (2002) Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 81:639–654
Hensing MCM, Rouwenhoorst RJ, Heijnen JJ, van Dijken JP, Pronk JT (1995) Physiological and Technological aspects of large scale heterologous-protein production with yeasts. Anton Leeuw Int J G 67:261–279
Tiwari SK, Srivastava S (2008) Purification and characterization of plantaricin LR14: a novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl Microbiol Biotechnol 79:759–767
Acknowledgments
The authors acknowledge financial assistance and the facilities supported by University Grant Commission (SAP) and Department of Science and Technology (FIST), Government of India in Department of Genetics, UDSC. SS acknowledges the financial assistance from Department of Biotechnology and GP acknowledges Council of Scientific and Industrial Research (CSIR), Government of India, for providing fellowship. Authors are thankful to Prof. R. K. Saxena, Department of Microbiology, for the Fermentor facility at TBI, South Campus, University of Delhi.
Conflict of interest
Gargi Pal and Sheela Srivastava declares that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pal, G., Srivastava, S. Scaling Up the Production of Recombinant Antimicrobial Plantaricin E from a Heterologous Host, Escherichia coli . Probiotics & Antimicro. Prot. 7, 216–221 (2015). https://doi.org/10.1007/s12602-015-9193-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-015-9193-7