Abstract
Late blight disease caused by Phytophthora infestans is an important yield reducing, prolific, and destructive pathogen of Solanaceae family members, mainly tomato and potato. P. infestans may cause entire crop loss unless controlled by chemical and cultural control measures. The Cukurova region of Turkey is one of the main potato-producing areas of Turkey and climatic conditions incite P. infestans to develop and cause severe yield losses in the region. A total of 186 isolates of P. infestans were obtained through survey studies conducted during the 2013–2014 potato-growing seasons in the Cukurova region comprised of Hatay, Adana and Mersin provinces in Turkey. All the isolates were analyzed for their metalaxyl resistance, mating type, mitochondrial DNA (mtDNA) haplotype and allozyme genotype diversity. P. infestans isolates were metalaxyl sensitive and both mating types were found in the potato-growing areas of the Cukurova region. The A1 mating type was more common (68.8%) than A2 (22.5%) and 8.6% of the P. infestans isolates were self-compatible. mtDNA haplotypes were diverse in the region and Ia was the most common type. Allozyme analyses revealed that the US-1 (Gpi 86/100) and US-6 (Gpi 100/100) patterns were prevalent in the potato-growing areas of the Cukurova region. This study contains the first data on molecular and biochemical characterization of potato late blight in Turkey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora infestans (Mont.) de Bary, is the causal agent of late blight disease primarily infecting potato and tomato in addition to wild species within the Solanaceae family. P. infestans has also a great potential to form new pathotypes and mutations, heterokaryosis and parasexual reproduction play parts in pathotype formation and genotypic differentiation (Goodwin 1997). The disease is known in Europe since the 1840s and caused epidemics in almost all potato growing regions. If the epidemics is not controlled, 100% yield loss may occur (Shtienberg et al. 1990; Fry et al. 1993).
P. infestans is a hemibiotrophic organism belongs to Oomycote class and a heterothallic species, i.e. the two mating types (A1 and A2) are necessary for the formation of oospores (Galindo and Gallegly 1960; Judelson 1997). The A2 mating type was known to exist only in central Mexico (Fry et al. 1993), until its detection in Switzerland in 1981, and report in 1984 (Hohl and Iselin 1984). In Western Europe the P. infestans population is dominated by aggressive genotypes including 13_A2 (also known as Blue_13) and others of either A1 or A2 mating types (Dey et al. 2018). The highly aggressive and dominant European genotype 13_A2 was first recorded in 2004 in the Netherlands and Germany and has subsequently been found in China and India (Cooke et al. 2012; Li et al. 2013; Chowdappa et al. 2013) In Estonia A1 and A2 mating type isolates were detected from every studied field indicating the high potential for sexual reproduction, which raises the genotypic diversity in P. infestans population (Runno-Paurson et al. 2016). In Turkey, the A2 mating type was first detected from west Turkey tomato growing areas during the survey studies conducted in 1999–2000 (Tosun et al. 2007).
Late blight disease is controlled by use of fungicide applications such as metalaxyl but it leads to development of resistance (Fry 2008). Metalaxyl was first used for control of late blight in Europe in the late 1970s (Dowley and O’Sullivan 1981). Some strains including the US-1 clonal lineage are reported sensitive to the fungicide metalaxyl and mefenoxam, while many other strains have developed resistance to these fungicides (Ristaino 2006). Previously it has been proposed that US-1 (A1 mating type and mitochondrial haplotype Ib) was the dominant genotype of potato late blight in the world until the end of the 1970s, or that it was a genotype closer to modern strains than to US-1. Yoshida et al. (2013) have ruled out both of these possibilities and showed that the lineage, which they call HERB-1, is clearly distinct from US-1, although they are closely related, and they conclude that both HERB-1 and US-1 might have dispersed from a common ancestor that existed outside of Mexico in the early 1800s. The increase of disease and associated yield losses has coincided with changes in populations of P. infestans, metalaxyl resistance, and migrations of new genotypes of the pathogen from Mexico (Goodwin et al. 1994a, b). Late twentieth century migrations of P. infestans from Mexico introduced new populations of the pathogen to Europe, North and South America and Asia (Fry et al. 1993). These are considered to be more aggressive, and may include both mating types and phenylamide fungicide-resistant strains, making disease control extremely difficult (Spielman et al. 1991; Drenth et al. 1994).
Total potato production of Turkey in 2016 was 4,750,000 tons in 1448.572 ha area wherein the Cukurova region produced 221,397 tons in 61,306 ha with 3611 kg/ha yield hence early in the season potato growth has an important economic impact in the region (TUIK 2016). Considering humid climatic conditions, yield losses occur due to potato late blight disease each growing season in the region and pesticide applications by growers’ result in damage both to environment and countries economy. In Europe the total annual cost of late blight of potato and tomato is estimated to be around €1 billion. That amount includes crop losses, as well as the cost of fungicides used for crop protection (Haverkort et al. 2009).
Potato late blight has emerged as the most important disease on potato in Turkey. Out of season potato growth and tuber production is important in the Cukurova Region potato agriculture. Isolates of P. infestans have not been characterized or the genetic structure of population has not been determined in Turkey. This study aimed to characterize the genetic diversity of the P. infestans populations in Cukurova region of Turkey. Biochemical and molecular characterization of P. infestans population were conducted through metalaxyl resistance, mating type, mitochondrial DNA (mtDNA) haplotype and allozyme analyses. This study contains the first data on molecular and biochemical characterization of potato late blight agent P. infestans in Turkey.
Materials and methods
Sample collection, isolation and culturing
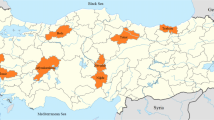
The isolates were collected during potato growing seasons between 2013 and 2014 and the blighted potato leaves, stems and tubers with freshly sporulating lesions of P. infestans were collected from standard production fields of Hatay, Adana and Mersin provinces (Fig. 1, Table 1). The sampling was started when the potato plants were at the 8–12 true leaf stage and continued until harvest. Each field was inspected carefully for blight symptoms and 1–15 samples per field were collected. The samples were then transported in plastic bags to the laboratory and labeled. Isolation of pure cultures was conducted according to Danies et al. (2013). The symptom exhibiting plant materials were placed into humid chambers in 9 cm Petri dishes, then were transferred onto disinfected potato tuber slices placed in water-agar medium in a growing chamber at 18 °C and 16 h in light and 8 h in dark for 5 to 7 days. After the formation of blight lesions on potato slices, the mycelia were inoculated to modified fresh rye agar (Rye A) media (Caten and Jinks 1968). The isolates were single spored and maintained on Rye A media in liquid nitrogen until use (Danies et al. 2013). More than 200 samples were collected from leaves, stems and tubers wherein 186 isolates were successfully purified and stored for further studies.
Pathogenicity assays
The pathogenicity assays were conducted on potato cv Marabel (high-medium foliage blight resistance https://www.europlant.biz/fileadmin/db_upload/Marabel_E_2016-06.pdf). The plants were grown under controlled climate chambers for 4–6 weeks, fully developed true leaves were excised, disinfected and were placed in 9 cm Petri dishes. Spore suspensions of 5 × 103 sporangia/ml were maintained at 4 °C for 2 h to release zoospores. Drops of P. infestans inoculum were then placed onto cv Marabel leaflets and the Petri dishes were maintained at 15–18 °C and 16 h in light and 8 h in dark until symptom development (Fig. 2). The P. infestans isolates causing late blight symptoms were recorded as pathogenic (Świeżyński et al. 2000; Fontem et al. 2005). The pathogenicity assays were conducted independently as a completely randomized design with three replicates wherein each Petri dish contained one leaf.
Mating type determination
Mating types of the P. infestans isolates were assigned according to Peters et al. (1998a), A1 (US-1) and A2 (US-8) reference isolates were kindly provided by Dr. William Fry (Cornell University, Ithaca, USA). The mating type of each isolate was determined by cutting a 5-mm agar disk from the margin of a 2 -week-old colony of P. infestans and placing it in the center of a Petri dish containing Rye A medium. One 5-mm disk of a known A1 (US-1) and A2 (US-8) isolate was placed on the agar surface on either side of the center disk of the unknown isolate. Isolates that produced oospores with the known A1 tester isolate were defined as the A2 mating type, and isolates that produced oospores with the known A2 tester isolate were assigned as the A1 mating type. Test isolates producing oospores with both A1 and A2 were scored as A1A2. The dual cultures were incubated at 15 °C in darkness for 7–14 days, and then examined microscopically for the presence of oospores where the two colonies interacted. Each trial was repeated twice under the same experimental conditions.
Metalaxyl response
In vitro testing for sensitivity to metalaxyl was by a modified protocol of Deahl et al. (1993). Metalaxyl (metalaxyl 90% w/w; technical grade; Novartis, Plant Protection Division, Cambridge, ON) was prepared as a 100 mg/mL stock solution in pure dimethyl sulfoxide (DMSO) and added to molten clarified Rye A after autoclaving to achieve a concentration of 100 μg /mL. Agar plugs (5 mm diameter) taken from the margin of 2- week-old cultures of P. infestans were transferred to 9 cm Petri plates containing clarified Rye A medium amended with metalaxyl concentrations of 0.1, 1.0, 10.0, 50.0, 100 (μg/ml) (Sujkowski et al. 1995; Peters et al. 1998b). Control Petri dishes contained sterile distilled water. Assessments were conducted by using the criteria of Shattock et al. (1990) and Deahl et al. (1993). Each isolate was tested three times. Three categories of fungicide sensitivity were scored on the basis of a percentage of mean growth (colony diameter) in the presence of 100 μg metalaxyl/ ml compared to the growth in the absence of metalaxyl: metalaxyl sensitive (MS) ≤ 10% growth, metalaxyl-intermediate or metalaxyl-moderately resistant (MMR) between 10 and 60% growth, and metalaxyl-highly resistant (MHR) ≥ 60% growth (Shattock 1988). All isolates were incubated in the dark, at 15 °C, for 7 days. Tester isolates provided by Dr. William Fry (Cornell University, Ithaca, USA) were used for comparison in the assays.
Mitochondrial haplotypes
Total genomic DNA was extracted from P. infestans mycelia using a modified CTAB method and mitochondrial haplotypes of P. infestans isolates were defined with PCR-RFLP using primer pairs P1 (P1 forward and P1 reverse), P2 (P2 forward and P2 reverse), P3 (P3 forward and P3 reverse) and P4 (P4 forward and P4 reverse) (Griffith and Shaw 1998). The reference isolates (Ia, Ib, IIa and IIb) were kindly provided by Dr. William Fry (Cornell University, Ithaca, USA). PCR (PTC-100TM MJ Research, Inc.) and amplification conditions were as follows for all primer combinations: 200 mM of each dNTP’s (Promega), PCR buffer 1X (1 M KCl, 1 M Tris HCl pH 8.3, 1 M MgCl2), 0.325 μM primers (forward and reverse) (Operon Technologies, Inc.), 1 U Taq DNA polymerase (Promega) and 10 ng of total DNA in 0.2 mL microcentrifuge tubes (final volume, 20 μL). PCR cycles were as follows: 1 cycle of 94 °C for 90 s; 40 cycles of 94 °C for 40s, 55 °C for 60s and 72 °C for 90s. The banding profile of each isolate was determined for each primer (1118 bp-P1; 1070 bp-P2; 1308 bp-P3; 946 bp-P4). The PCR products were digested with CfoI, MspI and EcoRI restriction endonucleases and separated on 1.5% agarose gel in 0.5 × TAE (20 mM Tris-acetate, 0.5 mM EDTA, pH 8). The gel was run at 100 V during 2 h. Restriction patterns were visualized with an UV transilluminator and the images were recorded by a gel documentation system.
Allozyme analyses
Allozyme genotypes were determined at the Glucose-6-phosphate isomerase (Gpi EC 5.3.1.9) loci (Goodwin et al. 1995a, b; McLoad et al. 2001). The clonal genotypes US-1 (Gpi 86/100), US-6 (Gpi 100/100), US-7 (Gpi 100/111) and US-8 (100/111/122) were used as standards on each cellulose acetate plate for the Gpi assay (Goodwin et al. 1995a, b; Forbes et al. 1998; McLoad et al. 2001). The reference isolates were kindly provided by Dr. William Fry (Cornell University, Ithaca, USA). Migration distances of proteins from the unknown isolates were compared with migration distances of proteins with that of the tester genotypes (Goodwin et al. 1995a, b).
Results
Sample collection and pathogenicity of Phytophthora infestans isolates
A total of 186 P. infestans isolates was obtained through survey studies conducted during the 2013–2014 potato growing seasons in three potato producing provinces of the Cukurova region were covered and the P. infestans isolates were recovered from 239 potato fields in an area of 61,306 ha (Table 1). All the isolates were pathogenic on the leaves cv Marabel (Fig. 2).
Mating type analyses
In 2013, 84% of the isolates were of A1 mating type 10% of A2 and 6% of A1A2 mating type, while in 2014 it was 51.2%, 36.3% and 12.5%, respectively. It was determined that most of the Adana isolates of Sarıçam, Tufanbeyli, Reyhanlı and Yüreğir districts were of the A2 mating type during both potato growing seasons. Chi square test was used to evaluate the differences between the regions of the isolates. The ratio of A2 to A1 isolates is significantly different from 1: 1 (χ2 = 41.24; α = 0.01).
Metalaxyl sensitivity
The studies were conducted on 186 P. infestans isolates collected during 2013 and 2014. In 2013, 98 P. infestans isolates were subjected to metalaxyl sensitivity analyses and all the isolates exhibited radial growth at 0.1 μg/ml metalaxyl dosage and 16 isolates were determined to grow on media containing 1.0 μg/ml metalaxyl. Dosages of 10 μg/ml, 50 μg/ml and 100 μg/ml completely restricted P. infestans growth (Table 2). Eighty-eight P. infestans isolates were used in metalaxyl sensitivity assays in 2014, and all the isolates grew at the 0.1 μg/ml dosage. Radial growth was determined with 64 and 20 P. infestans isolates at 1 μg/ml and 10 μg/ml dosages, respectively. Metalaxyl at all the dosages prevented sporulation of P. infestans isolates while sporangia formed in control Petri dishes. According to the applied criteria all the isolates were sensitive to metalaxyl.
Mitochondrial DNA (mtDNA) haplotype analyses
Four haplotypes (Ia, IIa, Ib and IIb) of mtDNA were determined in the potato-growing areas of the Cukurova region (Fig. 3, Table 1). The most common haplotype was Ia (33.87%) and was followed by Ib (27.42%), IIa (20.97%), IIb (17.74%).
Allozyme analyses
Allozyme analyses were conducted on 186 P. infestans isolates and the 86/100 genotype (146 isolates) as well as 100/100 (39 isolates) were determined to be widespread in the potato-growing areas of the Cukurova region (Fig. 4, Table 1). The US-8 genotype 100/111/122 was not detected.
Discussion
This study was conducted to explore the genotypic and phenotypic characteristics of the potato late blight disease agent P. infestans. The results obtained through this study contain the first reports on P. infestans population of potato from Turkey. Both mating types (A1 and A2) were determined in the potato-growing areas of the Cukurova region which is one of the major agricultural zones of Turkey and all the isolates were sensitive to metalaxyl. Genetic variation that exists within the P. infestans population was determined by mtDNA haplotype and allozyme analyses wherein Ia and Gpi 86/100 were determined as the most commonly genotypes in the region. The results obtained through this study revealed that the P. infestans population of potato-growing areas in the Cukurova region has heterogenic genetic structure.
Sensitivity of P. infestans to metalaxyl was determined according to criteria by Shattock et al. (1990) and Deahl et al. (1993) and all the isolates tested in this study were metalaxyl sensitive (Table 1). Griffin et al. (2002) tested 0.01–100 ppm concentrations of metalaxyl in Ireland and determined variation in sensitivity. They reported sensitive isolates that were able to grow at 0, 0.01, 0.1 ppm concentrations while the isolates that exhibited growth and sporulation in all the concentrations were classified as resistant to metalaxyl. Statsyuk et al. (2010) reported 98% of Mariy El (Russia) isolates as metalaxyl sensitive while isolates obtained from Kostroma and Moscow were in 58% metalaxyl sensitive. They reported that the three regions were planted with imported potato cultivars. In the USA, the P. infestans isolates were assigned as metalaxyl sensitive however in 1990, the US-6, US-7 and US-8 lineages were determined and the isolates of those lineages were found to be metalaxyl resistant (Goodwin et al. 1994a, b, 1998). Recently, due to seed potato export, aggressive and sometimes metalaxyl resistant strains of P. infestans were introduced to India, eastern Africa countries and Algeria (Rekad et al. 2017; Dey et al. 2018; Njoroge et al. 2018). In the Cukurova region of Turkey alternative fungicides beside metalaxyl are used to control potato late blight and the farmers prefer to grow registered potato varieties that in turn may be the explanation of the sensitivity of P. infestans population in the region. Metalaxyl has been used since the 1970s and the resistance was reported 20 years later in the 1990s.
P infestans is a heterothallic species that needs two mating types (A1, A2) to form sexual oospores and create genetic variation (Shattock 2002). A1 and A2 mating type strains are present in central Mexico, in the Nordic countries of Europe and in the Netherlands which has led to sexual reproduction and high genetic diversity (Wang et al. 2017; Fry et al. 2015; Drenth et al. 1993). Existence of both mating types allows genetic recombination that in turn might create new specifications exhibiting higher survival and virulence leading to heavy infections in the field (Barton and Charlesworth 1998). The presence of mating type A2 allows P. infestans to reproduce sexually, with subsequent effects on disease epidemiology and control. Studies conducted on the pathogen population in several potato growing areas reported an increased diversity (Drenth et al. 1994; Sujkowski et al. 1994; Andersson et al. 1998; Brurberg et al. 1999; Hermansen et al. 2000; Turkensteen et al. 2000; Flier et al. 2007; Widmark et al. 2007). High oospore production was reported to be related with epidemiological capacity of P. infestans (Hanson and Shattock 1998). However, the late blight of potato could effectively be controlled through fungicide applications (Evenhuis et al. 1996). Both of the mating types of P. infestans were identified in the Cukurova region (Table 1) and this result emphasizes the importance of protective fungicide applications and also of crop rotation that would ensure that oospores surviving in the soil will not find a suitable host in the following season.
Genetic differences determined through mtDNA revealed four groups in the Cukurova region wherein two groups (Ia, Ib) were the most common haplotypes. There are numerous reports indicating the entrance of Ia and IIa to Europe during the 1970s (Carter et al. 1991; Day and Shattock 1997; Griffith and Shaw 1998; Gavino and Fry 2002) while Ib was dispersed to potato-growing areas from Mexico (Carter et al. 1990; Gavino and Fry 2002). Erselius et al. (1998) studied P. infestans populations of Ecuador and determined four haplotypes (Ia, IIa, Ib and IIb) of mtDNA and two new genotypes (EC-2, EC-3) were reported that were isolated from different species of Solanaceae. Ib haplotype become widespread in Canada, the United Kingdom (Day et al. 2004), France (Lebreton et al. 1998) and the USA (Goodwin et al. 1994a, b). Chen et al. (2009) studied Taiwan P infestans tomato and potato population of 655 isolates collected through 1991–2006 to define genetic changes and determined a sudden difference in 1997–1998. They reasoned this change to potato imports to the country during 1997–1998. These studies indicate that the population change of P. infestans is closely related with migration, variety preference and seed potato export. Out of season and seedling potato production are actively conducted in the Cukurova region of Turkey. The potato varieties cultivated in the region are imported from different countries and this could be the reason for the existence of four mtDNA haplotypes.
The Glucose-6-phosphate isomerase (Gpi) and peptidase (Pep) loci are widely used to characterize allozyme genotypes of P. infestans populations (Goodwin et al. 1995a, b; Forbes et al. 1998). Seventy-six P. infestans isolates collected from tomato and potato-growing areas of France were subjected to mating type, Gpi, Pep and mtDNA haplotyping characterization (Lebreton et al. 1998). A majority of the isolates were Gpi 90/100 or 100/100, Pep 83/100 or 100/100) and their mtDNA haplotypes were Ia or IIa. These results showed that the isolates had the similar structure with those of the P. infestans population that entered to Europe in the 1970s.
Carlisle et al. (2001) studied P. infestans collections from 1995 to 1996 to define the genetic and phenotypic variation of north Ireland. The study revealed that the isolates were homozygous at Gpi 100/100 and Pep 100/100. Similarly, Chinese P. infestans isolates collected during 1998–2007 from the potato and tomato-growing areas were characterized through mating type, metalaxyl sensitivity, mtDNA genotyping, Gpi and Pep allozyme analyses. The common Gpi designs were 100/100/111 (176 isolates) and 100/100 (109 isolates). However, Gpi 86/100 and Gpi 100/111 were also determined in minor numbers. All the isolates analyzed in this study were homozygous at Pep loci (100/100) but the P. isolates exhibited high polymorphism with RAPD and ISSR markers (Li et al. 2009). Our data show that, the majority of the isolates from Tufanbeyli, Reyhanlı and Yuregir districts of Adana were determined to be of Gpi 86/100 and 100/100 profiles in both of the potato growing seasons.
The Gpi allozyme genotype of the Cukurova region were 86/100 (146 isolates), 100/100 (39 isolates) and 122/100 (1 isolate), whereas the 100/111/122 genotype was not determined. In Turkey, more than 50 potato varieties originating from the Netherlands, Germany and Great Britain were cultivated (TUIK 2015). The Tufanbeyli district of Adana is now an important seed potato tuber-producing area of Turkey. This area is free of potato wart disease caused by Synchytrium endobioticum and that it is a prerequisite to produce disease-free seed potato tubers in Turkey. This resulted in a high entrance of import potato varieties to the Tufanbeyli district. During the survey studies conducted in 2013–2014, a total of 239 potato fields were examined and P. infestans was isolated from each field (Table 1). This result revealed a high disease prevalence in the Cukurova region of Turkey and that the P. infestans population contains genetically diverse isolates determined through mtDNA haplotypes and Gpi allozyme analyses.
Biochemical and molecular characterization of P. infestans populations existing in the Cukurova region were determined for the first time in Turkey through metalaxyl resistance, mating type, mtDNA haplotype and allozyme analyses in this study. Characterization of genetic variation in P. infestans populations within the Cukurova region will assist in the development of strategies for potato late blight breeding efforts and rectify fungicide application systems.
References
Andersson, B., Sandstrom, M., & Stromberg, A. (1998). Indications of soil-borne inoculum of Phytophthora infestans. Potato Research, 41, 305–310.
Barton, N. H., & Charlesworth, B. (1998). Why sex and recombination? Science, 281, 1986–1989.
Brurberg, M. B., Hannukkala, A., & Hermansen, A. (1999). Genetic variability of Phytophthora infestans in Norway and Finland as revealed by mating type and fingerprint probe RG57. Mycological Research, 103, 1609–1615.
Carlisle, D. J., Cooke, L. R., & Brown, A. E. (2001). Phenotypic and genotypic characterization of Northern Ireland isolates of Phytophthora infestans. European Journal of Plant Pathology, 107, 291–303.
Carter, D. A., Archer, S. A., Buck, K. W., Shaw, D. S., & Shattock, R. C. (1990). Restriction fragment length polymorphisms of mitochondrial DNA of Phytophthora infestans. Mycological Research, 94, 1123–1128.
Carter, D. A., Archer, S. A., Buck, K. W., Shaw, D. S., & Shattock, R. C. (1991). DNA polymorphisms in Phytophthora infestans the U.K. experience (pp. 272–294). Cambridge University Press.
Caten, C. E., & Jinks, J. L. (1968). Spontaneous variability of single isolates of Phytophthora infestans. I. Cultural variation. Canadian Journal of Botany, 46(4), 329–348.
Chen, C. H., Wang, T. C., Black, L., Sheu, Z. M., Perez, F., & Deahl, K. (2009). Phenotypic and genotypic changes in the Phytophthora infestans population in Taiwan 1991 to 2006. Journal of Phytopathology, 157, 248–255.
Chowdappa, P., Kumar, N. B. J., Madhura, S., Kumar, M. S. P., Myers, K. L., Fry, W. E., Squire, J. S., & Cooke, D. (2013). Emergence of 13_A2 blue lineage of Phytophthora infestans was responsible for severe outbreaks of late blight on tomato in south-west India. Journal of Phytopathology, 161, 49–58.
Cooke, D. E. L., Cano, L. M., Raffaele, S., Bain, R. A., Cooke, L. R., Etherington, G. J., Deahl, K. L., Farrer, R. A., Gilroy, E. M., Goss, E. M., Grünwald, N. J., Hein, I., MacLean, D., & Kamoun, S. (2012). Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathogens. https://doi.org/10.1371/journal.ppat.1002940.
Danies, G., Small, I. M., Myers, K., Childers, R., & Fry, W. E. (2013). Phenotypic characterization of recent clonal lineages of Phytophthora infestans in the United States. Plant Disease, 97(7), 873–881.
Day, J. P., & Shattock, R. C. (1997). Aggressiveness and other factors relating to displacement of populations of Phytophthora infestans in England and Wales. European Journal of Plant. Pathology, 103, 397–391.
Day, J. P., Wattier, R. A. M., Shaw, D. S., & Shattock, R. C. (2004). Phenotypic diversity in Phytophthora infestans on potato in Great Britain, 1995-1998. Plant Pathology, 53, 303–315.
Deahl, K. L., Inglis, D. A., & DeMuth, S. P. (1993). Testing for resistance to metalaxyl in Phytophthora infestans isolates from northwestern Washington. American Potato Journal., 10, 779–795.
Dey, T., Saville, A., Myers, K., Tewari, S., Cooke, D., Tripaty, S., Fry, W., Ristaino, J. B., & Roy, S. (2018). Large sub-clonal variation in Phytophthora infestans from recent severe late blight epidemics in India. Article in Scientific Reports. February, 2018, 4429. https://doi.org/10.1038/s41598-018-22192-1.
Dowley, L. J., & O’Sullivan, E. (1981). Metalaxyl-resistant strains of Phytophthora infestans (Mont.) de Bary in Ireland. Potato Research., 24, 417–421.
Drenth, A., Tas, I. C. Q., & Govers, F. (1994). DNA fingerprinting uncovers a new sexually reproducing population of Phytophthora infestans in the Netherlands. European Journal of Plant Pathology, 100, 97–107.
Drenth, A., Goodwin, S. B., Fry, W. E., & Davidse, L. C. (1993). Genotypic diversity of Phytophthora infestans in The Netherlands revealed by DNA polymorphisms. Phytopathology, 83(10), 1087–1092.
Erselius, L.J., Hohl, H.R., Ordonez, M.E., Oyarzun, P.J., Jarrin, F., Velasco, A., Ramon, M.P, Forbes, G.A., (1998). Genetic diversity among isolates of from various host in Ecuador, CIP program report 1997–1998.
Evenhuis, A., Scheepers, H. T. A. M., Bus, C. B., & Stegeman, W. (1996). Synergy of cymoxanil and mancozeb when used to control potato late blight. Potato Research, 39, 551–559.
Flier, W. G., Kroon, L. P. N. M., Hermansen, A., van Raaij, H. M. G., Speiser, B., Tamm, L., Fuchs, J. G., Lambion, J., Razzaghian, J., Andrivon, D., Wilcockson, S., & Leifert, C. (2007). Genetic structure and pathogenicity of populations of Phytophthora infestans from organic potato crops in France, Norway, Switzerland and the United Kingdom. Plant Pathology, 56, 562–572.
Fontem, D. A., Olanya, O. M., Tsopmbeng, G. R., & Owona, M. A. P. (2005). Pathogenicity and metalaxyl sensitivity of Phytophthora infestans isolates obtained from garden huckleberry, potato and tomato in Cameroon. Crop Protection, 24, 449–456.
Forbes, G. A., Goodwin, S. B., Drenth, A., Oyarzun, P., Ordonez, M. E., & Fry, W. E. (1998). A global marker database for Phytophthora infestans. Plant Disease, 82, 811–818.
Fry, W. E. (2008). Phytophthora infestans: The plant (and R gene) destroyer. Molecular Plant Pathology, 9, 385–402.
Fry, W. E., Goodwin, S. B., Dyer, A. T., Matusazak, J. M., Drenth, A., Tooley, P. W., Sujkowski, L. S., Koh, Y. J., Cohen, B. A., Spielman, L. J., Deahl, K. L., & Inglis, D. A. (1993). Historical and recent migrations of Phytophthora infestans: Chronology, pathways, and implications. Plant Disease, 77, 653–661.
Fry, W. E., Birch, P. R. J., Judelson, H. S., Grünwald, N. J., Danies, G., Everts, K. L., Gevens, A. J., Gugino, B. K., Johnson, D. A., Johnson, S. B., McGrath, M. T., Myers, K. L., Ristaino, J. B., Roberts, P. D., Secor, G., & Smart, C. D. (2015). Five reasons to consider Phytophthora infestans a re-emerging pathogen. Phytopathology, 105, 966–981.
Galindo, J., & Gallegly, M. E. (1960). The nature of sexuality in Phytophthora infestans. Phytopathology, 50, 123–128.
Gavino, P. D., & Fry, W. E. (2002). Diversity in and evidence for selection on the mitochondrial genome of Phytophthora infestans. Mycologia, 94, 781–793.
Goodwin, S. B. (1997). The population genetics of Phytophthora. Phytopathology, 87, 462–473.
Goodwin, S. B., Cohen, B. A., Deahl, K. L., & Fry, W. E. (1994a). Migration from northern Mexico as the probable cause of recent genetic changes in populations of Phytophthora infestans in the United States and Canada. Phytopathology, 84, 553–558.
Goodwin, S. B., Cohen, B. A., & Fry, W. E. (1994b). Panglobal distribution of a single clonal lineage of the Irish potato famine fungus. Proceedings of the National Academy of Sciences of the United States of America, 91, 11591–11595.
Goodwin, S. B., Schineider, R. E., & Fry, W. E. (1995a). Use of cellulose-acetate electrophoresis for rapid identification of allozyme genotypes of Phytophthora infestans. Plant Disease., 79, 1181–1185.
Goodwin, S. B., Sujkowski, L. S., Dyer, A. T., Fry, B. A., & Fry, W. E. (1995b). Direct detection of gene flow and probable sexual reproduction of Phytophthora infestans in northern North America. Phytopathology, 83, 473–479.
Goodwin, S. B., Smart, C. D., Sandrock, R. W., Deahl, K. L., Punja, Z. K., & Fry, W. E. (1998). Genetic change within populations of Phytophthora infestans in the United States and Canada during 1994 to 1996: Role of migration and recombination. Phytopathology, 88(9), 939–949.
Griffin, D., O’Sullivan, E., Harmey, M. A., & Dowley, L. J. (2002). DNA fingerprinting, metalaxyl resistance and mating type determination of the Phytophthora infestans population in the Republic of Ireland. Potato Research, 45, 25–36.
Griffith, G. W., & Shaw, D. S. (1998). Polymorphisms in Phytophthora infestans: Four mitochondrial haplotypes are detected after PCR amplification of DNA from pure cultures or from host lesions. Appl. Environ. Microbiology, 64(10), 4007.
Hanson, K., & Shattock, R. C. (1998). Formation of oospores of Phytophthora infestans in cultivars of potato with different levels of race-nonspecific resistance. Plant Pathology, 47, 123–129.
Haverkort, A. J., Struik, P. C., Visser, R. G. F., & Jacobsen, E. (2009). Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Research., 52, 249–264.
Hermansen, A., Hannukkala, A., Hafskjold Nærstad, R., & Brurberg, M. B. (2000). Variation in populations of Phytophthora infestans in Finland and Norway: Mating type, metalaxyl resistance and virulence phenotype. Plant Pathology, 49, 11–22.
Hohl, H. R., & Iselin, K. (1984). Strains of Phytophthora infestans from Switzerland with A2 mating type behavior. Trans. Br. Mycoogyl. Soc., 83, 529–530.
Judelson, H. S. (1997). The genetics and biology of Phytophthora infestans: Modern approaches to a historical challenge. Fungal Genetics and Biology, 22, 65–76.
Lebreton, L., Laurent, C., & Andrivon, D. (1998). Evolution of Phytophthora infestans population in the two most important potato protection areas of France during 1992-96. Plant Pathology, 47, 427–439.
Li, B., Chen, Q., Lv, X., Lan, C., Zhao, J., Qiu, R., & Weng, Q. (2009). Phenotypic and genotypic characterization of Phytophthora infestans isolates from China. Journal of Phytopathology, 157, 558–567.
Li, Y., van-der Lee, T., Zhu, J. H., Jind, G. H., Lane, C. Z., Zhu, S. X., Zhang, R. F., Liu, B. W., Zhao, Z. J., Kessel, G., Huanga, S. W., & Jacobsen, E. (2013). Population structure of Phytophthora infestans in China – geographic clusters and presence of the EU genotype Blue_13. Plant Pathology., 62, 932–942.
McLoad, A., Denman, S., Sadie, A., & Denner, F. D. N. (2001). Characterization of south African isolates of Phytophthora infestans. Plant Disease., 85, 287–291.
Njoroge, A. W., Andersson, B., & Lees, A. K. (2018). Genotyping of Phytophthora infestans in eastern-Africa reveals a dominating invasive European lineage. Phytopathology. https://doi.org/10.1094/phyto-07-18-0234-r.
Peters, R. D., Plant, H. W., & Hall, R. (1998a). Characterization of changes in populations of Phytophthora infestans in Canada during mating types and metalaxyl sensitivity markers. Canadian Journal of Plant Pathology, 20, 259–273.
Peters, R. D., Plant, H. W., & Hall, R. (1998b). Use of allozyme markers to determine genotypes of Phytophthora infestans in Canada. Canadian Journal of Plant Pathology, 21, 144–153.
Rekad, F. Z., Cooke, L., Puglısı, I., Randall, E., Guenaouı, Y., Bouznad, Z., Evolı, M., Pane, A., Schena, L., Di Sanlio, G. M., & Caccıola, S. A. (2017). Characterization of Phytophthora infestans populations in northwestern Algeria during 2008-2014. Fungal Biology, 467–477.
Ristaino, J. B. (2006). Tracking the evolutionary history of the potato late blight pathogen with historical collections. Outlooks on Pest Management, 17(5), 228–231.
Runno-Paurson, E., Kiiker, R., Joutsjoki, T., & Hannukkala, A. (2016). High genotypic diversity found among population of Phytophthora infestans collected in Estonia. Fungal Biology, 120, 385–392.
Shattock, R. C. (1988). Studies on the inheritance of resistance to metalaxyl in Phytophthora infestas. Plant Pathology, 37, 4–11.
Shattock, R. C. (2002). Phytophthora infestans: Populations, pathogenicity and phenylamides. Pest Management Science, 58, 944–950.
Shattock, R. C., Shaw, D. S., Fyfe, A. M., Dunn, J. R., Loney, K. H., & Shattock, J. A. (1990). Phenotypes of Phytophthora infestans collected in England and Wales from 1995 to 1988: Mating type, response to metalaxyl and isozyme analysis. Plant Pathology, 39, 242–248.
Shtienberg, D. S., Bergeron, N., Nicholson, A. G., Fry, W. E., & Ewing, E. E. (1990). Development and evaluation of general model for yield loss assessment in potatoes. Phytopathology., 80(5), 466–472.
Spielman, L. J., Drenth, A., Davidse, L. C., Sujkowski, L. J., Gu, W., Tooley, P. W., & Fry, W. E. (1991). A second world-wide migration and population displacement of Phytophthora infestans? Plant Pathology, 40, 422–430.
Statsyuk, N.V., Kuznetsova, I.N., Kozlovskaya, B.E., Kozlovsky, S.N., Elansky, E.V., Valeva, E.V., Flippov, A.V. (2010). Characteristics of the Phytophthora infestans population in Russia. Twelfth EuroBlight workshop in France. EPPO. Special Report no.14,247–254.
Sujkowski, L. S., Goodwin, S. B., Dyer, A. T., & Fry, W. E. (1994). Increased genotypic diversity via migration and possible occurrence of sexual reproduction of Phytophthora infestans in Poland. Phytopathology, 84, 201–207.
Sujkowski, L., Fry, B. A., Power, R. J., Goodwin, S. B., Peever, T. L., Hamlen, R. A., & Fry, W. E. (1995). Sensitivities of Mexican isolates of Phytophthora infestans to chlorothalonil, cymoxanil, and metalaxyl. Plant Disease, 79, 1117–1120.
Świeżyński, K. M., Domański, L., Zarzycka, H., & Zimnoch-Guzowska, E. (2000). The reaction of potato differentials to Phytophthora infestans isolates collected in nature. Plant Breeding, 119, 119–126.
Tosun, N., Yıldırım, A., Türküsay, H., & Tanyolaç, B. (2007). Genetic variation among Phytophthora infestans (tomato blight) isolates from Western Turkey revealed by inter simple sequence repeat (ISSR) and random amplified polymorphic DNA (RAPD) markers. Pakistan Journal of. Botonical, 39(3), 897–902, 2007.
TUIK (2015). http://www.tuik.gov.tr. Accessed 23 Aug 2018.
TUIK (2016). http://www.tuik.gov.tr. Accessed 23 Aug 2018.
Turkensteen, L. J., Flier, W. G., Wanningen, R., & Mulder, A. (2000). Production, survival and infectivity of oospores of Phytophthora infestans. Plant Pathology, 49, 688–696.
Wang, J., Fernández-Pavía, S. P., Larsen, M. M., Garay-Serrano, E., Gregorio-Cipriano, R., Rodríguez-Alvarado, G., Grünwald, N. J., & Goss, E. M. (2017). High levels of diversity and population structure in the potato late blight pathogen at the Mexico Centre of origin. Molecular Ecology, 26, 1091–1107.
Widmark, A.-K., Andersson, B., Cassel-Lundhagen, A., Sandstr€om, M., & Yuen, J. E. (2007). Phytophthora infestans in a single field in Southwest Sweden early in spring: Symptoms, spatial distribution and genotypic variation. Plant Pathology, 56, 573–579.
Yoshida, K., Schuenemann, J. W., Cano, L. M., Pais, M., Mishra, B., Sharma, R., Lanz, C., Martin, F. N., Kamoun, S., Krause, J., Thines, N., Weige, D., & Burbano, H. A. (2013). The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. eLife, 2, e00731. https://doi.org/10.7554/eLife.00731.
Funding
This study is a part of the project fully supported by the Scientific and Technical Research Council of Turkey (TUBITAK) with project number, TOVAG-112O112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gunacti, H., Ay, T. & Can, C. Genotypic and phenotypic characterization of Phytophthora infestans populations from potato in Turkey. Phytoparasitica 47, 429–439 (2019). https://doi.org/10.1007/s12600-019-00737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-019-00737-y