Abstract
Brown rot (Phomopsis vexans) is a destructive disease of eggplant, which mainly causes fruit rot. It is currently managed through extensive foliar applications of fungicides, such as benzimidazole chemicals. In this research study, a total of 513 single-conidial isolates of P. vexans were collected between 2010 and 2013 for the purpose of characterizing their resistance to benzimidazole fungicides and diethofencarb. Two types of benzimidazole-resistant (Ben R) isolates, Ben R1 (benzimidazole-resistant and diethofencarb-sensitive), and Ben R2 (benzimidazole-resistant and diethofencarb-resistant), were detected. Ben R1 was associated with a point mutation from the GAG to GTG at codon 198 in the β-tubulin gene of the Ben S isolates, and was predicted to cause changes from glutamic acid to valine. Ben R2 was associated with a point mutation from the TTC to TAC at codon 200 in the β-tubulin gene of the Ben S isolates. It was determined that, whether the isolates of the P. vexans were sensitive or resistant to the benzimidazole fungicides, pyraclostrobin (a QoI fungicide) showed strong bioactivity against their mycelial growth, spore germination, and disease lesion development. Also, experiments in 2014 and 2015 at three testing sites showed that pyraclostrobin provided excellent control against brown rot disease in the eggplant fields, and significantly increased the yields of the eggplant fruits. Meanwhile, the applications of benzimidazole fungicides (carbendazim alone or mixed with diethofencarb) were found to aggravate the occurrences of the brown rot, and the eggplant yields were observed to decrease. The baseline sensitivity of the P. vexans to pyraclostrobin was further determined for these 513 isolates. In regard to inhibiting the mycelial growth, the mean EC50 values of the tested isolates were determined to be 3.19 ± 0.43 and 1.28 ± 0.14 mg l−1, respectively, in the absence and presence of the alternative oxidase specific inhibitor salicylhydroxamic acid (SHAM), and the range-of-variation factors were 49.6 and 35.1. For inhibiting spore germination, the mean EC50 values were 0.61 ± 0.12 and 0.39 ± 0.11 mg l−1, with the range-of-variation factors of 32.1 and 17.9, respectively. This study confirmed a serious resistance emergency to benzimidazole fungicides in the P. vexans populations, and indicated that pyraclostrobin may be a good alternative fungicide to control eggplant brown rot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eggplant (Solanum melongena) is one of the common fruit vegetables which are widely grown in Asia and Africa, as well as some of the world’s subtropical areas, including the southern USA and the Mediterranean region. On a global scale, it was recorded that the production of eggplant reached 42.9 million tons for the year 2009 (FAO 2010). Asia has the largest eggplant production in the world. It comprises more than 90 % of the world’s production area, and 87 % of the total world production (Choudhary and Gaur 2009). Eggplant brown rot (EBR) caused by Phomopsis vexans (Sacc. and Syd.) Harter is very devastating and widespread. It ranks second only to bacterial wilt in its destructiveness of eggplant crops. P. vexans is both externally and internally seed borne, and can remain viable for approximately 14 months in soil with plant debris, as well as in the seeds of infected fruits (Chaudhary and Hasija 1979; Wu et al. 2013). Besides fruit rot, this pathogen also causes damping off, seedling and stem blight, collar rot, stem cankers, and leaf spots. In China, eggplant crops may suffer from approximately 15 types of diseases, of which EBR has been treated as one of the major constraints of eggplant cultivation (Chen et al. 2015). The application of fungicides is an indispensable strategy for the control of EBR (Dharam and Chakrabarri 1982; Wu et al. 2013). Benzimidazole fungicides, including thiophanate-methyl and carbendazim (Nenad et al. 2015) have been widely applied in eggplant production for more than 10 years in China. However, a decrease in efficacy, and even failures to control P. vexans have been frequently reported by growers during recent years. However, no published reports of fungicide resistance for P. vexans. Currently, no other fungicides without positive cross-resistance with benzimidazole fungicides have been assessed or recommended for the management of EBR. Therefore, the management of EBR has become difficult, due to the lack of information. This study was conducted with the following goals:
(i) monitor the evolution of the resistance in the P. vexans to benzimidazole fungicides during a period ranging from 2010 to 2013, (ii) investigate the molecular mechanism involved in the resistance of the P. vexans to benzimidazole fungicides, (iii) assess the bioactivity of pyraclostrobin against the mycelial growth, spore germination, and disease lesion development in plants for both benzimidazole resistant and sensitive isolates,(iv) evaluate the control efficacy of pyraclostrobin controlling the EBR, and the effects on eggplant crop yields, through experiments in 2014 and 2015 at three testing sites, and (v) build the baseline sensitivity of the P. vexans to pyraclostrobin.
Materials and methods
Fungicides
In this study, technical-grade fungicides were adopted for the determination of sensitivity in vitro. Among these fungicides, the thiophanate-methyl, diethofencarb, and pyraclostrobin were dissolved in acetone, and the technical-grade carbendazim was dissolved in 0.1 mol l−1 hydrochloric acid, to prepare the stock solutions. The stock solutions were stored at 4 °C, under dark conditions. Three commercial formulations including 25 % carbendazin WP (Guoguang Agri-Chemical), 25 % pyraclostrobin EC (Carbrio, BASF), and 60 % carbendazim+ diethofencarb (1:1) (Guoguang Agri-Chemical), were used in the field experiments.
Isolates
During a period ranging from 2010 to 2013, isolates from diseased fruits and leaves were collected from Hangzhou, Shaoxing, and Huzhou in Zhejiang Province. A total of 513 single-conidial isolates of P. vexans were collected from 31 separate fields. These commercial fields had been annually treated with thiophanate-methyl and carbendazin for more than 10 years. The fields were separated from each other by at least 30 km. Between 5 and 12 isolates from each field were obtained on potato dextrose agar (PDA), and were identified on the basis of their morphology, as well as the DNA sequencing of the internal transcribed spacer (ITS) region, as described previously (Zhang et al. 2010). The isolates were kept on PDA slants at 4 °C.
Assessment of the resistance to benzimidazole fungicides
The inhibitions of the mycelia growth by carbendazim, thiophanate-methyl, and diethofencarb were assessed through measuring the radial growth on solid PDA plates which had been amended with 0, 0.025, 0.05, 1, 5, 20, 50, 100, and 200 mg a.i. l−1 of medium. Each of the fungicides was added to autoclaved PDA medium, cooled at approximately 60 °C, and the solvent concentrations were less than 1 ml per liter of medium. A 5-mm mycelial plug was then cut from the edge of a 5-day old colony, and was placed in the center of a PDA plate. Three plates for each of the fungicide concentrations were used. These plates were incubated at 27 °C for 5 days prior to measure each colony in two perpendicular directions. The diameter (5 mm) of the original mycelial plug was subtracted. For each isolate, the average of the colony diameters measured in the two perpendicular directions were used to calculate the EC50 (a fungicide concentration which results in 50 % radial growth inhibition) by the linearly regressing of the probit for percentage of the inhibiting radial growth as a function of the log10 of the fungicide concentration. This experiment was performed twice.
Isolation the β-tubulin gene fragments of P. vexans
Five isolates were randomly selected for each phenotype of benzimidazole sensitivity. Each of the isolates was grown on PDA plates for 5 days in the dark conditions in order to extract the DNA. The mycelia were harvested, washed in sterile water, frozen in liquid nitrogen, and lyophilized. DNA from each isolate was extracted using the UNIQ-10 Coloum DNA Extraction Kit SK1204, following the manufacturer’s protocol (Sangon, Shanghai). Then, in accordance with the known conserved regions of the β-tubulin gene, the PCR primer pair Pob-F (5-CACTGAGGGTGCTGAGCTTGT-3) and Pob-R (5-GAAGCGGCCATCATGTTCTTA-3) (Shi et al. 2013) was used to amplify the β-tubulin gene fragment containing codons 198 and 200. The PCR program of this study was as follows: 35 cycles at 94 °C for 4 min, 94 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. The PCR products were analyzed through agarose gel electrophoresis and purified using UNIQ-10 Coloum Agarose Gel DNA Purification Kit SK1131 (Sangon, Shanghai), following the manufacturer’s protocol. All of the PCR products were sequenced by Invitrogen Company, Shanghai, China. The sequences were aligned using Clustal W software (http://www.ebi.ac.uk).
Evaluation of the bioactivity of pyraclostrobin against P. vexans in eggplant
Eggplants were grown in pots in greenhouses, with a minimum temperature of 15 °C. The seed samples were disinfected using a 2 % sodium hypochlorite solution for 3 to 5 min, and were washed twice in distilled water before sowing. The eggplants were sprayed with pyraclostrobin at concentrations of 0.05, 0.2, 1, 2, 5, 10, and 50 mg a.i. l−1 at 30 days after sowing. Water containing Tween 80 but without pyraclostrobin, was used as the control. Three plants were used for each pyraclostrobin concentration, and the experiment was performed twice. The plants were artificially inoculated with P. vexans 24 h after the pyraclostrobin treatments. For each concentration, 20 leaves were inoculated. In briefly, each of the developed leaves of treated plants was inoculated with one mycelium disc. These discs were cut from the margin of a 5-day old colony, and were placed in the centre of leaves. In order to create favourable conditions for infections, the inoculated plants were maintained in dark conditions, with a humidity level of 96 % at 26 °C for 24 h. The plants were then moved back into the plant growth chambers at 26 °C with 90 % humidity, and a 12 h light and dark cycle. After 7 days following the inoculations, the EBR disease lesion development was determined. The mean diameters of the lesions were then calculated to represent the development of the disease lesions for each of the treatments. The fungicide concentration which inhibited the disease lesion diameters by 50 % (EC50 value) was determined for each of the isolates.
Assessment of the control efficacy for EBR in fields
During 2014 and 2015, experimental trials were carried out in three cities (Hangzhou, Shaoxing, and Huzhou) which had severe histories of EBR. Plants of the eggplant cultivar Hangqie No. 1 were grown in fields which were naturally infected by P. vexans. The cultivar was planted following normal agronomic practices. The treatments were carried out at all the experimental sites as followings: (i) 25 % carbendazin WP at a dosage of 600 mg a.i. l−1 (C600), (ii) 25 % pyraclostrobin EC at a dosage of 250 mg a.i. l−1 (P250), (iii) 25 % pyraclostrobin EC at a dosage of 150 mg a.i. l−1 (P150), (iv) 60 % carbendazim + diethofencarb (1:1) at 1000 mg a.i. l−1(CD1000), (v) water treatment control (CK). No other fungicides were applied to the experimental plots. The treatments were laid out as a randomized complete block design, with four replicates, and each plot had an area of 20 m2 (5*4 m). The tested fungicides were applied as leaf spray, at a volume of 450 litre ha−1. All the other treatments, such as fertilizers, were used in accordance with standard farming practices. A total of 4 fungicide applications (July 6th and 20th, and August 5th and 25th) were applied in 2014, and 3 applications (July 6th and 25th, and August 25th) were applied in 2015. Thirty plants from each plot were randomly selected and visually assessed for EBR incidence (%). The severity on the fruit was visually rated with a 9-class scale (Liu et al. 1994) on July 20th, August 5th and 25th, and September 10th in 2014, as well as July 25th, August 25th, and September 10th in 2015. The disease index (%) was calculated for each of the treatments. The fields were harvested normally, and yield in each treatment was weighed and recorded in order to estimate the tons of the commercial fruits which were produced.
Determination of the pyraclostrobin sensitivity: inhibiting mycelial growth
Pyraclostrobin stock solutions were added to the PDA medium after sterilization to produce concentrations of 0,0.39125, 0.7825,1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 mg a.i. pyraclostrobin per litre of medium. At this point, sensitivity tests were conducted in the absence and presence of salicylhydroxamic acid (SHAM), a specific inhibitor of alternative oxidase, at concentrations of 50 mg l−1 (Avila-Adame and Köller 2003; Olaya and Köller 1999), as described in our previous study (Zhang et al. 2011). In the absence of the SHAM, plates which had not been amended with either the fungicide or the SHAM were used as the control. In the presence of the SHAM, plates amended with 50 mg l−1 SHAM were used as the control. The concentration of the solvents in all of the media, including the media without the fungicide, was less than 1 ml per litre medium. Three plates for each pyraclostrobin concentration were used, and the tests were repeated twice. The diameter (cm) of the colony was measured in two perpendicular directions, with the original mycelial plug diameter (5 mm) subtracted for all of the replicates after an incubation period of 5 days at 27 °C, in dark conditions. For each of the isolates, the effective concentration which resulted in a 50 % reduction (EC50) of the mycelial growth was calculated.
Determination of pyraclostrobin sensitivity: inhibiting conidium germination
Pyraclostrobin was added to the 2.0 % water agar (WA) medium in order to produce concentrations of 0, 0.0005, 0.001, 0.005, 0.03125, 0.0625, 0.125, 0.25, 0.5, 1, and 2 mg a.i. pyraclostrobin per litre of medium. The concentrations of the solvents in all of the media, including the media without fungicide, were less than 1 ml per litre medium. The conidium suspensions (0.2 ml) containing 104 conidia per ml derived from the mycelia which had been grown for 9 days on PDA plates were poured onto the surface of each WA plate amended with the pyraclostrobin, SHAM, or both. In the absence of the SHAM, the plates which had not been amended with either the fungicide or SHAM were used as the control. In the presence of SHAM, the plates amended with 50 mg l−1 SHAM were used as the control (Zhang et al. 2011). The conidia were allowed to germinate at 25 °C for 14 h. The germination was quantified under a microscope at three sites by counting 100 conidia per site for each WA plate. In this study, a conidium was scored germinated if the germ tube had reached at least half the length of the conidium. Three plates for each concentration were used, and the experiment was performed twice. For each isolate, the EC50 (the fungicide concentration which resulted in a 50 % germination inhibition), was calculated.
Statistical analysis
In this study, multiple comparison tests were used to detect any differences among the means. Statistical analysis was performed using SAS version 9.1 software (SAS Co., USA).
Results
Benzimidazole resistance and diethofencarb sensitivity of the P. vexans
Two different types of benzimidazole-resistant isolates were detected in the tested populations (n = 513). The sensitive (S) isolates could not grow on the 1 mg l−1 carbendazim or thiophanate-methyl (MIC < 1 mg l−1), with EC50 values < 0.37 mg l−1. The moderately resistant (MR) isolates were able to grow on the 50 mg l−1. However, they were unable to grow on the 100 mg l−1 carbendazim or thiophanate-methyl (50 mg l−1 < MIC < 1 00 mg l−1), with EC50 values ranging from 15.2 to 37.5 mg l−1. The highly resistant (HR) isolates were able to grow on the 200 mg l−1 carbendazim or thiophanate-methyl (MIC > 200 mg l−1), and with EC50 values >100 mg l−1. In this study, between 2010 and 2013, it was determined that the total resistance frequency was 24.2, 39.7, 58.9, and 77.2 %, respectively (Fig. 1). These results suggested that a severe resistance of P. vexans to benzimidazoles was widespread in the eastern areas of China.
Two different levels of sensitivity to diethofencarb were observed, namely sensitive (S) and resistant (R). The S isolates could not grow on the 20 mg l−1 diethofencarb (MIC < 20 mg l−1), and had EC50 values ranging from 0.01 to 9.4 mg l−1. The R isolates could grow on 100 mg l−1 diethofencarb (MIC > 100 mg l−1). A close negative association between the benzimidazole resistance and the diethofencarb sensitivity was found (Table 1). All of the diethofencarb S isolates were determined to be benzimidazole HR isolates (hereafter referred to as Ben R1 isolates). The sensitivities of the diethofencarb R isolates to the benzimidazole fungicides was divided into two types, benzimidazole S, and MR isolates, respectively. In this study, the benzimidazole S-diethofencarb R isolates were referred to as the Ben S isolates, and the benzimidazole MR-diethofencarb R isolates were referred to as the Ben R2 isolates. From 2010 to 2013, the frequencies of the double resistance to benzimidazoles and diethofencarb (Ben R2) were determined to be 8.4, 19.6, 29.9, and 42.1 %, respectively (Fig. 1).
Fragments of the β-tubulin gene of P. vexans
The sequenced fragment of β-tubulin gene of P. vexans includes the positions known to affect sensitivity to benzimidazole fungicides. The sequenced Ben S isolates displayed a 99 % homology to that of the Phomopsis sp. in the Genbank (accession number HQ586944.1). As expected from the previous research results, all of the Ben S, Ben R1, and Ben R2 isolates had the respective sequence of GAG (glutamic acid), GCG (Alanine), and GAG (glutamic acid) at the codon 198 of the β-tubulin gene, and TTC (Phenylalanine), TTC (Phenylalanine), and TAC (Tyrosine) at the codon 200 of the β-tubulin gene (Table 1). Apart the differences at the 198 and 200 codons, all of the isolates had identical sequences.
Bioactivity of the pyraclostrobin against P. vexans
Pyraclostrobin showed strong bioactivity against P. vexans in vitro (Table 2). In the absence of the SHAM, the mean EC50 values of inhibiting mycelial growth and conidium germination were 2.13 and 0.72 mg l −1, respectively. In the presence of 50 mg l −1 of the SHAM, the mean EC50 values of inhibiting radial growth and conidium germination were 1.52 and 0.37 mg l −1, respectively. Pyraclostrobin also showed high activity against the disease lesion development of P. vexans, which was indicated by the corresponding mean EC50 values of 4.98 mg l −1. However, regardless of whether the isolates were sensitive or resistant to the benzimidazole fungicides, pyraclostrobin consistently showed good bioactivity against them (Table 2).
Efficacy of control EBR in the fields
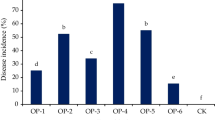
In this study, a total of 4 fungicide sprays (July 6th and 20th, and August 5th and 25th) were carried out in 2014. The first spray was carried out before the initiation of the EBR. The incidences of the EBR were assessed 4 times at 15, 32, 53, and 68 days after the first spray of the fungicides, respectively. The corresponding percentages of EBR incidence of fruit rot for the CK were determined to be 12.3, 26.7, 45.2, and 68.9 % in Hangzhou; 10.9, 24.2, 43.8, and 69.1 % in Shaoxing; and 8.6, 21.5, 44.7, and 70.3 % in Huzhou. The highest control for the EBR incidence of fruit rot was found to be 25 % pyraclostrobin EC at a dosage of 250 mg a.i. l−1 (P250), which showed an efficacy of 91.01 to 96.33 %. It was also determined that 25 % pyraclostrobin EC at a dosage of 150 mg a.i. l−1 (P150) provided the second control on the EBR incidence of fruit rot, with an efficacy of 77.21 to 87.53 %. However, a significant promotion of EBR incidence was observed for both the 25 % carbendazin WP at dosages of 600 mg a.i. l−1 (C600), and 60 % carbendazim: diethofencarb (1:1) at 1000 mg a.i. l−1(CD1000). The disease indexes of the EBR for CK in the experimental field situation were 1.20, 7.35, 15.84, and 29.13 % in Hangzhou; 0.96, 7.57, 18.28, and 26.24 % in Shaoxing, and 1.04, 6.94, 20.18, and 32.35 % in Huzhou. P250 had the highest control for the disease index, with an efficacy of between 87.47 and 96.57 %, and P150 had an efficacy of between 72.49 and 83.72 %, while the control efficacies of C600 and CD1000 were determined to be between −4.89 and −11.78 %, and −5.05 and −10.33 %, respectively (Table 3).
In 2015, the EBR was found to be weaker than that of 2014. Therefore, a total of 3 times of fungicide sprays (July 6th and 25th, and August 25th) were carried out. The first spray was before the incidence of EBR. The incidence of EBR was assessed 3 times at the 20th, 53rd and 68th day following the first spray of the fungicides, respectively. The corresponding percentages of EBR incidences for the CK were 6.1, 12.5, and 39.5 % in Hangzhou; 6.3, 13.3, and 36.7 % in Shaoxing; and 5.9, 14.2, and 41.1 % in Huzhou. P250 showed the highest control of the EBR incidence, with an efficacy of between 93.61 and 96.61 %. Also, P150 provided good control with an efficacy of between 82.37 and 87.53 %. The EBR disease indexes for the CK were determined to be 0.34, 3.47, and 10.23 % in Hangzhou; 0.35, 4.01, and 12.57 % in Shaoxing; and 0.42, 4.96, and 14.36 % in Huzhou. P250 also had the highest control, with an efficacy of between 91.43 and 97.06 %, and the P150 had an efficacy of between 79.05 and 86.05 %. Meanwhile, the efficacies of C600 and CD1000 were −4.89 to −11.78 %, and −5.05 to −10.33 %, respectively. Increases in the EBR incidences and disease indexes were observed for both C600 and CD1000 in all three of the experimental sites (Table 4).
In this experimental study, higher yields (ton/km2) were found for the treatments with pyraclostrobin. In 2014, P250 treatments had 13.55, 17.43, and 16.91 % higher yields of eggplant fruits than CK treatments, respectively in Hangzhou, Shaoxing, and Huzhou. Also, the yields for P150 treatments were 7.93, 8.46, and 7.97 % higher than those of CK. In 2015, the yields were 9.94, 12.94, and 14.68 % higher for P250 treatments, and 4.71, 6.07, and 7.37 % higher for P150 in Hangzhou, Shaoxing, and Huzhou, respectively. However, there were decreases observed in the yields in both the C600 and CD1000 for all of the experimental sites and years (Tables 3 and 4).
Sensitivity of P. vexans to pyraclostrobin
All the 513 isolates were tested for their sensitivities to pyraclostrobin. The sensitivities of the isolates collected from various cities and different years were compared. The results suggested that there was no evidence of geographical variations in the sensitivity of P. vexans to pyraclostrobin, and that the sensitivity remained unchanged from 2010 to 2013. In regard to the inhibition of the mycelial growth, in the absence of SHAM, the frequency distributions of the EC50 values for the 513 isolates were found to range from 0.17 to 8.43 mg l −1, with an average of 3.19 ± 0.43 mg l −1. The range-of-variation factor was 49.6 (Fig. 2a, Table 5). In the presence of SHAM at the concentration of 50 mg l −1, the frequency distributions of the EC50 values for the 513 isolates were found to range from 0.09 to 3.16 mg l −1, with an average of 1.28 ± 0.14 mg l −1. The range-of-variation factor was 35.1 (Fig. 2a, Table 5). Therefore, these sensitivity data could be used as a baseline for monitoring the shifts of sensitivity of P. vexans populations to pyraclostrobin during the growth stage.
For inhibiting the spore germination, in the absence of SHAM, the frequency distributions of the EC50 values were found to range from 0.09 to 2.89 mg l −1, with an average of 0.61 ± 0.12 mg l −1. The range-of-variation factor was 32.1. In the presence of SHAM at the concentration of 50 mg l −1, the frequency distributions of the EC50 values for 513 isolates ranged from 0.07 to 1.25 mg l −1, with an average of 0.39 ± 0.11 mg l −1. The range-of-variation factor was 17.9 (Fig. 2b, Table 5). Therefore, these sensitivity data could be used as a baseline for monitoring the shifts in sensitivity of the P. vexans populations to pyraclostrobin during the germination stage.
Discussion
In China, the frequent reports of control failures for P. vexans by carbendazim and thiophanate-methyl could be attributed to the dominance of the benzimidazole-resistant sub-populations in the fields. The totals of the resistance frequencies were determined to be 24.2, 39.7, 58.9, and 77.2 % from 2010 to 2013. Benzimidazole fungicides are anti-tubulin compounds which inhibit nuclear division by binding to β-tubulin (Davidse and Ishii 1995). In most cases, the resistances of benzimidazole fungicides are associated with single-point mutations in the β-tubulin gene, which result in altered amino acid sequences at the benzimidazole binding site (Davidson et al. 2006; Koenraadt et al. 1992; Ma et al. 2005; Maymon et al. 2006; Torres-Calzada et al. 2015). The majority of the field resistant isolates of the plant-pathogenic fungi displayed codon changes which seemed to be restricted to positions 50 (McKay et al. 1998), 198, 200 (Albertini et al. 1999; Koenraadt et al. 1992), and 240 (Albertini et al. 1999). Many fungi have only a single β-tubulin gene in their genomes. Two homologous β-tubulin genes have been reported in Aspergillus nidulans, Trichoderma spp., Colletotrichum spp., and Giberella zeae, and single-point mutations in one of the β-tubulin genes were found to be associated with the field resistances to benzimidazole fungicides (Ma and Michailides 2005; Chen et al. 2009). Also, two types of benzimidazole-resistant isolates, namely Ben R1 (benzimidazole-resistant and diethofencarb-sensitive) and Ben R2 (benzimidazole-resistant and diethofencarb-resistant), were detected in this study. Ben R1 was associated with a point mutation from GAG to GTG at codon 198 in the β-tubulin gene in Ben S isolates, which was predicted to cause a change from glutamic acid to valine. Ben R1 isolates were found to be highly resistant to benzimidazoles, and simultaneously more sensitive to diethofencarb than the wild isolates. This phenomenon was caused by the negative cross-resistance between the benzimidazole fungicides and diethofencarb (Elad et al. 1988; Leroux et al. 2002). Ben R2 was associated with a point mutation from TTC to TAC at codon 200 in the β-tubulin gene of the Ben S isolates. During the years of 2010 to 2013, the frequencies of the double resistance to benzimidazoles and diethofencarb (Ben R2) were determined to be successively 8.4, 19.6, 29.9, and 42.1 %. These two types of benzimidazole fungicide resistant isolates have been extensively recorded in other pathogenic fungi, such as Botrytis cinerea (Leroux et al. 2002; Ma et al. 2005; Zhang et al. 2010). Moreover, the results of field experiments of 2 years in three sites suggested that the applications of the benzimidazole fungicides (carbendazim alone or mixed with diethofencarb) aggravated EBR occurrences, and decreased the eggplant yields. These results confirmed that the benzimidazole chemical alone or mixed with diethofencarb should not be applied for the management of EBR, due to the seriousness of the emerging resistance. The stimulation of the pathogenicity of the benzimidazole fungicide resistant Sclerotinia sclerotiorum on rapeseed plants by carbendazim was recently reported (Di et al. 2015). Therefore, the possible stimulatory effects of a fungicide on the pathogen resistant to this group of fungicides should be taken into account during integrated disease management processes.
Pyraclostrobin is a novel fungicide of the QOI class, which has been developed from the natural derivatives strobilurin A, oudemansin A, and myxothiazol A. QOI fungicides inhibit mitochondrial respiration by binding to the QO site of the cytochrome b in fungi (Bartlett et al. 2002; Drabesova et al. 2013). One important feature of the QOIs is their extremely broad spectrum of activity (Estep et al. 2015). The mode of action of the QOIs is to block electron transport at the quinol-oxidation site of complex III in the mitochondrial respiration chain. Thereby, the QoI fungicides are effective in inhibiting the sporulation, spore germination, and mycelial growth of the target fungal pathogens. This study indicated that pyraclostrobin displayed strong activities in the inhibition of the mycelial growth, conidium germination, and disease lesion development, regardless of whether the isolates were sensitive or resistant to benzimidazoles and diethofencarb. The results of this study’s 2-year long three site experiments further confirmed that pyraclostrobin was able to provide excellent control efficacy against P. vexans in the fields, and resulted in significantly higher yield of eggplant fruits than that of the CK. Also, pyraclostrobin displayed significantly better control of the EBR and yield effect under high disease pressure (2014) than under medium disease pressure (2015). Therefore, these results suggested that pyraclostrobin was in fact a satisfactory alternative for the management of EBR in China.
When new fungicides are introduced for the purpose of controlling specific targets, it is theoretically required that the baseline sensitivity of each target is firstly established, so that the product-use strategies can be monitored, and possible resistance developments can be detected (Russell 2004). There are two main methodologies used to assess the sensitivity of a pathogen to a fungicide in vitro: radial growth and conidial germination. In this study, a total of 513 isolates of P. vexans were tested for their baseline sensitivity to pyraclostrobin. In regards to the inhibition of mycelial growth, the mean EC50 values were 3.19 and 1.28 mg l −1 in the absence and presence of SHAM, respectively, and the range-of-variation factor was 49.6 and 35.1. For spore germination, the mean EC50 values were 0.61 and 0.39 mg l −1, with a range-of-variation factor of 32.1 and 17.9, respectively. It was found that the stage of conidial germination was more sensitive than the radial growth for the assessment of sensitivity to pyraclostrobin. And, there was no resistant sub-population among the isolates used in this study. The isolates were sampled between 2010 and 2013, from origins where QOI compounds had never previously been applied to the eggplant crops. Therefore, the sensitivity data could effectively be used as the baseline for monitoring the shifts in sensitivity of the P. vexans populations to pyraclostrobin.
The establishment of the baseline sensitivity prior to the exposure of a pathogen population to an active ingredient in the fungicide is very useful for the subsequent sensitivity monitoring to detect the potential development of fungicide resistance (Brent and Hollomon 2007). Pyraclostrobin is a QOI chemical which has been widely used. However, it had not yet been intensively applied to eggplant crops in China. At the time of this study, no information regarding the risk level of the development of fungicide resistance in P. vexans was available (Brent and Hollomon 2007; Michael et al. 2015; Mikaberidze et al. 2014). Even for Phomopsis sp., only the resistance to benzimidazole fungicides, diethofencarb, and sterol demethylation inhibitors (DMIs) in P. obscurans on strawberry had been reported (Shi et al. 2013). The results of this study’s experiments indicated that pyraclostrobin could potentially provide excellent control of EBR. These findings were confirmed through a total of four sprayings under high disease pressure (2014), and three sprayings under medium disease pressure (2015). In some diseases, such as cucumber powdery mildew and downy mildew in Japan, and wheat powdery mildew in Europe, the practical resistance to QoIs were found to have developed more rapidly than expected (Sierotzki et al. 2000; Ishii et al. 2001). It has been widely accepted that QoIs are a class of fungicides with high risk of resistance development. Resistance to QoIs has been reported in some important plant-pathogenic fungi (Ma and Michailides 2005). Therefore, it is critical to further monitor any alterations in the sensitivity of P. vexans to QoIs under field conditions. Also, pyraclostrobin should be alternated or mixed with chemicals from different groups in order to control EBR (Mikaberidze et al. 2014).
References
Albertini, C., Gredt, M., & Leroux, P. (1999). Mutations of the β-tubulin gene associated with different phenotypes of benzimidazole resistance in the cereal eyespot fungi Tapesia yallundae and Tapesia acuformis. Pesticide Biochemistry Physiology, 64, 17–31.
Avila-Adame, C., & Köller, W. (2003). Impact of alternative respiration and target-site mutations on responses of germinating conidia of Magnaporthe grisea to Qo-inhibiting fungicides. Pest Management Science, 59, 303–309.
Bartlett, D. W., Clough, J. M., Godwin, J. R., Hall, A. A., Hamer, M., & Parr-Dobrzanski, B. (2002). The strobilurin fungicides. Pest Management Science, 58, 649–662.
Brent, K. J., & Hollomon, D. W. (2007). Fungicide resistance: the assessment of risk. FRAC Monograph 2, GCPF, Brussels (Now CropLife International). Available on line at www.frac.info.
Chaudhary, S. R., & Hasija, S. K. (1979). Phytopathological on Phomopsis vexans causing soft of brinjal fruits. Indian Phytopathology, 32, 495–496.
Chen, C. J., Yu, J. J., Bi, C. W., Zhang, Y. N., Xu, J. Q., Wang, J. X., & Zhou, M. G. (2009). Mutations in a β-tubulin confer resistance of Gibberella zeae to benzimidazole fungicides. Phytopathology, 99, 1403–1411.
Chen, S. S., Song, S. Y., Han, Y. Z., Zhao, J., Zhao, C. B., & Wen, T. (2015). Resistant responses in protective enzymes of different eggplant cultivars to Phomopsis vexans. Journal of Northwest A & F University - Natural Science, 43, 1–9.
Choudhary, B., & Gaur, K. (2009). The development and regulation of Bt brinjal in India (Eggplant/Aubergine). International Service for the Acquisition of Agri-biotech Applications (ISAAA) Brief No. 38. Ithaca: ISAAA. 15 p.
Davidse, L., & Ishii, T. (1995). Biochemical and molecular aspects of benzimidazoles, N-phenylcarbamates and N-phenylformamidoxines and the mechanisms of resistance to these compounds in fungi. In H. Lyr (Ed.), Modern selective fungicides (pp. 305–322). Jena: Gustav Fisher.
Davidson, R. M., Hanson, L. E., Franc, G. D., & Panella, L. (2006). Analysis of β-tubulin gene fragments from benzimidazole-sensitive and -tolerant Cercospora beticola. Journal of Phytopathology, 154, 321–328.
Dharam, S., & Chakrabarri, A. K. (1982). Chemical control of Phomopsis fruit rot of brinjal. Indian Phytopathology, 35, 314–315.
Di, Y. L., Zu, Z. Q., & Zhu, F. X. (2015). Pathogenicity stimulation of Sclerotinia sclerotiorum by subtoxic doses of carbendazim. Plant Disease, 99, 1342–1346.
Drabesova, J., Ryanek, P., Brunner, P., Mc Donald, B. A., & Croll, D. (2013). Population genetic structure of Mycosphaerella graminicola and Quinone Outside Inhibitor (Qo I) resistance in the Czech Republic. European Journal of Plant Pathology, 135, 211–224.
Elad, Y., Shabi, E., & Katan, T. (1988). Negative cross-resistance between benzimidazole and N-phenylcarbamate fungicides and control of Botrytis cinerea on grapes. Plant Pathology, 37, 141–147.
Estep, L. K., Torriani, S. F., Zala, M., Anderson, N. P., Flowers, M. D., Mc Donald, B. A., Mundt, C. C., & Brunner, P. C. (2015). Emergence and early evolution of fungicide resistance in North American populations of Zymoseptoria tritici. Plant Pathology, 64, 961–971.
FAO. (2010). Food and Agriculture Organization (FAO) database. http://www.faostat.fao.org/site/567/DesktopDefault.aspx.
Ishii, H., Fraaije, B. A., Sugiyama, T., Nogouchi, K., Nishimura, K., Takeda, T., Amano, T., & Hollomon, D. W. (2001). Occurrence and molecular characterization of strobilurin resistance in cucumber powdery and downy mildew. Phytopathology, 91, 1166–1171.
Koenraadt, H., Somerville, S. C., & Jones, A. L. (1992). Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology, 82, 1348–1354.
Leroux, P., Fritz, R., Debieu, D., Albertini, C., Lanen, C., Bach, J., Gredt, M., & Chapeland, F. (2002). Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Management Science, 58, 876–888.
Liu, X. M., Zhang, H. Q., & Bai, R. L. (1994). Control of eggplant brown rot (Phomopsis vexans) with fungicide mixtures. Journal of Vegetable, 2, 13–14.
Ma, Z., & Michailides, T. J. (2005). Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Protection, 24, 853–863.
Ma, Z. H., Yoshimura, M. A., Holtz, B. A., & Michailides, T. J. (2005). Characterization and PCR-based detection of benzimidazole-resistant isolates of Monilinia laxa in California. Pest Management Science, 61, 449–457.
Maymon, M., Zveibil, A., Pivonia, S., Minz, D., & Freeman, S. (2006). Identification and characterization of benomyl-resistant and -sensitive populations of Colletotrichum gloeosporioides from statice (Limonium spp.). Phytopathology, 96, 542–548.
McKay, G., Egan, J. D., Morris, E., & Brown, A. E. (1998). Identification of benzimidazole resistance in Cladobotryum dendroides using a PCR-based method. Mycological Research, 102, 671–676.
Michael, K., Grimmer, F. V. B., Stephen, J. P., & Neil, D. P. (2015). Fungicide resistance risk assessment based on traits associated with the rate of pathogen evolution. Pest Management Science, 71, 207–215.
Mikaberidze, A., McDonald, B. A., & Bonhoeffer, S. (2014). Can high-risk fungicides be used in mixtures without selecting for fungicide resistance? Phytopathology, 104, 324–331.
Nenad, T., Anja, M., Rade, S., Milana, M., Jelena, J., Ivo, T., & Jelena, B. (2015). Occurrence of Cercospora beticola populations resistant to benzimidazoles and demethylation-inhibiting fungicides in Serbia and their impact on disease management. Crop Protection, 75, 80–87.
Olaya, G., & Köller, W. (1999). Baseline sensitivities of Venturia inaequalis populations to the strobilurin fungicide kresoxim-methyl. Plant Disease, 83, 273–278.
Russell, P. E. (2004). Sensitivity baselines in fungicide resistance research and management. FRAC Monograph 3, CropLife International, Brussels. Available on line at www.frac.Info.
Shi, H. J., Wu, H. M., Zhang, C. Q., & Shen, X. (2013). Monitoring and Characterization of resistance development of strawberry Phomopsis leaf blight to fungicides. European Journal of Plant Pathology, 135, 655–660.
Sierotzki, H., Wullschleger, J., & Gisi, U. (2000). Point mutation in cytochrome b gene conferring resistance to strobilurin fungicides in Erysiphe graminis f.sp. tritici field isolates. Pesticide Biochemistry and Physiology, 68, 107–112.
Torres-Calzada, C., Tapia-Tussel, R., Higuera-CiaparaI, M. R., Nexticapan-Garcez, A., & Perez-Brito, D. (2015). Sensitivity of Colletotrichum truncatum to four fungicides and characterization of thiabendazole-resistant isolates. Plant Disease, 99, 1590–1595.
Wu, R. F., Yang, X. L., & Yang, D. Z. (2013). Studies on identification of eggplant Phomopsis rot caused by Phomopsis vexans and its biological characteristics. China Vegetables, 1(8), 80–85.
Zhang, C. Q., Liu, Y. H., & Zhu, G. N. (2010). Detection and characterization of benzimidazole resistance of Botrytis cinerea in greenhouse vegetables. European Journal of Plant Pathology, 126, 509–515.
Zhang, C. Q., Liu, Y. H., Ding, L., & Zhu, G. N. (2011). Shift of sensitivity of Botrytis cinerea to azoxystrobin in greenhouse vegetables before and after exposure to the fungicide. Phytoparasitica, 39, 293–302.
Acknowledgments
This research was partially supported by the Special Fund for Agro-Scientific Research in the Public Interest (No. 201303023).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Dai, D.J., Di Wang, H. et al. Management of benzimidazole fungicide resistance in eggplant brown rot (Phomopsis vexans) with pyraclostrobin. Phytoparasitica 44, 313–324 (2016). https://doi.org/10.1007/s12600-016-0534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0534-1