Abstract
Field trials conducted on a yellow-red latossol (pH 6.0), replicated in 2010 and 2011, sought to examine the effect of silicon, phosphite minerals, synthetic fungicides and genetic resistance for wheat blast management (Magnaporthe grisea) in Central Brazil. Disease intensity was measured on cvs. BRS 264 and BR18 subjected to the following Si treatments: pre-plant furrow application of Ca & Mg silicate (300 kg ha-1); post-plant scattered application of Ca & Mg silicate on top of the soil (1 ton ha-1); multiple foliar SiO2 applications (30 g l -1); and non-treated control. Blast incidence and severity were scored. Further experiments were conducted on cv. BR-264, for examination of the effect of potassium phosphite and synthetic fungicides on wheat blast intensity, with the following treatments: K2HPO3 (1ml l -1); epoxinazole + pyraclostrobin (700 ml ha-1); tebuconazole (600 ml ha-1); tebuconazole + trifloxystrobin (750 ml ha-1); and non-treated control. In 2010, disease intensity was lower than in 2011. In the silicate experiments, disease was significantly lower when plants were treated with foliar or furrow silicate. Si applications significantly reduced disease in BRS-264. While BR-18 consistently demonstrated lower disease levels, cv. BRS-264 generally responded more markedly to silicon applications. In the phosphite/fungicide experiment of 2010, all treatments reduced disease when compared with the control, and in 2011 phosphite efficiency was not significantly different from some fungicide treatments. Synthetic fungicides demonstrated an average blast control of 55% by severity values. Yields were increased in the phosphite-treated plots (by 9–80%), in the Si treatments (by 26–92%), and more so, and more consistently, with synthetic fungicides (by 90–121%). Combined results of all field studies, carried out under environmental conditions highly conducive to disease, indicated that control of wheat blast necessitates the joint integration of several alternatives for efficient disease management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat blast, caused by Magnaporthe grisea (Cook) Sacc. (anamorph: Pyricularia grisea (Hebert) Barr.) is arguably the most yield-limiting wheat disease in Brazil, both in the traditional planting regions of southern Brazil as well as in the nontraditional region of the mid-west (Goulart et al. 2007). Wheat blast was unknown to science up to its first detection in the southern Brazilian state of Paraná (Igarashi et al. 1986). Since then, wheat blast has spread to all areas cultivated with wheat in Brazil, Argentina, Bolivia and Paraguay (Fernandes & Pavan 2011; Kohli et al. 2011). Disease intensity varies widely from year to year, depending on environmental conditions. When precipitation, higher temperatures (highest at 300C) and extended leaf wetness periods (equal to or longer than 10 h) coincide with the heading stage, disease severity reaches maximum values and epidemic progress is rapid (Cardoso et al. 2008). The pathogen affects all aerial parts of the wheat plant, but the most characteristic symptom is observed in the spikes, which become bleached above dark lesions formed in the rachis, where the fungus sporulates abundantly. The bleached grain has no commercial value and yield losses of up to 74% can be incurred (Goulart et al. 2007). The dependency on fungicide sprays, which are not entirely effective, further reduces growers’ returns, threatening the industry´s economic viability. Nevertheless, a specific determination of yield loss has not been performed.
The scarcity of adequate levels of genetic resistance to the disease in Triticum aestivum is similar to the history of Magnaporthe adaptation to host resistance in rice (Oryza sativum). In addition, the fact that the disease is seed-transmitted, indicates that blast poses a potential threat to many wheat-growing regions of the world (Duveiller et al. 2007). In addition to South America, areas presumably at higher risk are parts of central India, Bangladesh and Ethiopia (Duveiller et al. 2011). Among 72 representative isolates of the wheat blast causal agent, 54 distinct virulence patterns have been observed (Urashima et al. 2004), which indicates a level of genetic diversity compatible with the one observed among rice isolates of M. grisea. Although most of the research on this disease has so far been done in Brazil, proactive cultivar screening tests are also being conducted elsewhere (Cruz et al. 2011a). The history of fungicide resistance among rice isolates of this fungus is also cause for concern.
Due to all of the complexities in wheat blast disease management, alternative forms of control, additional to genetic host resistance and synthetic fungicides, should be examined. The use of silicates and phosphites are among these alternatives. There are abundant references for the effect of silicon (Si) as a booster of plant disease resistance, especially in rice, such as on sheath blight (Rhizoctonia solani, Rodrigues et al. 2001), brown spot (Bipolaris oryzae, Zanão Junior et al. 2009), and especially, rice blast (e.g. Datnoff et al. 2007). Silicon has also been shown to increase sorghum resistance to anthracnose, caused by Colletotrichum sublineolum (Resende et al. 2009). In addition, measurable disease-controlling effects have been reported in hosts other than Poaceae (Chérif et al. 1992; French-Monar et al. 2010). In wheat, it has been reported to increase resistance to powdery mildew (Blumeria graminis f.sp. tritici, Bélanger et al. 2003) and spot blotch (Bipolaris sorokiniana, Domiciano et al. 2010), but a recent study under controlled conditions and with artificial inoculation has shown only a slight effect of the foliar application of silicate on wheat blast (Cruz et al. 2011b). Silicon effects in the host plant are structural, by deposition and accumulation under the cuticle (Cai et al. 2009; Datnoff et al. 2007) as well as an inductor of antifungal compounds (Rémus-Borel et al. 2005). Silicates are applied to the soil either in powder or in granulated form or directly to the leaves in liquid form (Prabhu et al. 2001).
Phosphites are very mobile in the xylem and phloem and act as resistance inductors (Deliopoulos et al. 2010; Reuveni 1997). Potassium phosphite, K2HPO3, has been widely used. They have been first used for control of oomycetes (Forster et al. 1998; Rohrbach & Schenck 1985), but they are also applied against true fungi (Lovatt & Mikkelsen 2006). There has been a recent report that K2HPO3 reduced the severity of wheat blast under controlled conditions (Cruz et al. 2011b).
This paper reports the field efficacy of alternative methods for wheat blast control and their impact on grain yield, under natural environmental conditions favorable for blast progress, using cultivars with different reactions to the disease.
Materials and methods
Experiments were carried out in Planaltina, DF, mid-west Brazil, latitude 17º35'03" S, longitude 47º42'30" W, altitude 1100 m, with natural wheat blast inoculum present in the region. This is part of the Brazilian Cerrado Biome, which harbors areas of high pathotype diversity for Magnaporthe (Dias Neto et al. 2010; Urashima et al. 2004). Soil of the experimental fields is classified as yellow-red latossol, pH 6.0, 3.54% organic matter, 0.43 mg kg-1 silicon dioxide (SiO2), and the following chemical characteristics, (cmolc dm -3): Ca: 2.9; Mg: 0.7; K: 0.72; Na: 0.05; Al: 0.0; Acidity (H + Al): 4.3; and (mg dm -3): P: 49.0; B: 0.61; Cu: 1.36; Fe: 69.2; Mn: 16.7; Zn: 13.4; S: 6.8. Temperature, relative humidity and precipitation data were taken from Embrapa Cerrados’ meteorological station, located 2.4 km from the site of the field trials. Two independent field studies were instituted in the same experimental area, 100 m apart, on November 15, 2010 and repeated on February 17, 2011. Sowing was done manually, with 80 seeds/m. Four hundred kg of NPK 4-30-16 + boron fertilizer was applied at planting, followed by 180 kg ha-1 of CO(NH2)2 at 25 and 45 days after emergence. Weeds and pests were controlled by metsulfuron and chlorpyrifos, at recommended dosages. Two applications of propiconazole (750 ml ha-1, not recommended for wheat blast management), were made for the preventive control of other foliar pathogens. Supplemental low-pressure overhead irrigation was applied when necessary, in order to make the environment most favorable to the disease.
Efficiency of silicates for blast control in wheat cultivars of different resistance levels
Two wheat cultivars were selected for the study of the efficiency of silicates for wheat blast control: BRS 264, highly susceptible to blast (HS), and BR18-Terena, reported by others as moderately resistant (MR) to the disease (Arruda et al. 2005; Goulart & Paiva 1992; Urashima et al. 2004). The effects of three forms of silicate and one non-treated control were compared: pre-plant application of Ca (CaSiO3) & Mg (MgSiO3) silicate to the plant furrows (300 kg ha-1); post-plant application of Ca & Mg silicate, scattered once on top of soil (1 ton ha-1); multiple (three) foliar silicate clay applications (17.43% SiO2, 30 g l -1, Rocksil®), starting at the flag leaf stage (see below). Blast incidence (percentage of symptomatic spikes) and severity (percentage of the spike area with symptoms (see below), were scored. Ca & Mg silicate applied to the soil had the following composition: Ca: 25.0%; CaO: 34.9%; Mg: 6.0%; MgO: 9.9%; Si: 10.5%; SiO2: 22.4%. Composition of the ground silicate clay applied to leaves was: Al203: 20.6%; SiO2: 17.4%; Ca: 12.0%; S: 9.8%; K: 3.0% CaO: 1,3%; TiO2: 0.34%; Mg: 0.18%; Fe203: 0.16%; P2O5: 0.10%. The three foliar applications were done at 15-day intervals, starting when 50% of the leaves were at stage 47 (flag leaf sheath opening, Zadoks et al. 1974). Experimental units were composed of five 3.0-m plant lines, spaced at 0.20 m. Data were collected from the three central lines, discarding 0.50 m at extremities. The experiment and its repeat were conducted as a 2 x 4 factorial in a randomized complete block design (RCBD), with four replicates.
Efficiency of phosphite and synthetic fungicides for wheat blast control
The study on the efficiency of phosphite and fungicides was conducted on cv. BRS-264 (HS) with the following treatments: K2HPO3 (1 ml l -1); epoxinazole + pyraclostrobin (700 ml ha-1); tebuconazole (600 ml ha-1); tebuconazole + trifloxystrobin (750 ml ha-1); and non-treated control. Two applications were done at a 15-day interval, corresponding to Zadoks' phenological stages 50 (inflorescence emergence) and 70 (milk). Experimental units, assessment of incidence and severity were as described above. The experiment and its repeat were conducted in a RCBD with five treatments and four replicates.
Disease variables, yield estimates and statistical analysis
Blast incidence was estimated as the rate of symptomatic spikes over total number of spikes (healthy + symptomatic) in percentage points. Severity was estimated with the aid of the scale proposed by Trindade et al. (2006), which assigns notes according to the point at which the pathogen has penetrated the rachis, and consequently affects the area of the spike above the point of entry, where 0 = symptoms absent, 1 = 25 % of the spike symptomatic; 2 = 50%, 3 = 75%, and 4 = 100%. Productivity per ha was estimated based on grain yield in treated and control plots. For statistical analysis, normality of the percentage values was verified by the Kolmogorov-Smirnov procedure, followed by analysis of variance and mean separation by Tukey´s test (P ≤ 0.05).
Results
Mean maximum and minimum temperatures were very similar in the first (November 2010) and second (February 2011) planting periods (27.7, 17.4oC vs 27.3, 17.6oC, respectively). Maximum and minimum relative humidity (r.h.) was also comparable in the course of the first and second experiments (96.2–52.4% vs 93.7–49.9%, respectively). Inflorescence emergence (Zadoks' stages 50-59) occurred circa 50 days after planting. Rains were more frequent in the first (15. XI. 2010) than in the second (17. II. 2011) planting period, (647 mm vs 297 mm, respectively), but additional overhead irrigation approached precipitation totals of both periods. Overall disease levels were very high in both experimental periods, but reached extremely high levels in the experiments conducted in the second planting date as compared with the first planting date.
Efficiency of silicates for blast control in wheat cultivars with different resistance levels
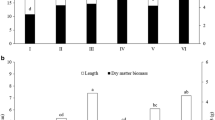
A highly significant interaction was found between the Si treatments and plant genotypes for incidence in 2010 (Table 1) and for severity in 2011 (Table 2). For cv. BRS 264 (HS), foliar and furrow Si applications reduced disease incidence significantly in 2010 (64.4–64.8% vs 99.4% in control plots, Fig. 1A). Foliar Si applications clearly reduced severity in 2011 (75.9% vs 97.7% in the control plots, Fig. 2B). A trend towards lower severity is also apparent in 2010 (Fig. 2A).
Incidence of blast, caused by Magnaporthe grisea, on wheat cultivars highly susceptible (BRS 264) and moderately resistant (BR 18) to the disease and treated with foliar silicon applications (starting at flag leaf stage), pre-plant furrow Si application, or post-plant scattered Si application, under conditions less (A, 2010) and more (B, 2011) favorable to the disease
Severity of blast, caused by Magnaporthe grisea, on wheat cultivars highly susceptible (BRS 264) and moderately resistant (BR 18) to the disease and treated with foliar silicon applications (starting at flag leaf stage), pre-plant furrow Si application, or post-plant scattered Si application, under conditions less (A, 2010) and more (B, 2011) favorable to the disease
Disease intensity was generally lower in the moderately resistant cv. BR 18, and the effects of silicate treatments were not significant (P ≤ 0.05, Figs. 1, 2). Nevertheless, a trend towards reduced incidence was discerned in 2010 with foliar Si applications in BR 18 (69% in foliar treated plots vs 81% in control plots, Fig. 1A) and severity (50.8% vs 84.1%, respectively, Fig. 2A).
Efficiency of phosphite and synthetic fungicides for wheat blast control
Wheat blast intensity in the phosphite and fungicide assays was extremely high in the susceptible variety, reaching 100% incidence in the untreated plots (both years) and 89.7% and 98.6% severity in 2010 and 2011, respectively. Nevertheless, significant differences were found (Table 3). In 2010, all treatments reduced disease intensity when compared with the untreated plots, with the epoxiconazole+ pyraclostrobin treatment decreasing disease incidence by 52% and severity by 72% (Table 3). In 2010, the phosphite treatment was as efficient as the synthetic fungicides, reducing blast incidence and severity by 36% and 56%, in that order, when compared with the untreated plots.
Blast levels in 2011 exceeded the already high levels observed in 2010. Synthetic fungicides reduced disease incidence by a factor of 32% to 38%, but no significant reduction was found with the phosphite treatment in that year (Table 3). Blast severity was reduced by synthetic fungicides, whereas K2HPO3 applications had an intermediate response, and did not differ significantly from either the untreated plots or from two out of the three synthetic fungicide treatments.
Impact on grain yield
Yields in the synthetic fungicide/phosphite treatments differed widely from non-sprayed control plots, reflecting the relative effect of each treatment (Table 3). In 2010, phosphite application significantly increased yield by 80%, while synthetic fungicides increased yields by 90–121%. In 2011, respective values were 9% for phosphite (not significant, P > 0.05) and 93–111% for synthetic fungicides (P < 0.05). Yields in the silicon experiments were uniformly reduced to extremely low levels by high disease intensity, especially in the 2011 season. Therefore, only data from the silicon experiment with BRS 264 in 2010 were compared. Yields in the control and scattered silicon treatments were the lowest and did not differ significantly from each other (194.8 kg ha-1 in the control plots and 245.2 kg ha-1 in the scattered silicon plots), while yields in the furrow and foliar treatments were significantly higher (371.8 and 373.7 kg ha-1, respectively, not significantly different from each other). BR 18 yield did not respond significantly to Si applications in either year.
Discussion
Environment conditions were conducive to wheat blast in both experimental seasons. The higher disease levels found in all field plots planted on February 2011 can be ascribed to the presence of inoculum sources on the debris of the first planting (November 2010). Although all field tests relied on natural inocula, the later experiments were conducted on areas at distances up to 100 m from the earlier experiments, which were taken down only days before sowing the second experiments.
Silicate treatments, especially foliar sprays, were effective in reducing wheat blast. Several earlier studies have demonstrated the potential of Si to reduce rice leaf or panicle blast (e.g. Berni & Prabhu 2003; Datnoff et al. 2007; Prabhu et al. 2001; Santos et al. 2011a). In wheat, silicates were shown to increase plant response to infection by powdery mildew (Blumeria graminis f.sp. tritici, Bélanger et al. 2003) and spot blotch (Bipolaris sorokiniana, Domiciano et al. 2010). However, reported effects on wheat blast are ambiguous: While Xavier-Filha et al. (2011) argued for the potential of Si to decrease susceptibility to blast in controlled studies, Cruz et al. (2011b) found only very limited effects; although both studies were conducted under controlled environment conditions, the methodologies used to evaluate plant–pathogen interactions were very different. This is the first report of field studies on the effect of Si on wheat blast relying on natural inoculum, conducted in a hot spot for Magnaporthe diversity. Environmental conditions, including supplemental irrigation, were extremely favorable to the disease. Even under such conditions, a measurable effect of foliar or furrow Si applications was found. Seebold et al. (2004) observed that under conditions relatively unfavorable to rice blast, the application of 1000 kg ha-1 of silicon reduced symptoms better than did the fungicide tricyclazole in Colombia. Accordingly, our results indicate that efficacy of silicate treatments is expected to be more valuable when conditions for the wheat blast development are less favorable. When disease pressure was high, the observed effects of silicate treatments were less marked. Nevertheless, a combination of silicate and synthetic fungicides, not tried here, may display additive or even synergistic effects.
Regarding the reaction of cvs. BRS 264 and BR 18-Terena, the latter seems to possess a higher level of quantitative resistance. Previous assessment of resistance of cv. BR 18 varied. This genotype has been referred to as resistant (Arruda et al. 2005; Goulart & Paiva 1992), was found to have a broad resistance spectrum to M. grisea isolates, and was recommended for wheat resistance breeding programs (Urashima et al. 2004). However, it has also been grouped together with susceptible genotypes in a test by Cruz et al. (2010). Our results indicate a moderate quantitative level of resistance to blast in BR 18, clearly inadequate to withstand high disease pressure in the field, without concurrence of other disease-reducing methods. Hypothetically, BR 18 could be recommended in an integrated system in combination with other management strategies.
Nonetheless, it should be pointed out that cv. BR 18, being partially resistant, obviously responded less efficiently to treatment with silicates. Clearly, there is a significant interaction of disease variables and genotypes (Tables 1, 2). This is due to the differential response of genotypes to silicon, either in anionic or atomic form (Cruz et al. 2011b). A greater predisposition of cv. BRS 264 to the enhanced resistance by Si application seems to be the case, even if more studies are necessary to confirm this hypothesis. Some earlier works demonstrated that Si concentration in wheat leaves is naturally low, circa 1% to 2%, whereas Si in rice leaves can reach 10% (Dallagnol et al. 2009; Rafi & Epstein 1999). However, Xavier-Filha et al. (2011) observed that in wheat plants where this percentage was increased by artificial application, it significantly enhanced plant response to infection by M. grisea, reducing susceptibility to wheat blast. The ability to absorb Si may differ among wheat genotypes, as has been verified in rice (Winslow 1992), and can play an important role in the effectiveness of Si as a tool for wheat blast control. In general, our results indicate that cv. BRS 264 is apparently more predisposed to the beneficial effects of silicate treatment than BR 18. The different responses of BR 18 and BRS 264 to Si found in this study demonstrate the importance of testing T. aestivum genotypes for response to silicon, since genetic differences in Si uptake probably exist.
Although synthetic fungicides measurably reduced wheat blast, control by fungicide alone was not complete on cv. BRS 264 at either planting date. More interestingly, a positive effect of phosphite was observed, at times equal to the effect of synthetic fungicides (Table 3). The effect of phosphite as a disease resistance booster has been known on many crops, against oomycetes (Deliopoulos et al. 2010) and true fungi (e.g. Dallagnol et al. 2012), and includes resistance against rice blast (Manandhar et al. 1998), but to our knowledge, it has not been tested against wheat blast in the field. In wheat, Santos et al. (2011b) demonstrated positive effects for the control of Drechslera tritici-repentis and Bipolaris sorokiniana, but the response to phosphite depends on the crop species and on the pathosystem. Our results also indicate that the potential of fungicides to diminish blast intensity is much less evident on wheat than on rice, where 84–90% decreases in panicle infection have been reported (Scheuermann & Eberhardt 2011; Swamy et al. 2009). Urashima & Kato (1994) and Goulart & Paiva (1993) have already indicated that chemicals with good performance against rice blast were not equally efficient in the protection of wheat panicles to blast. Indeed, international prices of wheat grain, costs of synthetic fungicides and low efficacy of chemicals indicate that chemical control of wheat blast may not be economical (Goulart et al. 1996; Urashima & Kato 1994). Our results (Table 3) tend to corroborate these findings. Although yields were significantly increased in the chemical and phosphite treatments when compared with the non-treated control (80% to 121% in 2010 and 9% to 111% in 2011), these increases may not be economically feasible, due to the overall very low production observed in the trials. The same holds true for the foliar or furrow silicon treatments, which increased production over control plots by 92% and 91%, respectively, albeit over an excessively low basis in the control plots. Nevertheless, the value of synthetic fungicides, as well as of the alternative compounds phosphite and silicon, are established as useful tools in the development of integrated pest management practices for wheat blast control.
Generally, very high intensities of wheat blast were observed in all field plots and planting dates. Even if significant reductions in disease were recorded with foliar or furrow applications of Si, potassium phosphite and synthetic fungicides, and a partial field resistance is present in BR 18, disease levels would render wheat crops uneconomical at these planting dates in the Brazilian Cerrado. The main reasons for this are the exceedingly favorable environment for the disease, compounded by use of irrigation, the high pathogen diversity in the Cerrado region, and the presence of inoculum in the plant debris of previous crops. Incidentally, the inadequate level of field resistance of BR 18 shown here further confirms that the natural diversity of M. grisea is indeed very high in the Brazilian mid-west. Clearly, although none of these disease management methods should be relied upon as isolated forms of disease control, they can be integrated, in a complementary fashion, especially under conditions less favorable to blast epidemics.
References
Arruda, M. A., Bueno, C. R. N. C., Zamprogno, K. C., Lavorenti, N. A., & Urashima, A. S. (2005). Reação do trigo à Magnaporthe grisea nos diferentes estádios de desenvolvimento. [Reaction of wheat to Magnaporthe grisea at different stages of host development]. Fitopatologia Brasileira, 30, 121–126.
Bélanger, R. R., Benhamou, N., & Menzies, J. G. (2003). Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f.sp. tritici). Phytopathology, 93, 402–412.
Berni, R. F., & Prabhu, A. S. (2003). Eficiência relativa de fontes de silício no controle de brusone nas folhas de arroz. [Relative efficiency of silicon sources on rice leaf blast control]. Pesquisa Agropecuária Brasileira, 38, 195–201.
Cardoso, C. A. A., Reis, E. M., & Moreira, E. M. (2008). Development of a warning system for wheat blast caused by Pyricularia grisea. Summa Phytopathologica, 34, 216–221.
Cai, K., Gao, D., Chen, J., & Luo, S. (2009). Mini-Review: Probing the mechanisms of silicon-mediated pathogen resistance. Plant Signaling and Behavior, 4, 1–3.
Chérif, M., Menzies, J. G., Benhamou, N., & Bélanger, R. R. (1992). Studies of silicon distribution in wounded and Pythium ultimum infected cucumber plants. Physiological and Molecular Plant Pathology, 41, 371–85.
Cruz, C. D., Bockus, W., Pedley, K., Peterson, G., Stack, J., Tang, X., et al. (2011a). Resistance among U.S. wheat (Triticum aestivum) cultivars to the wheat pathotype of Magnaporthe oryzae. Phytopathology, 101, S220. Abstract.
Cruz, M. F. A., Diniz, A. P. C., Rodrigues, F. A., & Barros, E. G. (2011b). Aplicação foliar de produtos na redução da severidade da brusone do trigo. [Foliar application of products on the reduction of wheat blast severity]. Tropical Plant Pathology, 36, 424–428.
Cruz, M. F. A., Prestes, A. M., Maciel, J. L. N., & Scheeren, P. L. (2010). Resistência parcial à brusone de genótipos de trigo comum e sintético nos estádios de planta jovem e de planta adulta. [Partial resistance to blast on common and synthetic wheat genotypes in seedling and in adult plant growth stages]. Tropical Plant Pathology, 35, 24–31.
Dallagnol, L. J., Rodrigues, F. A., Mielli, M. V. B., Ma, J. F., & Datnoff, L. E. (2009). Defective active silicon uptake affects some components of rice resistance to brown spot. Phytopathology, 99, 116–121.
Dallagnol, L. J., Rodrigues, F. A., Tanaka, F. A. O., Amorim, L., & Camargo, L. E. A. (2012). Effect of potassium silicate on epidemic components of powdery mildew on melon. Plant Pathology, 61, 323–330.
Datnoff, L. E., Rodrigues, F. A., & Seebold, K. W. (2007). Silicon and plant disease. In L. E. Datnoff, W. H. Elmer, & D. M. Huber (Eds.), Mineral nutrition and plant disease (pp. 233–246). St. Paul, MN, USA: APS Press.
Deliopoulos, T., Kettlewell, P. S., & Hare, M. C. (2010). Fungal disease suppression by inorganic salts: A review. Crop Protection, 29, 1059–1075.
Dias Neto, J. J., Santos, G. R., Silva, L. M. A., Cunha, A. C. R., Rangel, P. H. N., & Ferreira, M. E. (2010). Hot spots for diversity of Magnaporthe oryzae physiological races in irrigated rice fields in Brazil. Pesquisa Agropecuaria Brasileira, 45, 252–260.
Domiciano, G. P., Rodrigues, F. A., Vale, F. X. R., Xavier, M. S., Moreira, W. R., Andrade, C. C. L., et al. (2010). Wheat resistance to spot blotch potentiated by silicon. Journal of Phytopathology, 158, 334–343.
Duveiller, E., Singh, R. P., & Nicol, J. M. (2007). The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica, 157, 417–430.
Duveiller, E., Hodson, D., Sonder, K., & von Tiedemann, A. (2011). An international perspective on wheat blast. Phytopathology, 101, S220. Abstract.
Fernandes, J. C., & Pavan, W. (2011). Risk mapping wheat blast potential in Brazil. Phytopathology, 101, S221. Abstract.
French-Monar, R. D., Rodrigues, F. A., Korndorfer, G. H., & Datnoff, L. E. (2010). Silicon suppresses Phytophthora blight development on bell pepper. Journal of Phytopathology, 158, 554–560.
Forster, H., Adaskaveg, J. E., Kim, D. H., & Stanghellini, M. E. (1998). Effect of phosphite on tomato and pepper plants and susceptibility of pepper to Phytophthora root and crown rot in hydroponic culture. Plant Disease, 82, 1165–1170.
Goulart, A. C. P., & Paiva, F. A. (1992). Incidência de brusone (Pyricularia oryzae) em diferentes cultivares de trigo (Triticum aestivum) em condições de campo. [Incidence of blast (Pyricularia oryzae) in different wheat cultivars under field conditions]. Fitopatologia Brasileira, 17, 321–325.
Goulart, A. C. P., & Paiva, F. A. (1993). Avaliação de fungicidas no controle da brusone (Pyricularia oryzae) do trigo (Triticum aestivum). [Evaluation of fungicides in the control of wheat (Triticum aestivum) blast (Pyricularia oryzae)]. Fitopatologia Brasileira, 18, 167–173.
Goulart, A. C. P., Paiva, F. A., Melo-Filho, G. A., & Richetti, A. (1996). Efeito da época e do número de aplicações dos fungicidas tebuconazole e mancozebe no controle da brusone (Pyricularia grisea) do trigo. Viabilidade técnica e econômica. [Effect of timing and number of applications of the fungicides tebuconazole and mancozeb on the control of wheat blast disease (Pyricularia grisea) – economical and technical viability]. Fitopatologia Brasileira, 21, 381–387.
Goulart, A. C. P., Souza, P. C., & Urashima, A. S. (2007). Danos em trigo causados pela infecção por Pyricularia grisea. [Damage in wheat caused by infection of Pyricularia grisea]. Summa Phytopathologica, 33, 358–363.
Igarashi, S., Utiamada, C. M., Igarashi, L. C., Kazuma, A. H., & Lopes, R. S. (1986). Ocorrência de Pyricularia sp. em trigo no estado do Paraná. [Occurrence of Pyricularia sp. on wheat in the state of Paraná]. Fitopatologia Brasileira, 11, 351–352. Abstract.
Kohli, M. M., Mehta, Y. R., Guzman, E., De Viedma, L., & Cubilla, L. E. (2011). Pyricularia blast - a threat to wheat cultivation. A review. Czech Journal of Genetics and Plant Breeding, 47, 130–134.
Lovatt, C. J., & Mikkelsen, R. L. (2006). Phosphite fertilizers: What are they? Can you use them? What can they do? Better Crops, 90, 11–13.
Manandhar, H. K., Jørgensen, H. J. L., Mathur, S. B., & Smedegaard-Petersen, V. (1998). Resistance to rice blast induced by ferric chloride, di-potassium hydrogen phosphate and salicylic acid. Crop Protection, 17, 323–329.
Prabhu, A. S., Barbosa Filho, M. P., Filippi, M. C., Datnoff, L. E., & Snyder, G. H. (2001). Silicon from rice disease control perspective in Brazil. In L. E. Datnoff, G. H. Snyder, & G. H. Korndörfer (Eds.), Silicon in agriculture (pp. 293–311). Amsterdam, the Netherlands: Elsevier.
Rafi, M. M., & Epstein, E. (1999). Silicon absorption by wheat (Triticum aestivum L.). Plant and Soil, 211, 223–230.
Rémus-Borel, W., Menzie, J. G., & Bélanger, R. R. (2005). Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiological and Molecular Plant Pathology, 66, 108–115.
Resende, R. R., Rodrigues, F. A., Soares, J. M., & Casela, C. R. (2009). Influence of silicon on some components of resistance to anthracnose in susceptible and resistant sorghum lines. European Journal of Plant Pathology, 124, 533–541.
Reuveni, M. (1997). Post-infection applications of K3PO3, phosphorous acid and dimethomorph inhibit development of downy mildew caused by Plasmopara viticola on grapes. Journal of Small Fruit & Viticulture, 5, 27–38.
Rodrigues, F. A., Datnoff, L. E., Korndörfer, G. H., Seebold, K. W., & Rush, M. C. (2001). Effect of silicon and host resistance on sheath blight development in rice. Plant Disease, 85, 827–832.
Rohrbach, K. G., & Schenck, S. (1985). Control of pineapple heart rot, caused by Phytophthora parasitica and P. cinnamomi, with fosetyl-Al and phosphorous acid. Plant Disease, 69, 320–323.
Santos, G. R., Castro Neto, M. D., Ramos, L. N., Sarmento, R. A., Korndörfer, G. H., & Ignácio, M. (2011a). Effect of silicon sources on rice diseases and yield in the State of Tocantins, Brazil. Acta Scientiarum Agronomy, 33, 451–456.
Santos, H. A. A., Pria, M. D., Silva, O. C., & Mio, L. L. M. (2011b). Controle de doenças do trigo com fosfitos e acibenzolar-s-metil isoladamente ou associados a piraclostrobina + epoxiconazole. [Control of wheat diseases using phosphites and acibenzolar-s-methyl alone or associated with piraclostrobina + epoxiconazole]. Semina: Ciências Agrárias, 32, 433–442.
Scheuermann, K. K., & Eberhardt, D. S. (2011). Avaliação de fungicidas para o controle da brusone de panícula na cultura do arroz irrigado. [Fungicide evaluation for the control of panicle blast in irrigated rice]. Revista de Ciências Agroveterinárias, 10, 23–28.
Seebold, K. W., Datnoff, L. E., Correia-Victoria, F. J., Kucharek, T. A., & Snyder, G. H. (2004). Effects of silicon and fungicides on the control of leaf and neck blast in upland rice. Plant Disease, 88, 253–258.
Swamy, H. N., Sannaulla, S., & Kumar, M. D. (2009). Evaluation of new fungicides against rice blast in Cauvery delta. Karnataka Journal of Agricultural Sciences, 22, 450–451.
Trindade, M. G., Prabhu, A. S., Sá e Silva, M. (2006). Resistência parcial de genótipos de trigo a brusone nas folhas. .[Partial resistance of wheat genotypes to foliar blast]. Passo Fundo, BR: Embrapa Trigo (Comunicado Técnico, 201).
Urashima, A. S., & Kato, H. (1994). Varietal resistance and chemical control of wheat blast fungus. Summa Phytopathologica, 20, 107–112.
Urashima, A. S., Lavorenti, N. A., Goulart, A. C. P., & Mehta, Y. R. (2004). Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatologia Brasileira, 29, 511–518.
Winslow, M. D. (1992). Silicon, disease resistance and yield of rice genotypes under upland cultural conditions. Crop Science, 32, 1208–1213.
Xavier Filha, M. S., Rodrigues, F. A., Domiciano, G. P., Oliveira, H. V., Silveira, P. R., & Moreira, W. R. (2011). Wheat resistance to leaf blast mediated by silicon. Australasian Plant Pathology, 40, 28–38.
Zadoks, J. C., Chang, T. T., & Konzac, C. F. A. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421.
Zanão Junior, L. A. Z., Rodrigues, F. A., Fontes, R. L. F., Korndörfer, G. H., & Neves, J. C. L. (2009). Rice resistance to brown spot mediated by silicon and its interaction with manganese. Journal of Phytopathology, 157, 73–78.
Acknowledgments
A.C. Café-Filho is a research fellow of the Brazilian National Research Council, grant no. CNPq 301.095/2009-4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagani, A.P.S., Dianese, A.C. & Café-Filho, A.C. Management of wheat blast with synthetic fungicides, partial resistance and silicate and phosphite minerals. Phytoparasitica 42, 609–617 (2014). https://doi.org/10.1007/s12600-014-0401-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-014-0401-x