Abstract
Powdery mildew fungi are parasitized by strains of the genetically distinct Ampelomyces quisqualis. To investigate whether differences in the phylogeny and other cultural, morphological and physiological characteristics of these different strains are related to differences in their geographic origins or the host species from which they were isolated, several A. quisqualis strains isolated from different species of Erysiphaceae collected in different countries and possessing different ITS rDNA sequences were selected and characterized. The results revealed some significant variation among the selected strains, which provides evidence for the existence of different physiological forms within the A. quisqualis species. Two groups that display differential growth on artificial media were identified. These groups also differ in the morphology of their mycelium, but not in the morphology of their pycnidia and conidia. Temperature greatly affected the in vitro growth of the A. quisqualis strains and growth rate was closely correlated to colony color. Differences in the conidial germination of distinct strains were observed during the recognition phase of the parasitic relationship. The germination of each of the investigated strains was greatly stimulated by all of the examined powdery mildew species and not only by the conidia of their original hosts. An Italian strain isolated from grapevine in the Trentino Alto-Adige region was identified as the strain that germinates the most quickly in the presence of powdery mildew conidia. Phylogenetic analysis revealed that these A. quisqualis strains can be classified into five different genetic groups, which generally correlate with the fungal host of origin and morphological and growth characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ampelomyces quisqualis is a widespread hyperparasite of powdery mildews (Falk et al. 1995a; Kiss 1998, 2003). The natural occurrence of A. quisqualis on various Erysiphaceae species has been reported in different geographic regions (Angeli et al. 2009b; Kiss 1997; Kiss et al. 2004; Rankovic 1997). Most research on A. quisqualis has focused on its potential use as a biocontrol agent against powdery mildews of various crops (Bélanger and Labbé 2002; Paulitz and Bèlanger 2001; Sundheim and Tronsmo 1988; Sztejnberg et al. 1989). Its biology and biocontrol potential were reviewed by Kiss et al. (2004).
This mycoparasite invades and destroys host cytoplasm, killing the parasitized powdery mildew cells (Falk et al. 1995a, b; Hashioka and Nakai 1980; Kiss et al. 2004). Intracellular pycnidia of A. quisqualis are commonly found in hyphae, conidiophores and immature ascomata of powdery mildews (Kiss et al. 2004). These pycnidia vary in shape depending upon the fungal structure in which they are formed (Sundheim and Krekling 1982). Pycnidia contain cylindrical to spindle-shaped conidia, which are occasionally curved and two-spotted (Falk et al. 1995a). Recently, the microcyclic conidiogenesis of powdery mildews has been investigated (Kiss et al. 2009). When mildew colonies are treated with a suspension of A. quisqualis conidia, pycnidia are formed in microcyclic conidiophores, thereby accelerating the asexual reproduction of A. quisqualis.
The concentration of A. quisqualis conidia is an important factor affecting their germination. Germination has been shown to decrease dramatically when conidia are at a concentration of more than 106 conidia ml-1, due to the production of self-inhibitory substances (Gu and Ko 1997). A. quisqualis conidia do not germinate well in sterile, distilled water. However, their germination is significantly enhanced in the presence of conidia of powdery mildew fungi (Sundheim 1982). The presence of host fungi is recognized by A. quisqualis and a water-soluble substance from conidia of powdery mildew fungi has been shown to stimulate the germination of A. quisqualis conidia in vitro (Gu and Ko 1997). After penetration, the hyphae of the mycoparasite continue to grow and produce their intracellular pycnidia after 5 to 8 days in the mycelia of their fungal host (Hashioka and Nakai 1980; Sundheim and Krekling 1982). High relative humidity and temperatures between 20° and 25°C enhance the growth and sporulation of A. quisqualis, but this mycoparasite survives and is active against powdery mildew even at temperatures below 12°C (Jarvis and Slingsby 1997; Philipp and Cruger 1979).
Ampelomyces quisqualis is known to be a slow-growing fungus with an in vitro radial growth rate of 0.5–1.0 mm d−1 on Czapek-Dox agar supplemented with 2% malt extract (MCzA) at 23°C (Kiss 1997; Kiss and Nakasone 1998). Until recently, A. quisqualis was often confused with several other species: A. quercinus, A. humuli, A. heracli and Phoma glomerata. Unlike A. quisqualis, these other powdery mildew mycoparasites do not produce intracellular pycnidia and they also grow more quickly, with in vitro radial growth rates of 3–4 mm d−1 (Kiss et al. 2004; Sullivan and White 2000). In the past, scientists identified all of these fast-growing strains as A. quisqualis (e.g. Kiss 1997; Mhaskar 1974). Later, a molecular phylogenetic study of rDNA ITS sequences (Kiss and Nakasone 1998) revealed that the slow-growing and fast-growing strains belong to two distinct groups. The slow-growing strains are genetically more diverse, whereas the fast-growing ones show a close phylogenetic relationship with Epicoccum nigrum, which is known to have a pycnidial, Phoma-like state (Kiss et al. 2004). It seems likely that the fast-growing strains are, in fact, Phoma species, whereas true A. quisqualis strains always grow slowly in culture and produce intracellular pycnidia in powdery mildew mycelium.
Several A. quisqualis strains are available from culture collections and one strain has been formulated, registered and commercialized under the trade name of AQ10 (Kiss 1997; Sztejnberg 1993). Many molecular analyses based on the internal transcribed spacer (ITS) region of the nuclear ribosomal RNA gene (nrDNA) have revealed considerable genetic diversity among A. quisqualis strains (Angeli et al. 2009a; Liang et al. 2007; Park et al. 2010; Szentiványi et al. 2005). Recently, ITS sequences and microsatellite markers have been used to show that a set of A. quisqualis populations found in apple powdery mildew (Podosphaera leucotricha) are quite genetically distinct from populations collected from several other powdery mildew species infecting other plant species (Kiss et al. 2011). Although the genetic diversity within the A. quisqualis species has already been characterized, we still do not know whether the differences in the phenotypic characteristics of different strains of A. quisqualis are related to their phylogeny.
The morphological and cultural characteristics of this hyperparasite on different fungal hosts and plants have been reported to be extremely variable (Belsare et al. 1980; Shin and Kyeung 1994; Speer 1978). Some older studies focused on the shape and size of the pycnidia and conidia on culture media (e.g. Mhaskar 1974; Mhaskar and Rao 1974) and fungal hosts (e.g. Kiss 1997; Rankovic 1997). However, all of these studies were carried out when fast-growing strains (Phoma glomerata and Ampelomyces spp.) were still classified as A. quisqualis. Therefore, a thorough investigation of A. quisqualis sensu strictu is lacking. More physiological and phenotypic information is required to complete the picture of the genetic differences that have been identified in previous studies (Kiss et al. 2011; Liang et al. 2007; Park et al. 2010).
In the present work, we selected several A. quisqualis strains from different hosts and geographic regions, all characterized by the formation of intracellular pycnidia and slow radial growth at room temperature in vitro (defined as Type II; Kiss 1997), features corresponding to the characterization of A. quisqualis sensu strictu. We investigated the cultural, morphological and growth characteristics and phylogenetic relations (ITS) of these strains. The objectives of this study were to determine whether the host or site of origin of the strains or their cultural, morphological and/or growth characteristics are related to their phylogenetic group, which would indicate an adaptation to the host or geographic area. Furthermore, some physiological tests were performed as we looked for evidence of any host specificity or temperature adaptation of strains affecting the recognition phase of the host–parasite interaction.
Materials and methods
A total of 28 A. quisqualis strains isolated from different crops, sites and powdery mildew species were included in this study (Table 1). We selected strains isolated from grapevine (Vitis vinifera), cucumber (Cucumis sativus) and apple (Malus domestica) in different geographic regions, as well as strains isolated from wild and/or ornamental species. Only slow-growing strains, e.g. Type II (0.5–1.0 mm d−1) (Kiss 1997), producing intracellular pycnidia within powdery mildew mycelia were selected. The strains were obtained from culture collections (ATCC, American Type Culture Collection, Rockville, MD, USA; CBS, Centraalbureau voor Schimmelcultures, Baarn, the Netherlands; CABI, Commonwealth Agricultural Bureaux International, Egham, UK; DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen Gmbh, Braunschweig, Germany) or provided by individual scientists (L. Kiss, PPI, Plant Protection Institute of the Hungarian Academy of Sciences, Budapest, Hungary; D. Angeli, FEM, Fondazione Edmund Mach, S. Michele all’Adige, Italy). For phylogenetic comparison, 15 sequences of ITS rDNA of strains of A. quisqualis were retrieved from GenBank (Table 2). Strains were selected according to the fungal host of origin. The selected strains represent the four major clades reported in a recent study by Park et al. (2010).
Cultural, morphological and physiological characteristics
The cultural behavior and morphological characteristics of the pycnidia and conidia of the 28 selected A. quisqualis strains were evaluated on six different types of media: potato dextrose agar [PDA] (Oxoid, Hampshire, UK, 39 g l −1 twice-distilled water), sucrose nutrient agar (50 g sucrose, 28 g nutrient agar, twice-distilled water to 1 l), yeast malt dextrose agar (10 g dextrose, 5 g peptone, 3 g yeast extract, 3 g malt extract, 20 g agar, twice-distilled water to 1 l), nutrient agar (Oxoid, 28 g l −1 twice-distilled water), Czapek agar (Oxoid, 33.4 g l −1 twice-distilled water) and cornmeal agar (Oxoid, 17 g l −1 twice-distilled water)
The petri dish cultures of fungus were prepared with 100 μl of a suspension of sterile distilled water containing 102 conidia ml−1. The color of the mycelia and pycnidia was assessed by visual observation of colonies grown for 14 days at 25°C in the dark. We measured the length and width of 60 pycnidia and 60 conidia per strain (20 per replicate) on glass slides under a light microscope (Hund Wetzlar H 600LL, Wetzlar, Germany). The radial growth rate of the A. quisqualis strains was evaluated at temperatures of 10°, 15°, 20°, 25° and 30°C on petri dishes containing PDA that were inoculated as described above. Cultures were incubated in the dark for 30 days and the radial growth of the colonies (20 per replicate) was evaluated by measuring the diameter of each colony twice a week. All of the experiments were conducted with three replicates (plates) per strain.
The recognition of the fungal host by A. quisqualis strains at different temperatures was studied. The effects of the conidia of four powdery mildew species, Podosphaera xanthii (from cucumber), Erysiphe necator (from grapevine), Podosphaera aphanis (from strawberry) and Podosphaera leucotricha (from apple), on the germination rates of five selected A. quisqualis strains were evaluated. Each of the tested strains belongs to a different genetic (ITS) group (identified in this study) and was collected from a different host plant in a different geographic area (AQ10, ITA3, CBS129.79, MYA-3391 and MYA-3401). Each strain was paired with an isolate of the fungal host from which it was originally isolated and three other powdery mildews. Sterile distilled water suspensions of the conidia of each powdery mildew strain (1 × 105 conidia ml−1) together with each A. quisqualis strain (1 × 105 conidia ml-1) were prepared, for a total of 20 combinations. Suspensions of only A. quisqualis conidia in sterile distilled water were used as controls. Six drops (10 μl each) per combination (three replicates) were put onto a glass slide, which was placed in a petri dish (r.h. = 100%) and stored in a dark incubator kept at 25°C, which is the temperature that Gu and Ko (1997) used in their study of the factors affecting the germination of A. quisqualis conidia. The germination rates and germ-tube elongation of 150 conidia per strain (three replicates, 50 conidia per replicate) were evaluated under a light microscope after 24 h.
The effect of temperature on the germination of A. quisqualis conidia in the presence of the fungal host was evaluated, by testing two powdery mildew species (P. xanthii from cucumber and E. necator from grapevine) and the two A. quisqualis strains that were the most and least responsive to powdery mildew conidia in the previous experiment. Both the germination rate and the tube elongation of those strains were recorded at three temperatures (15°, 20° and 25°C) using the method described above.
Phylogenetic analysis
The phylogenetic relations of the 28 strains analyzed in the present study and another 15 strains selected for phylogenetic analysis were inferred from maximum-likelihood (ML) and maximum-parsimony (MP) analyses in which the sequences of their respective ITS rDNA regions were compared (White et al. 1990). The additional 15 sequences of ITS rDNA from A. quisqualis strains that were analyzed by Park et al. (2010) were retrieved from GenBank (Table 2). The strains isolated by Park et al. (2010), whose GenBank accession numbers begin with GQ and GU, were identified here with the same isolate numbers used by Park et al. (2010), to make it easier to recognize them across the two studies (SMKC22963 = GQ324149, SMKC22519 = GQ324144, SMKC22511 = GQ324095, SMKC23914 = GU329997).
The 28 selected strains (Table 1) were grown on PDA for 21 days at 25°C. DNA was extracted from approximately 200 μg of homogenized, lyophilized mycelium, using the Nucleo Spin Plant Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. The ITS region of the nuclear ribosomal DNA was amplified using ITS1 and ITS4 fungal-specific primers (Gardes and Bruns 1993). PCR was performed using the Gene Amp PCR System 9700 (PerkinElmer, Waltham, MA, USA). PCR products were purified using ExoSAP-IT enzymes (USB Corporation, Staufen, Germany) and sequenced using a Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Both DNA strands were sequenced with the primers that had been used for PCR amplification and electrophoresis was carried out using an ABI 3130xl Genetic Analyzer (PerkinElmer). The consensus sequence was assembled using Pregap4 and Gap4 (Staden et al. 2000; http://staden.sourceforge.net/). Blast searches were performed in the NCBI/GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/) using the program Megablast to find long stretches of alignment between very similar nucleotide sequences. The ClustalW2 program (Larkin et al. 2007; http://www.ebi.ac.uk/Tools/clustalw2/index.html) was used to construct multiple sequence alignments and to identify evolutionary relationships among the input sequences. ML and MP analyses based on ITS region sequences were conducted using the Mega4 program (Tamura et al. 2007; http://www.megasoftware.net/mega.html) and a p-distance model (Nei and Kumar 2000) was used to calculate within-group and between-group distances. A phylogenetic tree inferred from the ITS rDNA region sequences was constructed using the TreeView program, version 1.6.6 (Page 1996; http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). P. glomerata (AF126816), A. quercinus (AF035778) and A. humuli (AF035779) were also included in the model.
Clustering and statistical analysis
Morphological experiments (size of pycnidia and conidia, Table 3) were performed in three replicates per strain and medium and standard deviations of the averages were calculated. A one-way analysis of variance (ANOVA) was performed on log-transformed and arcsin-transformed data. If significant differences were detected, treatment means (strains and media) were separated using Tukey’s test (α = 0.05).
Growth tests involved two independent experiments with three replicates per experiment. A two-way ANOVA was performed on log-transformed and arcsin-transformed data from two experiments and revealed no significant Experiment × Treatment (temperature) interaction. Therefore, cluster analyses were performed using a software package for multivariate data analysis capable of performing K-means cluster analyses (Multi-Experiment Viewer, TMEV). The Euclidean distance between each of the samples within a given cluster and their respective cluster centroid was calculated. The angular coefficients (a) and the correlation coefficients (r) of the linear regression curves were also calculated. Data from the two experiments were pooled and averages of six replicates are presented in Table 4 and Fig. 1. Statistical analyses were performed using Statistica software 7.0 (Statsoft, Tulsa, OK, USA). Significant differences between treatments (strains and temperatures) were separated using Scheffè’s test (α = 0.05).
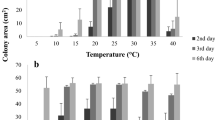
Effect of temperature (10°, 15°, 20°, 25° and 30°C) on the growth of Ampelomyces quisqualis strains over 30 days on potato dextrose agar. The radial growth rates of 28 strains, which divided into slow-growing and very slow-growing groups at each temperature, are presented (pooled data). K-means clustering using the software TMEV (Multi-Experiment Viewer) was based on the Euclidean distance between each of the strains at each temperature. Significant differences between groups were separated by ANOVA. Presented values are means of six replicates derived from two independent experiments with three replicates per experiment. Bars represent the standard deviation of the six replicates
Germination tests involved two independent experiments with three replicates per experiment and 50 conidia per replicate. Two-way ANOVA was performed on both the log-transformed and arcsin-transformed data from the two experiments and revealed no significant Experiment × Treatment (temperature) interaction. Therefore, data from the two experiments were pooled. Averages of six replicates are presented in Figs. 2 and 3. Significant differences between treatments (strains and powdery mildews) were separated using Tukey’s test (α = 0.05).
Effects of different powdery mildew species (Podosphaera xanthii from cucumber, Erysiphe necator from grapevine, Podosphaera aphanis from strawberry and Podosphaera leucotricha from apple) on the (a) conidial germination (%) and (b) tube elongation (μm) of five selected strains of Ampelomyces quisqualis. Measurements were taken in aqueous suspensions containing mixtures of A. quisqualis and powdery mildew conidia that had been incubated together for 24 h at 25°C. Identical column designs indicate exposure to the same powdery mildew species. Within strains, columns labeled with a common letter (a-d) do not differ significantly (P ≤ 0.05) according to Tukey’s test. Three replicates (50 conidia per replicate) of each strain were evaluated. Presented values are means of six replicates derived from two independent experiments with three replicates per experiment
Effect of temperature on the (a) conidial germination (%) and (b) tube elongation (μm) of two selected strains of Ampelomyces quisqualis in the presence of two different powdery mildew species (Podosphaera xanthii from cucumber and Erysiphe necator from grapevine). Measurements were taken in aqueous suspensions containing mixtures of A. quisqualis and powdery mildew conidia that had been incubated for 24 h at three different temperatures (15°, 20° and 25°C). Identical column designs indicate incubation at the same temperature. Within strains, columns labeled with the same letter (a-e) do not differ significantly (P ≤ 0.05) according to Tukey’s test. Three replicates (50 conidia per replicate) of each strain were evaluated. Presented values are means of six replicates derived from two independent experiments with three replicates per experiment
Results
Cultural, morphological and physiological characteristics
Cultural and morphological analyses of A. quisqualis strains indicated that two different colony types can be distinguished after 14 d on all tested media: A. quisqualis strains that form dark-brown colonies and those that form olive-green colonies (mycelium and pycnidia; Table 3). Furthermore, mycelial growth and pycnidial and conidial formation were all greatly increased on three of the tested media (PDA, sucrose nutrient agar and yeast malt dextrose agar), in comparison with the other media. The study of morphology of pycnidia and conidia (Table 3) revealed differentiation in color and dimensions of the fruiting bodies of the tested strains. In all of the examined strains, the color and shape of the pycnidia varied from light to dark (brown or green) and from ovoid to ellipsoid, with increasing age of the culture. The length and width of the pycnidia ranged from 30 to 95 μm and from 25 to 55 μm, respectively. A great deal of variation in width and length was observed among the pycnidia of individual strains, but no significant differences were found between strains (P = 0.112). The conidia of the different strains also varied in size. They ranged from 5.5 to 14.5 μm in length and from 2.3 to 3.5 μm in width. However, there were no significant differences between strains according to Tukey’s test (P = 0.073).
Temperature greatly affected the growth rate of A. quisqualis growth on PDA. The highest radial growth rates for all strains were measured between 15° and 25°C. At the extreme temperatures of 10° and 30°C, sharp reductions were observed in the growth rates (Fig. 1). K-means clustering based on Euclidean distances showed that at temperatures of 15°, 20° and 25°C, the slow-growing Type II strains (Kiss 1997) could be further clustered into two different growth-rate groups that were significantly different from each other. At all of these temperatures, the two groups included the same isolates, which we refer to as slow-growing (0.70–0.82 mm d−1) and very slow-growing (0.34–0.63 mm d−1) (Table 4). At 10° and 30°C, K-means clustering analysis revealed two growth-rate classes, which do not include exactly the same strains as the groups observed at the other tested temperatures (Table 4). However, no statistically significant differences were observed between the two classes (P = 0.09). At 10°C, the strains grew at a rate of 0.18–0.43 mm d−1, whereas at 30°C they grew at a rate of 0.07–0.33 mm d−1.
Our results indicate that, for all of the tested strains except MYA-3401, there is a relationship between the growth rates at 15°, 20° and 25°C and colony color. Strains that grew fairly slowly at these temperatures formed dark-brown colonies and strains that grew very slowly at these temperatures formed olive-green colonies. However, even with the limited number of strains examined, it is possible to demonstrate a relationship between growth rate and the original fungal host. Strains isolated from apple, grapevine and cucumber plants belonged to the same growth rate group at temperatures between 15° and 25°C. On the other hand, no relationship between the growth rate and the geographic origin of the strains was observed.
Physiological tests with different powdery mildew species and A. quisqualis strains showed that the germination rate and germ-tube length of A. quisqualis conidia increased greatly in the presence of powdery mildew conidia. This stimulation of germination is not powdery mildew species-specific. All of the tested powdery mildews increased the germination rate and tube elongation of the A. quisqualis strains isolated from different host species (Fig. 2a, b). However, in some cases, significant differences in the induction of germination were detected among the different powdery mildews. For example, the conidial germination of CBS129.79 (originally isolated from cucumber) was stimulated more strongly by grapevine powdery mildew and cucumber powdery mildew than by strawberry powdery mildew. The conidial germination of MYA-3391 (originally isolated from apple) was most strongly induced by grapevine powdery mildew and was less affected by strawberry and apple powdery mildews. We observed big differences among the conidial germination rates of the different strains, independent of the powdery mildew species. The conidial germination of ITA3 and MYA-3401 was strongly induced by conidia of all of the tested powdery mildew species (both in terms of germination rate and tube elongation), whereas AQ10 was generally the least responsive.
In the second experiment, investigating the effect of temperature, it was again observed that, in the absence of powdery mildew conidia, the germination rates and germ-tube elongation of strains ITA3 and AQ10 were very poor (Fig. 3a, b). Both parameters were strongly enhanced in the presence of powdery mildew conidia, with no statistical differences between the effects of E. necator and those of P. xanthii. As in the previous experiment, ITA3 was significantly more induced by powdery mildew conidia than AQ10 (Fig. 3a, b). We did not observe any changes in the general behavior of the two A. quisqualis strains at the different tested temperatures. In fact, temperature (within the tested range) had no effect on germination rate, but did seem to affect tube length. In the presence of powdery mildew conidia, tube elongation for both strains was always greater at the two higher temperatures (20° and 25°C) than at 15°C.
In these two experiments, AQ10 exhibited significantly lower germination rates and less tube elongation in the presence of powdery mildew conidia, as compared to the strain ITA3 (Fig. 3a, b). However, on agar plates incubated at the examined temperatures, AQ10 and ITA3 were classified as slow-growing and very slow-growing, respectively. Thus, the growth rates of strains of A. quisqualis on artificial media are not correlated with their germination rates and tube elongation in the presence of a host fungus.
Phylogenetic analysis
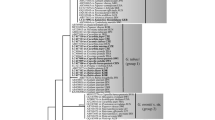
In the phylogenetic analysis, the 43 A. quisqualis strains clustered into five different groups (1–5), revealing genetic diversity among the A. quisqualis strains included in the present work (Fig. 4). Those strains with similar ITS sequences were often isolated from the same powdery mildew host. In fact, strains isolated from powdery mildew of apple (clade 3), powdery mildew of cucumber (clade 1) and powdery mildew of grapevine (clades 4 and 5) with bootstrap (BS) values between 98% and 99% mainly clustered into four different genetic groups. However, different sequence similarity was observed among strains in clade 1 (92–100%), clade 3 (99–100%), clade 4 (95–100%) and clade 5 (97–100%). Strains isolated from apple powdery mildew and classified within clade 3 displayed the least ITS diversity, even though they were collected in three different geographical areas.
The maximum-likelihood tree for the ITS rDNA sequences of 43 Ampelomyces quisqualis strains. Sequences were inferred with the Mega4 software package, using the Jukes-Cantor substitution model and rate uniformity among sites. The gaps in the 450-character-long alignment were handled as missing characters. The bootstrap values were obtained from maximum-likelihood and maximum-parsimony analyses. The values shown above the branches are percentages of 1000 replicates; scores below 50% are not shown. The scale bar represents 0.1 substitutions per nucleotide position. The five groups (1–5) discussed in the text are indicated on the tree. Different colors are used to indicate the different powdery mildew host species: blue = apple, green = grapevine, orange = cucumber, no color = other species). In brackets: geographic origin, colony color and growth pattern of each strain. Geographic origin: DE = Germany, UK = United Kingdom, HU = Hungary, CA = Canada, CH = Switzerland, IT = Italy, IL = Israel, EC = Ecuador, CN = China, KR = South Korea, US = United States of America. Colony color and growth pattern: bs = brown, slow-growing; bvs = brown, very slow-growing; gvs = green, very slow-growing)
Comparing clades, clade 1 containing strains isolated from cucumber had the greatest ITS sequence diversity. In fact, these strains can be divided into two different subclades, the first composed of strains collected in Canada (1A) and the second composed of strains collected in China (1B). The strains within each of these subclades share exactly the same ITS sequence (100%). Comparing strains according to their host, the strains isolated from grapevine powdery mildew clearly displayed the highest level of ITS diversity. These strains fell into two different clades: clade 4, which includes strains isolated in China, and clade 5, which includes strains isolated in the USA and Italy. Taking a closer look at the ITS sequence clustering in clade 5, it is seen that, with the exception of ATCC200250, the Italian strains seem to differ from the American strains. The only ITS clade which includes strains isolated from different mycohosts and in different geographical regions was clade 2. This clade contains two strains from grapevine powdery mildew (ITA1 and ITA2), one strain from cucumber powdery mildew (DSM2222) and nine strains isolated from several different ornamental plants. The 12 strains in clade 2 (BS value of 83%) were further divided into two subgroups: subclade 2A (nine sequences) and subclade 2B (three sequences), which were supported by BS values of 83% and 99%, respectively, and which each had ITS-similarity scores of 98–100%. Among the strains included in subclade 2A, only the ITS sequence of strains DSM2225 and DQ490762 differed from that of the commercial strain AQ10.
Interestingly, strains isolated from the same fungal host and assigned to the same ITS group (clades 1, 3, 4 and 5) showed similar colony morphology and growth characteristics. Strains belonging to clades 3, 4 and 5 are green and very slow-growing, whereas strains belonging to clade 1 are brown and grow more quickly. Again, clade 2 is an exception in that it includes strains that differ in the color of their colonies and their in vitro growth rates.
Discussion
Ampelomyces quisqualis strains have been studied mostly for use in the biological control of powdery mildew on different plant species. Data on the morphology and cultural patterns of A. quisqualis strains found in the literature are contradictory and incomplete. There is a need for further cultural and morphological investigations aiming at the identification of phenotypic markers that can be used to differentiate genetically distinct groups (Park et al. 2010) within the true A. quisqualis [e.g. Type II (Kiss 1997)].
The aim of this study was to understand whether there are relationships between the origins (host, geographic location) of different strains of A. quisqualis and their phylogeny and/or other characteristics (cultural, growth, morphology, physiology), in order to establish the existence of any host/geographic adaption of this mycoparasite. Our results provide solid evidence for the existence of different physiological forms among slow-growing A. quisqualis strains [e.g. Type II (Kiss 1997)] isolated from different fungal hosts. These physiological forms vary in terms of their cultural, morphological and physiological characteristics. In the present study, the A. quisqualis strains cultured on artificial media did not differ in the dimensions or shape of their fruiting bodies (pycnidia and conidia). Thus, these morphological characters are insufficient for accurate identification of different strains in the laboratory. Based on colony characteristics and growth rates on artificial media, two fairly homogeneous groups can be clearly distinguished: dark-brown, slow-growing strains and olive-green, very slow-growing strains. Thus, a clear relationship was detected between the growth rate and colony color of A. quisqualis strains. Moreover, we demonstrated the existence of a relationship between the original fungal host and cultural and growth characteristics. In contrast, we did not identify any relationship between the geographic origins of the different strains and their in vitro growth rates.
As expected, temperature greatly affects the growth rate of A. quisqualis. We observed differences in the behavior of different strains at different temperatures and six strains (DSM4624, CBS128.79, CBS129.79, CBS130.79, CBS131.79, CABI272851) grew faster than the others at all of the tested temperatures.
Powdery mildew strongly affects the germination of A. quisqualis conidia during the recognition phase of parasitism, but specialization is indistinct. In fact, the germination rates and tube elongation of all of the examined A. quisqualis strains could be stimulated by all of the examined powdery mildew species and not only by the conidia of their original fungal host. However, we did observe statistically significant differences among the tested strains. Strain ITA3 was most strongly stimulated and, interestingly, among all the tested strains, the commercial strain AQ10 was stimulated the least by the presence of conidia of different powdery mildew species.
It was found that the growth rate of A. quisqualis on artificial media is not related to the conidial germination or the germ-tube elongation of A. quisqualis during the recognition phase of parasitism. Therefore, we think that a screening program based on in vitro growth rates is not the right way to select strains of this mycoparasitic fungus for use in biocontrol programs.
Finally, phylogenetic analysis of the ITS rDNA sequence revealed a high level of genetic diversity among Type II strains of A. quisqualis and suggested that ITS groups could be related to the host fungus. In most cases, a degree of mycohost specialization was present in the A. quisqualis strains, as demonstrated by the phylogenetic studies conducted by Park et al. (2010). Our study revealed that strains isolated from the same host species had similar cultural, morphological and growth characteristics and grouped into the same ITS group. Moreover, among the strains isolated from cucumber powdery mildew and grapevine powdery mildew, we observed differentiation among A. quisqualis strains that was congruent with differences in geographic origin. Therefore, it appears that, in some cases, powdery mildew fungi and A. quisqualis might have coevolved through the processes of specialization and adaptation without the mycoparasite losing the ability to be stimulated by other hosts.
The present results indicate that the Type II strains of A. quisqualis examined in this study are widely scattered among four clades and two subclades identified by Park et al. (2010). Furthermore, the presence of an additional clade (5) was revealed. This clade includes the Type II strains isolated from grapevine powdery mildew whose morphology and physiology were analyzed in this experiment, as well as all of the American strains of grapevine powdery mildew. Clade 5 appears homogeneous in regard to the mycohost (E. necator), as well as colony color and growth pattern. However, the clustering of strains within clade 5 may point to a certain tendency toward geographic–genetic differentiation, suggesting possible local adaptations of this mycoparasite that should be verified in wider studies. Clade 2A includes the commercial AQ10 strain, as well as five strains (CABI272851, MYA-3401, ITA1, ITA2, SMKC22511) that were isolated after the commercialization of AQ10 and have identical ITS sequences, suggesting that those five strains may have originated from AQ10 treatments applied in the same area. This would indicate that the commercial strain can spread and persist in nature. On the other hand, strain DQ490762 belonging to Clade 2A, which was isolated after the commercialization of AQ10 and having a different nucleotide sequence, probably represents a distinct strain.
Screening is a crucial step in the selection of strains capable of providing highly effective biocontrol. Future studies should attempt to clarify whether the conidial germination rates of A. quisqualis strains are related to differences in virulence against powdery mildew, which would be a relevant factor in the selection of biocontrol agents.
References
Angeli, D., Pellegrini, E., Micheli, S., Ress, D., Maurhofer, M., Pertot, I., et al. (2009a). Molecular characterization of Ampelomyces spp. strains from different hosts and geographic origins and evaluation of their potential to control powdery mildew of cucumber. IOBC/WPRS Bulletin, 43, 40–44.
Angeli, D., Pellegrini, E., & Pertot, I. (2009b). Occurrence of Erysiphe necator chasmothecia and their natural parasitization by Ampelomyces quisqualis. Phytopathology, 99, 704–710.
Bélanger, R. R., & Labbé, C. (2002). Control of powdery mildews without chemicals: prophylactic and biological alternatives for horticultural crops. In R. R. Bélanger, W. R. Bushnell, A. J. Dik, & T. L. W. Carver (Eds.), The powdery mildews: a comprehensive treatise (pp. 256–267). St. Paul, MN, USA: APS Press.
Belsare, S. W., Moniz, L., & Deo, V. B. (1980). The hyperparasite Ampelomyces quisqualis Ces. from Maharashtra State, India. Biovigyanam, 6, 173–176.
Falk, S. P., Gadoury, D. M., Cortesi, P., Pearson, R. C., & Seem, R. C. (1995a). Parasitism of Uncinula necator cleistothecia by the mycoparasite Ampelomyces quisqualis. Phytopathology, 85, 794–800.
Falk, S. P., Gadoury, D. M., Pearson, R. C., & Seem, R. C. (1995b). Partial control of grape powdery mildew by the mycoparasite Ampelomyces quisqualis. Plant Disease, 79, 483–490.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Gu, Y. H., & Ko, W. H. (1997). Water agarose medium for studying factors affecting germination of conidia of Ampelomyces quisqualis. Mycological Research, 101, 422–424.
Hashioka, Y., & Nakai, Y. (1980). Ultrastructure of pycnidial development and mycoparasitism of Ampelomyces quisqualis parasitic on Erysiphales. Transactions of the Mycological Society of Japan, 21, 329–338.
Jarvis, W. R., & Slingsby, K. (1997). The control of powdery mildew of greenhouse cucumber by water spray and Ampelomyces quisqualis. Plant Disease Reporter, 61, 728–730.
Kiss, L. (1997). Genetic diversity in Ampelomyces isolates, hyperparasites of powdery mildew fungi, inferred from RFLP analysis of the rDNA ITS region. Mycological Research, 101, 1073–1080.
Kiss, L. (1998). Natural occurrence of Ampelomyces intracellular mycoparasites in mycelia of powdery mildew fungi. The New Phytologist, 140, 709–714.
Kiss, L. (2003). A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Management Science, 59, 475–483.
Kiss, L., & Nakasone, K. K. (1998). Ribosomal DNA internal transcribed spacer sequences do not support the species status of Ampelomyces quisqualis, a hyperparasite of powdery mildew fungi. Current Genetics, 33, 362–367.
Kiss, L., Pintye, A., Kovacs, G. M., Jankovics, T., Fontaine, M. C., Harvey, N., et al. (2011). Temporal isolation explains host-related genetic differentiation in a group of widespread mycoparasitic fungi. Molecular Ecology, 20, 1492–1507.
Kiss, L., Pintye, A., Zséli, G., Jankovics, T., Szentivànyi, O., Hafez, Y. M., et al. (2009). Microcyclic conidiogenesis in powdery mildews and its association with intracellular parasitism by Ampelomyces. European Journal of Plant Pathology, 126, 445–451.
Kiss, L., Russell, J. C., Szentivanyi, O., Xu, X., & Jeffries, P. (2004). Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonist of powdery mildew fungi. Biocontrol Science and Technology, 14, 635–651.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). ClustalW2 and ClustalX version 2. Bioinformatics, 23, 2947–2948.
Liang, C., Yang, J., Kovacs, G. M., Szentivanyi, O., Li, B., Xu, X. M., et al. (2007). Genetic diversity of Ampelomyces mycoparasite isolated from different powdery mildew species in China inferred from analyses of rDNA ITS sequences. Fungal Diversity, 24, 225–240.
Mhaskar, D. N. (1974). Mycoparasite—Ampelomyces in artificial culture I. Morphology and cultural behaviour. Mycopathologia et Mycologia applicata, 52, 55–64.
Mhaskar, D. N., & Rao, V. G. (1974). The mycoparasite Ampelomyces quisqualis Ces. in artificial culture. II. Effect of environmental factors. Phytopathologia Mediterranea, 13, 147–154.
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. New York, NY: Oxford University Press.
Page, R. D. M. (1996). Tree View: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences, 12, 357–358.
Park, M. J., Choi, Y. J., Hong, S. B., & Shin, H. D. (2010). Genetic variability and mycohost association of Ampelomyces quisqualis isolates inferred from phylogenetic analyses of ITS rDNA and actin gene sequences. Fungal Biology, 114, 235–247.
Paulitz, T. C., & Bèlanger, R. R. (2001). Biological control in greenhouse systems. Annual Review of Phytopathology, 39, 103–133.
Philipp, W. D., & Cruger, G. (1979). Parasitismus von Ampelomyces quisqualis auf echten mehltaupilzen an gurken und anderen gemusearten. Zeitchrift fur Pflkanzenkrankheiten und Pflanzenschutz, 86, 129–142.
Rankovic, B. (1997). Hyperparasites of the genus Ampelomyces on powdery mildew fungi in Serbia. Mycopathologia, 139, 157–164.
Shin, H. D., & Kyeung, H. Y. (1994). Isolation of hyperparasitic fungi to control powdery mildews and selection of superior isolates for biocontrol of cucurbit powdery mildew. RDA Journal of Agricoltural Science, 36, 141–151.
Speer, E. O. (1978). Beitrag zur morphologie von Ampelomyces quisqualis Ces. Sydowia, 31, 242–246.
Staden, R., Beal, K. F., & Bonfield, J. K. (2000). The Staden package, 1998. Methods in Molecular Biology, 132, 115–130.
Sullivan, R. F., & White, J. F., Jr. (2000). Phoma glomerata as a mycoparasite of powdery mildew. Applied and Environmental Microbiology, 66, 425–427.
Sundheim, L. (1982). Control of cucumber powdery mildew by the hyperparasite Ampelomyces quisqualis and fungicides. Plant Pathology, 31, 209–214.
Sundheim, L., & Krekling, T. (1982). Host–parasite relationships of the hyperparasite Ampelomyces quisqualis and its powdery mildew host Sphaerotheca fuliginea. I. Scanning electron microscopy. Phytopathology, 104, 202–210.
Sundheim, L., & Tronsmo, A. (1988). Hyperparasites in biological control. Vol. I. In K. G. Mekerji & K. L. Garg (Eds.), Biocontrol of plant diseases (pp. 53–69). Boca Raton, FL, USA: CRC Press.
Szentiványi, O., Kiss, L., Russell, J. C., Kovács, G. M., Varga, K., Jankovics, T., et al. (2005). Ampelomyces mycoparasites from apple powdery mildew identified as a distinct group based on single-stranded conformation polymorphism analysis of the rDNA ITS region. Mycological Research, 109, 429–438.
Sztejnberg, A. (1993). Ampelomyces quisqualis AQ10, CNCM I-807, for biological control of powdery mildew. US patent no. 5190754.
Sztejnberg, A., Galper, S., Mazar, S., & Lisker, N. (1989). Ampelomyces quisqualis for biological and integrated control of powdery mildews in Israel. Journal of Phytopathology, 124, 285–295.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). San Diego, CA, USA: Academic Press.
Acknowledgments
The authors thank Dr. Levente Kiss (Plant Protection Institute of the Hungarian Academy of Science, Budapest, Hungary) for valuable comments and suggestions and for providing fungal strains. We are grateful to the following colleagues for their helpful suggestions and assistance with the data analysis: Lorenzo Tosi, Valerio Mazzoni and Alberto Pellegrini. We would also like to thank Susanna Micheli and Denise Ress for their technical support and assistance. This work was supported by the AMPELO project (morphological and genetic analysis) and the ENVIROCHANGE project (effect of temperature), both funded by the Autonomous Province of Trento.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angeli, D., Maurhofer, M., Gessler, C. et al. Existence of different physiological forms within genetically diverse strains of Ampelomyces quisqualis . Phytoparasitica 40, 37–51 (2012). https://doi.org/10.1007/s12600-011-0197-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-011-0197-x