Abstract
Indian citrus ringspot disease is an important viral disease in kinnow mandarin orchards where disease incidence up to 100% has been recorded. The disease is caused by Indian citrus ringspot virus (ICRSV), a positive sense flexuous RNA virus. The transmission of ICRSV is generally through budwood. Association of ICRSV with pollens of naturally infected flowers from cv. ‘Kinnow’ mandarins has been shown previously and this study demonstrates the presence of ICRSV in seed tissues. DAC-ELISA revealed the presence of virus in seed coats but not in embryo and endosperm of seeds collected from the fruits of ICRSV-infected Kinnow plants. Of the infected seed coats, 18% were found to harbor the virus. The seedlings in the grow-out test did not show any symptom for 2 years and the virus could not be detected in seedlings by DAC-ELISA and RT-PCR. The present study indicated that ICRSV could be localized in the testa of seeds but its transmission to progeny was not observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transmission of virus from diseased plants to their progenies via seeds was long thought to be a rare phenomenon, but today it is known that approximately one-fifth of the known viruses are seed-transmitted in one or more of their hosts (Conti 2008). Seed transmission can occur directly when the embryo is infected or indirectly when the seed coat is contaminated (Mink 1993; Stace-Smith and Hamilton 1988). Seed transmission of viruses has been reported in many fruit trees such as Prunus sp. (Amari et al. 2007), cocoa (Quainoo et al. 2008), cherry (Rumbou et al. 2009) and fig (Castellano et al. 2009). Citrus tristeza virus was detected in seeds of many of the citrus species (Davino et al. 1991). Citrus psorosis virus (D’Onghia et al. 2000) and Citrus variegation virus (Davino et al. 1991) in citrus are seedborne but not transmitted to seedlings. An infectious viral disease of citrus caused by Citrus leaf blotch virus was seed transmitted (Guerri et al. 2004). Seed transmission of Citrus tatter leaf virus was recently reported in citrus (Tanner et al. 2010).
Indian citrus ring spot disease is an emerging viral disease and a limiting factor in ‘Kinnow’ mandarin (Citrus reticulata cv. ‘Blanco’, a hybrid between ‘King’ and ‘Willow’ mandarins) (Ahlawat and Pant 2003). Indian citrus ringspot disease is caused by Indian citrus ringspot virus (ICRSV), a positive sense flexuous RNA virus approximately 7.5 kb in size with six Open Reading Frames (ORFs) (Rustici et al. 2002) belonging to the genus Mandarivirus in the Alphaflexiviridae family of order Tymovirales (http://ictvonline.org/virusTaxonomy.asp?version=2009). Conspicuous yellow rings on mature leaves are characteristic symptoms of ICRSV infection in Kinnow mandarin (Pant and Ahlawat 1998). ICRSV has been reported only from India and occurs predominantly in northern India. Maximum incidence of the ring spot disease was observed in Kinnow mandarin trees, with disease incidence up to 100% in most of the Kinnow mandarin orchards in northern India, especially in Punjab (Byadgi and Ahlawat 1995). However, its spread to states in the southern and western parts of the country has also been observed (Byadgi and Ahlawat 1995). This may be due to the movement of budwood, as there is no domestic quarantine for this virus. As electron microscopy has shown an association of ICRSV particles in the pollen of flowers from naturally infected citrus plants (R. P. Pant, 1995, Ph.D. thesis, IARI, New Delhi, India), it was important to determine the presence of ICRSV in seed and its seed transmissibility. In this paper we have discussed the localization of ICRSV in seed tissues and its transmission to the progeny.
Materials and methods
Seed collection
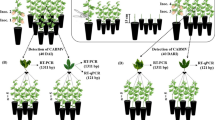
Seeds were collected from mature fruits of ICRSV-infected as well as healthy trees of Kinnow mandarin from the citrus orchard of the Indian Agricultural Research Institute. The presence of ICRSV infection in the Kinnow trees and its absence in healthy trees were confirmed by ELISA and RT-PCR. Seeds were thoroughly washed with running tap water and dried at room temperature. From fruits of infected plants, 300 seeds were collected and divided into two lots and one lot of 100 seeds was placed in plastic bags and stored at 4°C until detection of ICRSV. The other lot with 200 seeds was taken to conduct a grow-out test.
Grow-out test
Seeds were surface treated with Bavistin (2 g kg-1 seed) for a few minutes before planting. Treated seeds were planted in small trays with steam-sterilized medium consisting of one-third soil, one-third sand and one-third vermiculite. They were kept in a glasshouse under insect-free conditions for 2 years at 25–28°C day and 15–18°C night temperatures with 70–80% r.h. and observed for symptom appearance at weekly intervals. Six-month-old seedlings were transplanted to small pots of 6” diameter with one seedling per pot in soil sterilized by 0.1% formaldehyde.
Detection of ICRSV in seeds
Testae of the seeds were separated carefully from endosperm and embryos and were used for virus detection. Since the embryo was too small, the embryo and endosperm were used together for virus testing. Detection of ICRSV was done through Direct Antigen Coated – Enzyme Linked Imunosorbent Assay (DAC-ELISA) (Baranwal et al. 2000). The testa and endosperm with embryo from both healthy and infected Kinnow plants were homogenized in coating buffer containing 2% PVP (w/v, 1:10) and incubated at 37°C for 1 h and thereafter blocking was performed with 0.5% BSA. Subsequently wells were coated with specific antibody (produced in our laboratory) at 1:500 dilutions and incubated at 37°C for 90 min. Enzyme conjugate (antirabbit IgG–alkaline phosphatase) was added and incubated at 37°C for 1 h. ELISA plate was washed three times thoroughly with PBS-T between all incubation steps. Optical density (OD) reading was taken at 405 nm at 15 min, 30 min and 60 min after adding substrate (PNPP, 0.6 mg ml-1) in a micro plate titre reader (TECAN A-5082, Sunrise, Salzburg, Austria). All samples were used in duplicate and were rated positive if the mean OD of a sample was at least three times the mean OD of a healthy sample (seeds from ICRSV-negative mother plants).
Detection of ICRSV in seedlings
Leaves from the seedlings selected at random were used individually for detection of ICRSV at 6-month intervals. Detection of ICRSV was done by DAC–ELISA protocol (20–25% of seedlings) as described above by homogenizing leaf tissues in coating buffer at 1: 2 ratios. The presence of ICRSV was also confirmed by RT-PCR (11–17.5% of seedlings) with virus-specific primers after isolating RNA from leaf samples collected at random. Seedlings from ICRSV-negative mother plants were used as the healthy control. ICRSV-infected leaves were used as the positive control in both ELISA and RT-PCR.
Total RNA from the seedlings was isolated using RNeasy Mini Kit (QIAGEN, Hilden, Germany, Cat. No. 74903), following the manufacturer’s protocol. cDNA synthesis was performed using M-MuLV Reverse Transcriptase (Fermentas Life Sciences, Burlington, ON, Canada) by incubating at 42°C for 45 min. RT–PCR detection of ICRSV was done in a MyCycler thermal cycler (Bio-Rad, Hercules, CA, USA) using ICRSV-specific primers ICRSVF ACCCCTTTCAACACTTAAACAG (ORF5) and ICRSVR GTCAATGACCTAATCGGTCCC (ORF6) at 94°C denaturation for 30 s, an annealing temperature of 56°C for 30 s and an extension at 72°C for 1 min using Taq DNA Polymerase (Fermentas); the products were analyzed by 1% agarose gel electrophoresis.
Data analysis
Percentage seed transmission was calculated from sample test results using STpro (version 1.0), a computer program for estimating the proportion of infected seeds using the results of tests with confidence intervals and goodness-of-fit test (Albrechtsen 2006; www.planthealth.co.uk/downloads).
Results
Detection of ICRSV in seed coat, embryo and endosperm
Ninety-three seeds were used in ELISA for detection of ICRSV. ICRSV was detected in seed coats of 17 seeds (18%) and no virus was detected in the embryo and endosperm (Fig. 1, Table 1).
Detection of ICRSV in seedlings
In a grow-out test, germination of 160 seeds (80%) of the 200 seeds sown was observed. No symptom was produced by any of the seedlings during 24 months. Seedlings were healthy throughout the season and showed normal growth rate like the seedlings from healthy mother plants. None of the seedlings tested was positive for ICRSV in ELISA or RT-PCR (Table 2).
Discussion
Seed trade and trans boundary exchange of germplasm play a key role in the distance dissemination of destructive virus and its strains, and therefore detection and diagnosis of virus are crucial for healthy seed trade (Chalam and Khetarpal 2008). Citrus is susceptible to a large number of virus and virus-like pathogens and most of them are bud- and vector-transmitted. ICRSV is the type species of Mandarivirus genus in the family Alphaflexiviridae. Seed transmission is uncommon in members of Alfaflexiviridae. A few members of Betaflexiviridae –such as members of Carlavirus, Trichovirus (Fig latent virus I) and Citrivirus (Citrus leaf blotch virus) are seed transmitted (Castellano et al. 2009; Johansen et al. 1994; Martelli et al. 2007). Seed transmission in citrus has been investigated for a few viruses: Citrus tristeza virus of Closteroviridae and Citrus variegation virus of the Bromoviridae (Davino et al. 1991); Citrus psorosis virus of the Ophioviridae (D’Onghia et al. 2000); Citrus leaf blotch virus (CLBV) (Guerri et al. 2004) and Citrus tatter leaf virus (Tanner et al., 2010) of the Betaflexiviridae family.
In the seed transmission study of Citrus psorosis virus in psorosis-affected mandarin and sour orange, the virus was more common in seed coats (20.0–83.0% incidence) than endosperm/embryos (3.0–20.0% incidence) (D’Onghia et al. 2000). Even though virus was localized in the seed coat as well as in endosperm and embryos, no vertical transmission to seedlings was observed. In the indexing of citrus seeds for Citrus variegation virus, the virus concentration was higher (90.9%) in seed coats and the presence of virus was observed in peeled seeds also (66%), but no seed transmission was observed (Davino et al. 1991). In our study ICRSV was localized only in the testa of Kinnow seeds (18%) and not in the embryo or endosperm and the virus could not be transmitted to the progeny. In the case of other trees like Prunus, Plum pox virus (PPV) was detected in 19–35% of the seed coats and in 5–23% of the cotyledons, but virus was absent in the embryos. PPV was detected in germinating seeds, in up to 34% of the seed coats and in up to 8.3% in the cotyledons but seedlings were symptomless and virus free (Milusheva et al. 2008). Another virus infecting citrus, CLBV of the Betaflexiviridae family, is seedborne in several citrus species or hybrids and seed transmission of 2.50%, 2.52% and 2.46% of this virus was observed in seedlings from CLBV-infected Troyer citrange, Nagami kumquat and sour orange (Citrus aurantium L.), respectively (Guerri et al. 2004). However, no virus localization was studied in this case but it is possible that the virus might be present in the embryo, leading to transmission of virus to progeny. In the case of phloem limited ‘Candidatus Liberibacter asiaticus’, the pathogen was readily detected in the seed coat but not in embryos of citrus seeds (Tatineni et al. 2008). This indicates infection of the embryo is required for transfer of virus to seedlings, which may be the reason why ICRSV in Kinnow could not be transmitted to progeny in our study.
Host factors are important in the seed transmission of viruses. Seed transmissibility in any plant is determined by host genotype, virus strain, presence of other viruses in the plant and environmental factors (Timmerman-Vaughan et al. 2009), and the three-way interplay of genome of virus, the maternal host and the progeny (Maule and Wang 1996). The higher percentage of the virus in the seed coat indicates testa as a possible source of viral infection of embryo and other seed tissues, which was not observed in the case of ICRSV in this study. The other explanations given for the impossibility of seed transmission are that – as in the case of PPV in plum and apricot – the virus is inactivated due to particle break down in the mature seeds (Eynard et al. 1991) or in the seeds; PPV localizes in the integuments, the unabsorbed parts of the endosperm and nucellus, i.e., the tissues which burst and fall during stratification and germination (Dulic-Markovic and Rankovic 1997). No embryo invasion of ICRSV was observed, nor was virus detected in other parts of the seed-like endosperm, thereby eliminating the chance of transfer of virus to seedlings germinated through polyembryony, i.e., nucellar seedlings. The seedlings in the grow-out test were symptom-less and ICRSV was negative for the entire 24 months. As favorable conditions were maintained and observations were taken for a long time, we can exclude the chances of latency of the virus. Even though pollen was invaded by ICRSV (R. P. Pant, 1995 thesis), this could not be translated into seed transmission. For successful transmission, the virus would have to go across the vascular system in the seed coat through the chalazal cup, which is a permeable layer of cells that separate embryo from the vascular system of the mother plant in citrus (Schneider 1968). Since ICRSV is not highly stable in the environment (N. V. Hoa, 2003, Ph.D. thesis, IARI, New Delhi, India), the virus could not have survived in the contaminated seed coat, eliminating the possibility of seedling infection through contact between germinating seedlings and virus-contaminated seed coat. Based on our observations in the present study, it seems plausible to conclude that ICRSV is localized in seed testa. Since we could include only 160 seedlings in our transmission study of ICRSV, the results indicated the absence of a substantial rate of transmission to progeny. A far larger number of seedlings will have to be examined in order to establish the absolute absence of seed transmission.
References
Ahlawat, Y. S., & Pant, R. P. (2003). Major viruses and virus like diseases of citrus in India, their diagnosis and management. Annual Review of Plant Pathology, 2, 447–474.
Albrechtsen, S. E. (2006). Testing methods for seed transmitted viruses: Principles and protocols. Wallingford, UK: CABI Publishing.
Amari, K., Burgos, L., Pallás, V., & Sánchez-Pina, M. A. (2007). Prunus necrotic ringspot virus early invasion and its effects on apricot pollen grain performance. Phytopathology, 97, 892–899.
Baranwal, V. K., Chakraborty, N. K., Giri, B. K., & Ahlawat, Y. S. (2000) Purification, production of polyclonal antibodies and detection of Indian citrusringspot virus (ICRSV) (pp. 1036–1040). Hitech citrus management: Proceedings of the International Symposium on Citriculture (1999, Nagpur, India).
Byadgi, A. S., & Ahlawat, Y. S. (1995). A new viral ringspot disease of citrus (Citrus species) in India. Indian Journal of Agricultural Sciences, 65, 61–68.
Castellano, M. A., de Stradis, A., Minafra, A., Boscia, D., & Martelli, G. P. (2009). Seed transmission of Fig latent virus 1. Journal of Plant Pathology, 91, 697–700.
Chalam, V. C., & Khetarpal, R. K. (2008). A critical appraisal of challenges in exclusion of plant viruses during trans-boundary movement of seeds. Indian Journal of Virology, 19, 139–149.
Conti, M. (2008). Seed transmission of plant viruses and diagnosis. Protezione delle Coiture, 1, 7–9.
Davino, M., Areddia, R., Pelicani, L., & Grimaldi, V. (1991). Indexing of seeds of different citrus species for tristeza and variegation viruses. Proceedings of the Eleventh Conference of the International Organization of Citrus Virologists (Orlando, FL, USA), pp. 368–372.
D’Onghia, A. M., Djelouah, K., & Savino, V. (2000). Serological detection of Citrus psorosis virus in seeds but not in seedlings of infected mandarin and sour orange. Journal of Plant Pathology, 82, 233–235.
Dulic-Markovic, I., & Rankovic, M. (1997). An experiment with plum pox virus transmission by apricot and peach seeds. Proceedings of Middle European Meeting ‘96 on Plum Pox (Budapest, Hungary, 1996), pp. 117–119.
Eynard, A., Roggero, P., Lenzi, R., Conti, M., & Milne, R. G. (1991). Test for pollen and seed transmission on Plum pox virus (Sharka) in two apricot cultivars. Advances in Horticultural Science, 5, 104–106.
Guerri, J., Pina, J. A., Vives, M. C., Navarro, L., & Moreno, P. (2004). Seed transmission of Citrus leaf blotch virus: Implications in quarantine and certification programs. Plant Disease, 88, 906.
Johansen, E., Edwards, M. C., & Hampton, R. O. (1994). Seed transmission of viruses: current perspectives. Annual Review of Phytopathology, 32, 363–386.
Martelli, G. P., Adams, M. J., Kreuze, J. F., & Dolja, V. V. (2007). Family Flexiviridae: a case study in virion and genome plasticity. Annual Review of Phytopathology, 45, 73–100.
Maule, A. J., & Wang, D. (1996). Seed transmission of plant viruses: a lesson in biological complexity. Trends in Microbiology, 4, 153–158.
Milusheva, S., Gercheva, P., Bozhkova, V., & Kamenova, I. (2008). Experiments on transmission of Plum pox virus through Prunus seeds. Journal of Plant Pathology, 90, S1.23–S1.26.
Mink, G. I. (1993). Pollen- and seed-transmitted viruses and viroids. Annual Review of Phytopathology, 31, 375–402.
Pant, R. P., & Ahlawat, Y. S. (1998). Partial characterization of a filamentous virus associated with ringspot disease of citrus. Indian Phytopathology, 51, 225–232.
Quainoo, A. K., Wetten, A. C., & Allainguillaume, J. (2008). Transmission of cocoa swollen shoot virus by seeds. Journal of Virological Methods, 150, 45–49.
Rumbou, A., Von Bargen, S., & Büttner, C. (2009). A model system for plant-virus interaction—infectivity and seed transmission of Cherry leaf roll virus (CLRV) in Arabidopsis thaliana. European Journal of Plant Pathology, 124, 527–532.
Rustici, G., Milne, R. G., & Accotto, G. P. (2002). Nucleotide sequence, genome organization and phylogenetic analysis of Indian citrus ringspot virus. Archives of Virology, 147, 2215–2224.
Schneider, H. (1968). The anatomy of citrus. In W. Reuther, L. D. Batchelor, & H. J. Webber (Eds.), The citrus industry, vol. 2 (pp. 1–85). Berkeley, CA, USA: University of California Press.
Stace-Smith, R., & Hamilton, R. (1988). Inoculum thresholds of seed borne pathogens: viruses. Phytopathology, 78, 875–880.
Tanner, J. D., Kunta, M., da Graca, J. V., Skaria, M., & Nelson, S. D. (2010). Evidence for citrus tatter leaf seed transmission in citrus. Citrus Research & Technology: XVIII Conference of the IOCV (Campinas (SP), Brazil), 31:50.
Tatineni, S., Shankar Sagaram, U., Gowda, S., Robertson, C. J., Dawson, W. O., Iwanami, T., et al. (2008). In planta distribution of ‘Candidatus Liberibacter asiaticus’ as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology, 98, 592–599.
Timmerman-Vaughan, G., Larsen, R., Murray, S., McPhe, K., & Coyne, C. (2009). Analysis of the accumulation of Pea enation mosaic virus genomes in seed tissues and lack of evidence for seed transmission in pea (Pisum sativum). Phytopathology, 99, 1281–1287.
Acknowledgments
The first author is grateful to the Indian Agricultural Research Institute for a Junior Research Fellowship. The authors are thankful to the Department of Biotechnology for financial support and to Dr. R. K. Jain, Head, Division of Plant Pathology, IARI, New Delhi, for the facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
K., P., Baranwal, V.K. Indian citrus ringspot virus: localization of virus in seed tissues and evidence for lack of seed transmission. Phytoparasitica 39, 491–496 (2011). https://doi.org/10.1007/s12600-011-0181-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-011-0181-5