Abstract
The insecticidal effects of pyrogallol were studied by treating eggs and larvae of the melon fruit fly, Bactrocera cucurbitae (Coquillett) (Tephritidae: Diptera), with various concentrations (1, 5, 25, 125, 625 and 3125 ppm) of the phenolic compound. Although egg hatching decreased following treatment of 0–8-h old eggs with pyrogallol, the decrease was not significantly different from the control. Larval period and total development period declined significantly in 64–72-h-old and 88–96-h-old B. cucurbitae larvae fed on pyrogallol-treated diet. However, in the 44–48-h-old larvae, the larval period and total development period were not affected by pyrogallol treatment at any of the tested concentrations. None of them survived up to the pupal stage at the highest concentration. Number of pupae formed and adult emergence decreased significantly in all larval instars following feeding on pyrogallol-treated diet. The analysis of enzymes in 64–72-h-old larvae treated with LC40 concentration (16.21 ppm) of pyrogallol at three time intervals, i.e., 24 h, 48 h and 72 h, showed significant induction in the activities of ascorbate peroxidase (APOX) and glutathione S-transferases (GSTs) at 24 h but a decrease was observed following prolonged treatment. On the other hand, superoxide dismutase (SOD) and peroxidases (POX) activity remained suppressed during the initial treatment interval but increased with prolonged treatment in 136–144-h-old larvae. The catalase (CAT) activity was suppressed at all treatment durations whereas glutathione reductase (GR) activity was not affected by pyrogallol treatment. An increase in the activities of ascorbate peroxidase, superoxide dismutase, peroxidases and glutathione S-transferases indicates an induction of defensive response of the melon fruit fly to the toxic effects produced by ingestion of pyrogallol. Although the effects of the compound on enzyme activity were tested on second instar, it would be interesting to see the effects on other instars too.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last few decades have witnessed a major advance in the field of crop protection, achieved largely through organic pesticides. These chemicals no doubt have contributed greatly to increasing crop yield but have raised a number of ecological and medical problems. Excessive use of pesticides has resulted in faster evolution of resistant forms of pests, destroyed natural enemies, harmed non-target organisms, and contaminated food. This has necessitated a need for new pest control agents that are ecologically safe, less toxic and biodegradable.
Plants produce a wide array of biologically active products, some of which have only an insignificant role in primary physiological processes in plants that synthesize them. These compounds represent the secondary metabolites whose major role in plants is mainly defensive. The behavioral and physiological effects of some of these compounds on insect pests have already been reported (Berenbaum 1978; Malik et al. 1983; Puttick and Bowers 1988). Phenols are one of the prominent classes of plant secondary metabolites, which are characterized by the presence of an aromatic ring bearing one or more hydroxyl groups and are present throughout the plant kingdom. Recently phenolic compounds have been gaining attention because of their anticarcinogenic, antiallergic and anti-inflammatory properties (Daniel et al. 1999; Hollman 2001; Parr and Bolwell 2000). Insecticidal effects of phenols have been reported against many insect orders including Coleoptera and Lepidoptera (Akhtar and Isman 2004; Weissenberg et al. 1997). Upon oxidation, phenolic compounds produce hydrogen peroxide and organic hydroperoxides which in the presence of reduced metal ions form hydroxyl and alkoxyl radicals (Barbehenn et al. 2005a,b). These radicals oxidize cellular components, damage lipids, proteins and nucleic acids, and degrade the nutritional quality of food in the gut lumen of insects. Some phenols, like tannins, bind to proteins, acting as protein precipitating agents, thus reducing their digestibility (Appel 1993). Insects are greatly susceptible to the ravages of these radicals and possess a suite of antioxidant enzymes that form a concatenated response to an onslaught of these dietary and endogenously produced oxidants (Felton and Summers 1995).
In order to ascertain the potential of phenolic compounds as promising candidates in pest management strategies we investigated the insecticidal effects of pyrogallol, a simple phenol, on the growth and development of melon fruit fly, Bactrocera cucurbitae (Coquillett). We also looked at the role of enzyme defense of the melon fruit fly by measuring the activity of major antioxidant and detoxifying enzymes including superoxide dismutase, catalase, peroxidases, ascorbate peroxidase, glutathione reductase and glutathione S-transferases (GST) in response to pyrogallol. General esterases, GST and cytochrome P450-dependent monooxygenases are common detoxification enzymes that metabolize pesticides in arthropods.
Bactrocera cucurbitae is a serious pest of cucurbit crops. In India alone it destroys about 60% of vegetables (Kapoor 1993). Approximately 125 species of host plants, including cucurbits, tomatoes and many other vegetables, have been recorded as hosts of the melon fly. Preferred hosts include watermelon, pumpkin, squash, gourd, cucumber, tomato and cowpea (Dhillon et al. 2005).
Materials and methods
The cultures of B. cucurbitae were maintained on pumpkin in wire mesh cages kept in the insect culture room at 25 ± 2°C, 70–80% r.h. and a photoperiod of 10 h:14 h L:D. Pyrogallol (98.5% pure) was purchased from Loba Chemie Private Ltd. (Mumbai, India).
Experiment 1: Insecticidal effects of pyrogallol
Eggs treatment
Bioassays were conducted using six different concentrations (1, 5, 25, 125, 625 and 3125 ppm) of pyrogallol. The eggs were dipped in solutions of various concentrations of pyrogallol for 1 min. Distilled water was used as the control. The eggs were then placed in a petri dish containing moist filter paper and observed for hatching at intervals of 24 h. There were 20 eggs in each petri dish with six replications for each concentration and control and the experiment was repeated twice.
Larval treatment
Fresh pumpkin pieces were kept in wire mesh cages with approximately 100 gravid females for 6–8 h. These charged (egg laid) pumpkin pieces were removed from the cages and dissected after 44 (for 1st instar), 64 (for 2nd instar) and 88 h (for 3rd instar) to harvest the larvae. The harvesting was done in saline water and the larvae were washed in distilled water before shifting them into culture vials (25 O.D. × 100 mm length) containing culture medium incorporated with various concentrations of pyrogallol or culture medium without pyrogallol (control). The artificial culture medium used in the bioassay was prepared according to the methodology suggested by Srivastava (1975) for this fruit fly. The diet consisted of agar-agar (1.0 g), casein (3.0 g), cholesterol (0.04 g), ribonucleic acid (0.10 g), McCollum’s salt mixture no. 185 (0.10 g), 10% potassium hydroxide (0.4–0.5 ml) along with the antibiotic chloramphenicol (0.10 g), a vitamin–sucrose mixture (1.10 g) and distilled water (50 ml). Observations were made daily for recording the time taken for pupae formation, number of pupae formed, larval and pupal weight, time taken for emergence of flies and number of flies emerged. There were six replications of 15 larvae per treatment. The nutritional indices for the melon fruit fly could not be determined but the weight of the pupae was recorded in order to determine the extent to which the compound affects the fitness of the melon fruit fly. This experiment was carried out by releasing newly molted 64–72-h-old larvae on artificial diet containing different concentrations of pyrogallol using the same number of larvae and replications as mentioned before; the weight of the pupae formed was recorded.
Experiment 2: Biochemical analysis of enzymes
The biochemical analysis was done to gain some insight into the role played by enzymes in combating the deleterious flux of oxidative radicals that might have been generated by the oxidation of pyrogallol. In this experiment we focused on the major enzymes involved in detoxification and antioxidant mechanisms including superoxide dismutase, catalase, peroxidases, ascorbate peroxidase, glutathione reductase and GST. Second instar larvae (64–72 h old) were fed on pyrogallol-treated diet (16.21 ppm = LC40) at 24 h, 48 h and 72 h. LC40 is the concentration which kills 40% of the insect population.
Superoxide dismutase was estimated and extracted by homogenizing the larvae of B. cucurbitae (10% w/v) in 50 mM sodium carbonate buffer (pH 10.0) (Kono 1978). The assay mixture contained 1.3 ml of 50 mM sodium carbonate buffer (pH 10.0), 0.5 ml of NBT, 0.1 ml of 0.6% Triton-X100 and 0.1 ml of 20 mM hydroxylamine hydrochloride (pH 6.0). The absorbance was recorded at 540 nm. The method of Bergmeyer (1974) was used for extraction and estimation of catalase. The homogenates (5% w/v) were prepared in 0.05 M potassium phosphate buffer (pH 7.0). The enzyme extract (0.1 ml) was added to 0.05% H2O2 (2.9 ml) and the decrease in absorbance was recorded at 240 nm.
Peroxidases were extracted by homogenizing the larvae (1% w/v) in 0.05 M Tris-HCL buffer (pH 7.0) containing 1% β-mercaptoethanol (Kar and Mishra 1976). The assay mixture consisted of the enzyme extract (0.25 ml), 2.0 ml of 0.1 M sodium phosphate buffer (pH 7.0), 0.25 ml of 0.005 M H2O2 and 0.25 ml of 0.005 M pyrogallol. The mixture was incubated at 25°C followed by addition of 5% H2SO4. The absorbance was recorded at 420 nm. The extraction of ascorbate peroxidase was done in 50 mM potassium phosphate buffer (pH 7.0) containing 50 mM ascorbic acid using 10% homogenates of the larvae (Asada 1984). The assay mixture comprised the extract (0.1 ml), 0.6 ml of 50 mM ascorbic acid and 0.125 ml of 0.3% H2O2. The decrease in absorbance was recorded at 290 nm at 25°C.
Glutathione reductase extraction was done by homogenizing the larvae (10% w/v) in 50 mM potassium phosphate buffer (pH 7.6) (Carlberg and Mannervik 1975). The reaction mixture comprised 600 μl of 50 mM potassium phosphate buffer (pH 7.6), 100 μl of 3 mM ethylenediaminetetraacetic acid (EDTA), 100 μl of 0.1 mM nicotinamideadenine dinucleotide tetra sodium salt (NADPH), 100 μl of 1 mM oxidized glutathione, 50 μl of distilled water and 50 μl of enzyme sample. The decrease in absorbance was recorded at 340 nm at 30°C. GSTs were extracted and estimated according to the method of Chien and Dauterman (1991). The homogenate (2% w/v) was prepared in 0.1 M sodium phosphate buffer (pH 7.6) containing 0.1 mM phenyl thiourea. The assay mixture consisted of 30 μl of 10 mM ethanolic 1-chloro-2,4-dinitrobenzene, 100 μl glutathione, 50 μl of enzyme extract and 20 μl of distilled water. The increase in absorbance was recorded at 340 nm at 25°C.
Statistical analysis
The data were analyzed using one way ANOVA followed by least significant difference (LSD) to determine whether the difference between the control and treatments exceeded the computed LSD value.
Results and discussion
Pyrogallol had no significant effect on egg hatching (F = 0.73) but exercised a repressive influence on the development of B. cucurbitae. The larval period (data not shown) and total development period were shortened significantly in 64–72-h-old (P 0.01 = 17.89, 14.03) and 88–96-h-old (P 0.01 = 4.45) larvae (Table 1). Contrary to the present findings, the development period of B. cucurbitae was prolonged when it was treated with coumarin, another phenolic compound (Kaur and Rup 2003). The pupal weight of B. cucurbitae declined significantly (P 0.01 = 6.86) from 7.32 ± 0.32 mg in control to 5.13 ± 0.25 mg at 3125 ppm with pyrogallol treatment. Likewise, a significant decline in pupal weight has been reported in Bactrocera oleae by Manoukas (1996). The decrease in pupal weight of B. cucurbitae could be due to the shortening of the larval period (data not shown), which significantly reduced the feeding period. Pyrogallol had an adverse effect on percentage pupation and percentage emergence of B. cucurbitae, which declined significantly in all the treated instars (Table 1). The percentage pupation was reduced by more than half in the 1st instar at 625 ppm; at 3125 ppm all larvae died before pupation. Manoukas (1996) too had reported failure of the pyrogallol-treated 1st instar larvae of B. oleae to develop into pupae. Maximum inhibitory effects of pyrogallol were observed at higher concentrations, where emergence was inhibited by 63.2% at 625 ppm in the 44–48-h-old larvae and by 55.85% and 57.56% in the 64–72-h-old and 88–96-h-old larvae, respectively, at 3125 ppm. These findings are in accordance with the results of Manoukas (1996), who reported a decrease in percentage pupation and percentage emergence in the olive fruit fly B. oleae upon treatment with pyrogallol as well as with other simple phenols, i.e., hydroquinone and phloroglucinol. Kaur and Rup (2003) had also perceived an inhibitory influence of coumarin on percentage pupation and emergence in B. cucurbitae.
It is evident from the findings that whereas egg hatching was not significantly affected, the development and survival of the larvae were influenced significantly by pyrogallol treatment. This could be due to the fact that phenolics upon ingestion reduce the nutritional quality of the diet. The egg is a non-feeding stage and so was not significantly affected by the pyrogallol treatment. Plant phenolics interact with protein through several physical or chemical mechanisms including hydrogen bonding, hydrophobic interaction and covalent bonding (McManus et al. 1983; Pierpoint 1983). The covalent interactions between oxidized phenolics (i.e., quinones) and dietary proteins are deleterious to insects (Duffey and Felton 1989; Felton et al. 1989). Also pyrogallol had more of an effect on the 2nd and 3rd instars as compared with the 1st instar, which could be due to a greater consumption of the diet by these larvae, thereby producing a toxic effect.
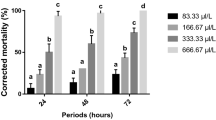
Enzymatic investigations carried out in the larvae of B. cucurbitae under the influence of pyrogallol showed that superoxide dismutases, a group of metalloenzymes, which catalyze the dismutation of O2-, became suppressed when the 2nd instar larvae were treated with pyrogallol for 48 h (Fig. 1). However, prolongation in treatment time for another 24 h resulted in a significant increase in enzyme activity in the melon fly. An increase in SOD activity has been perceived in lepidopterans – Spodoptera eridania, Trichoplusia ni and Spodoptera littoralis – when fed on diet incorporated with phenolic compounds, quercetin, xanthotoxin and tannic acid, respectively (Krishnan and Sehnal 2006; Lee and Berenbaum 1989; Pritsos et al. 1990). Elevated levels of superoxide dismutase were also perceived in Rhopalosiphum padi and Sitobion avenae in response to plant O-dihydroxyphenols (Lukasik 2007). The increase in superoxide dismutase activity in the larvae of B. cucurbitae could be a form of adaptation to metabolic increase in superoxide anions in response to prolonged pyrogallol treatment. Catalase activity which is widespread in insect cell organelles was reduced significantly in all treatment intervals (Fig. 1). Inhibitory effects of phenolic compounds such as quercetin, xanthotoxin and tannic acid on catalase activity have been reported in S. eridania and S. littoralis (Krishnan and Sehnal 2006; Pritsos et al. 1990). Lukasik (2007) had also observed a decrease in catalase activity in the aphids R. padi and S. avenae after treatment with O-dihydroxyphenols. Kono and Fridovich (1982) and Pritsos et al. (1988) had reported that treatment of insects with a metabolic generator of superoxide anions, such as flavonoid quercetin, reduced catalase activity.
Peroxidases whose activity was suppressed significantly during initial treatment intervals was induced only after prolonged exposure of the larvae to pyrogallol (Fig. 1). Arora et al. (2008) had perceived an induction in peroxidases in Lipaphis erysimi activity after coumarin treatment at all treatment intervals. The role of peroxidases in oxidizing dietary phenolic-catechin in the gut of rose aphid, Macrosiphum rosae, has been highlighted by Peng and Miles (1991). A significant induction in ascorbate peroxidase activity was observed when the 64–72-h-old larvae were treated with pyrogallol for 24 h (Fig. 1). Lukasik (2007) suggested that ascorbate peroxidase activity plays an important role in removing hydrogen peroxide from the insect body. Thus, the low levels of catalase observed with pyrogallol treatment might have been compensated for by the induction of ascorbate peroxidase activity in larvae of B. cucurbitae. Mathews et al. (1997) had also proposed that catalase was inefficient at reducing hydrogen peroxide to low levels and ascorbate peroxidase better serves the role. However, the induction in enzyme activity could not be sustained and further treatment caused the activity to decrease significantly. Pyrogallol had no effect on glutathione reductase activity, indicating that this enzyme had no role in scavenging the radicals formed during oxidation of pyrogallol ingested in the insect body (Fig. 1). Phenolic compounds quercetin and xanthotoxin likewise had no effect on glutathione reductase activity in T. ni and Papilio polyxenes (Lee and Berenbaum 1989, 1993).
Glutathione S-transferases are among the most studied detoxifying enzymes because of their role in chemical detoxification (Yu 1996) and insecticide resistance (Huang et al. 1998; Prapanthadara et al. 1993; Vontas et al. 2001). The levels of GST were elevated when the 64–72-h-old larvae of B. cucurbitae were fed pyrogallol-treated diet for 48 h but the induction was not very significant (Fig. 1). Shen et al. (2003) had demonstrated elevated levels of GST in the fruit fly Drosophila melanogaster exposed to phenol. A significant induction in GST activity has been observed in Papilio glaucus canadensis and Lymantria dispar treated with phenolic glycosides (Hemming and Lindroth 2000; Lindroth 1989) and in Spodoptera frugiperda fed on flavones-treated diet (Wheeler et al. 1993; Yu 1983). The present findings indicated that GST might have played an insignificant role in the metabolism of pyrogallol.
Ingested phenolics undergo oxidation (Appel 1993) to products that curb digestion by cross linking amino acids and proteins (Harborne 2001) and enhance the load of reactive oxygen species in the digestive tract (Barbehenn et al. 2005a,b; Krishnan and Sehnal 2006). High reactive oxygen species concentration impairs the absorption of ingested nutrients (Bi and Felton 1995). The antioxidant and detoxification enzymes in insects play an important role in combating the deleterious flux of oxidative radicals that are generated by the oxidation of allelochemicals. However, among the different enzymes investigated for their activity in the larvae of B. cucurbitae under the influence of pyrogallol, a significant initial induction was observed only in the ascorbate peroxidase and GSTs. The activities of superoxide dismutase and peroxidases were induced only after a prolonged treatment interval. The catalase activity was suppressed at all the treatment intervals whereas glutathione reductase activity was not affected much by treatment. These findings indicate that the antioxidant enzymes and the detoxification enzyme did not play a significant role in counteracting the pro-oxidant challenges presented by the ingestion of pyrogallol in the larvae of B. cucurbitae, which could be the reason for the adverse effect of pyrogallol on development of the melon fruit fly. The present study revealed the anti-insect potential of pyrogallol, but further work needs to be carried out on the molecular biology and metabolic engineering of the phenolic pathway with respect to insect resistance in plants.
References
Akhtar, Y., & Isman, M. B. (2004). Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. Journal of Applied Entomology, 128, 32–38.
Appel, H. M. (1993). Phenolics in ecological interactions: The importance of oxidation. Journal of Chemical Ecology, 19, 1521–1552.
Arora, G. K., Rup, P. J., & Sohal, S. K. (2008). Influence of coumarin on the enzymatic activity in nymphs of Lipaphis erysimi (Kalt.). Allelopathy Journal, 22, 221–230.
Asada, K. (1984). Chloroplast: Formation of active oxygen and its scavenging. Methods in Enzymology, 105, 422–429.
Barbehenn, R. V., Cheek, S., Gasperut, A., Lister, E., & Maben, R. (2005a). Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. Journal of Chemical Ecology, 31, 969–988.
Barbehenn, R., Dodick, T., Poopat, U., & Spencer, B. (2005b). Fenton-type reaction and iron concentrations in the midgut fluids of tree-feeding caterpillars. Archives of Insect Biochemistry and Physiology, 60, 32–43.
Berenbaum, M. (1978). Toxicity of a furanocoumarin to armyworms: A case of biosynthetic escape from insect herbivores. Science, 201, 532–534.
Bergmeyer, H. U. (1974). Reagents for enzymatic analysis. In H. U. Bergmeyer & K. Gawehn (Eds.), Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie.
Bi, J. L., & Felton, G. W. (1995). Foliage oxidative stress and insect herbivory: Primary compounds, secondary metabolites and reactive oxygen species as components of induced resistance. Journal of Chemical Ecology, 21, 1511–1530.
Carlberg, I., & Mannervik, B. (1975). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. The Journal of Biological Chemistry, 250, 5475–5480.
Chien, C., & Dauterman, W. C. (1991). Studies on glutathione S-transferases in Helicoverpa (=Heliothis) zea. Insect Biochemistry, 21, 857–864.
Daniel, O., Meier, M. S., Schlatter, J., & Frischknecht, P. (1999). Selected phenolic compounds in cultivated plants: Ecological functions, health implications, and modulation by pesticides. Environmental Health Perspectives, 107, 109–114.
Dhillon, M. K., Singh, R., Naresh, J. S., & Sharma, H. C. (2005). The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. Journal of Insect Science, 5, 1–16.
Duffey, S. S., & Felton, G. W. (1989). Role of enzymes in agriculture. In J. Whitaker & P. Sonnet (Eds.), Biocatalysis in agricultural biotechnology (pp. 289–313). Washington, DC: American Chemical Society.
Felton, G. V., Donato, K. K., Del Vecchio, R. J., & Duffey, S. S. (1989). Activation of plant polyphenol oxidases by insect feeding damage reduces the nutritive quality of foliage. Journal of Chemical Ecology, 15, 2667–2694.
Felton, G. W., & Summers, C. B. (1995). Antioxidant systems in insects. Archives of Insect Biochemistry and Physiology, 29, 187–197.
Harborne, J. B. (2001). Twenty-five years of chemical ecology. Natural Product Reports, 18, 361–379.
Hemming, J. D. C., & Lindroth, R. L. (2000). Effects of phenolic glycosides and protein on gypsy moth (Lepidoptera: Lymantriidae) and forest tent caterpillar (Lepidoptera: Lasiocampidae) performance and detoxification activities. Environmental Entomology, 29, 1108–1115.
Hollman, P. C. H. (2001). Evidence for health benefits of plant phenols: Local or systemic effects? Journal of the Science of Food and Agriculture, 81, 842–852.
Huang, H. S., Hu, N. T., Yao, Y. E., Wu, C. Y., Chiang, S. W., & Sun, C. N. (1998). Molecular cloning and heterologous expression of a glutathione S-transferase involved in insecticide resistance from the diamondback moth, Plutella xylostella. Insect Biochemistry and Molecular Biology, 28, 651–658.
Kapoor, V. C. (1993). Economic fruit flies. In: Indian fruit flies (Insecta: Diptera: Tephritidae) (pp. 130–131). New Delhi, India: Oxford and DBH Publishing Co. Pvt. Ltd.
Kar, M., & Mishra, D. (1976). Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiology, 57, 315–319.
Kaur, R., & Rup, P. J. (2003). Influence of four plant growth regulators on development of the melon fruit fly, Bactrocera cucurbitae (Coquillett). Insect Science and its Application, 23, 121–125.
Kono, Y. (1978). Generation of superoxide radical during auto-oxidation of hydroxylamine and an assay for superoxide dismutase. Archives of Biochemistry and Biophysics, 186, 189–195.
Kono, Y., & Fridovich, I. (1982). Superoxide radical inhibits catalase. The Journal of Biological Chemistry, 275, 5751–5754.
Krishnan, N., & Sehnal, F. (2006). Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Archives of Insect Biochemistry and Physiology, 63, 1–10.
Lee, K., & Berenbaum, M. R. (1989). Action of antioxidant enzymes and cytochrome p-450 monooxygenases in the cabbage looper in response to plant phototoxins. Archives of Insect Biochemistry and Physiology, 10, 151–162.
Lee, K., & Berenbaum, M. R. (1993). Food utilization and antioxidant enzyme activities of black swallowtail in response to plant phototoxins. Archives of Insect Biochemistry and Physiology, 23, 79–89.
Lindroth, R. L. (1989). Differential esterase activity in Papilio glaucus subspecies: Absence of cross-resistance between allelochemicals and insecticides. Pesticide Biochemistry and Physiology, 35, 185–191.
Lukasik, I. (2007). Changes in activity of superoxide dismutase and catalase within cereal aphids in response to plant o-dihydroxyphenols. Journal of Applied Entomology, 131, 209–214.
Malik, R. S., Anand, I. J., & Srinivasachar, D. (1983). Effects of glucosinolates in relation to aphid (Lipahis erysimi Kalt.) fecundity in crucifers. International Journal of Tropical Agriculture, 4, 273–278.
Manoukas, A. G. (1996). The influence of four phenolics on the olive fruit fly. In B. A. McPheron & G. J. Steck (Eds.), Fruit fly pests, a world assessment of their biology and management (pp. 433–436). Delray Beach, FL, USA: St. Lucie Press.
Mathews, M. C., Summers, C. B., & Felton, G. W. (1997). Ascorbate peroxidase: A novel antioxidant enzyme in insects. Archives of Insect Biochemistry and Physiology, 34, 57–68.
McManus, J., Lilley, T. H., & Haslam, E. (1983). Plant polyphenols and their associations with proteins. In P. A. Hedin (Ed.), Plant resistance to insects (pp. 123–137). Washington, DC: American Chemical Society.
Parr, A. J., & Bolwell, G. P. (2000). Phenols in plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. Journal of the Science of Food and Agriculture, 80, 985–1012.
Peng, Z., & Miles, P. W. (1991). Oxidases in the gut of an aphid, Macrosiphum rosae (L.) and their relation to dietary phenolics. Journal of Insect Physiology, 37, 779–787.
Pierpoint, W. S. (1983). Reactions of phenolic compounds with proteins. In L. Telek & H. D. Graham (Eds.), Leaf protein concentrates (pp. 235–267). Westport, CT, USA: Avi Publishing Comp. Inc.
Prapanthadara, L. A., Hemingway, J., & Ketterman, A. J. (1993). Partial purification and characterization of glutathione S-transferases involved in DDT resistance from the mosquito Anopheles gambiae. Pesticide Biochemistry and Physiology, 47, 119–133.
Pritsos, C. A., Ahmad, S., Bowen, S. M., Elliott, A. J., Blomquist, G. J., & Pardini, R. S. (1988). Antioxidant enzymes of the black swallowtail butterfly, Papilio polyxenes, and their response to the prooxidant allelochemical, quercetin. Archives of Biochemistry and Biophysics, 8, 101–112.
Pritsos, C. A., Ahmad, S., Elliott, A. J., & Pardini, R. S. (1990). Antioxidant enzyme level response to prooxidant allelochemicals in larvae of the southern armyworm moth, Spodoptera eridania. Free Radical Research Communications, 9, 127–133.
Puttick, G. M., & Bowers, M. D. (1988). Effect of qualitative and quantitative variation in allelochemicals on a generalist insect: Iridoid glycosides and the southern armyworm. Journal of Chemical Ecology, 14, 335–351.
Shen, S., Chein, Y., & Chein, C. (2003). Induction of glutathione S-transferases activities in Drosophila melanogaster exposed to phenol. Archives of Insect Biochemistry and Physiology, 53, 80–91.
Srivastava, B. G. (1975). A chemically defined diet for Dacus cucurbitae (Coq.) larvae under aseptic conditions. Entomology Newsletter, 5, 24.
Vontas, J. G., Small, G. J., & Hemingway, J. (2001). Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. The Biochemical Journal, 357, 65–72.
Weissenberg, M., Meisner, J., Klein, M., Schaeffler, I., Eliyahu, M., Schmutterer, H., & Ascher, K. R. S. (1997). Effect of substituent and ring changes in naturally occurring naphthoquinones on the feeding response of larvae of the Mexican bean beetle, Epilachna varivestis. Journal of Chemical Ecology, 23, 3–18.
Wheeler, G. S., Slansky, F., Jr., & Yu, S. J. (1993). Fall armyworm sensitivity to flavone: Limited role of constitutive and induced detoxifying enzyme activity. Journal of Chemical Ecology, 19, 645–667.
Yu, S. J. (1983). Induction of detoxifying enzymes by allelochemicals and host plants in the fall armyworm. Pesticide Biochemistry and Physiology, 19, 330–336.
Yu, S. J. (1996). Insect glutathione S-transferases. Zoological Studies, 35, 9–19.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohal, S.K., Sharma, R. Bioactivity of pyrogallol against melon fruit fly, Bactrocera cucurbitae . Phytoparasitica 39, 361–367 (2011). https://doi.org/10.1007/s12600-011-0169-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-011-0169-1