Abstract
The occurrence and prevalence of bark beetle pathogens in forest stands in Bulgaria were investigated in 944 specimens belonging to 21 bark beetle species. Protozoa, microsporidia, fungi and nematodes occurred in 19 of all investigated species. The infections were found in the gut (nematodes, gregarines, microsporidia), gonads (microsporidia) and hemolymph (nematodes) of the infected insects. Protozoan species (Gregarina typographi, Gregarina spp.) were detected in eight bark beetle species. Morphometric data about G. typographi and Gregarina spp. are presented. The prevalence of the gregarines varied between 1.4% and 64.2%. Microsporidia of the genera Nosema and Chytridiopsis were revealed in three bark beetle species. The prevalence of microsporidia ranged between 1.5% and 11.8%. This is the first report of a microsporidium in Taphrorychus villifrons and of gregarines in T. villifrons, Pityogenes bistridentatus, P. conjunctus, and Orthotomicus erosus. The fungus Beauveria bassiana was found in 3.4% of Hylurgops palliatus specimens. Nematodes (in gut and haemolymph) were revealed in 19 bark beetle species and their prevalence varied between 10% and 98.5%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bark beetles (Coleoptera: Curculionidae: Scolytinae) are among the most dangerous insect pests in forests. Usually they colonize recently dry-topped and physiologically exhausted trees.
There is still lack of knowledge about the potential of pathogens in population regulation or as biological control agents of bark beetles (Händel et al. 2003). So far the most commonly used controls against these pests are sanitation measures, which are limited to the removal of infested host trees (Wermelinger 2004).
The investigations of bark beetle pathogens started at the beginning of last century when Fuchs (1915) described the first protozoan, Gregarina typographi, parasitizing Ips typographus. Later several authors studied bark beetle pathogens, e.g. Händel et al. (2003), Holuša et al. (2009), Wegensteiner (2004), and Yaman (2007).
In Bulgaria, economically important bark beetle species of coniferous trees (Pinus sylvestris, Pinus nigra, Picea abies) are the European spruce bark beetle (Ips typographus L.), the engraver beetle (Ips acuminatus Gyllenhal), the six-toothed bark beetle (Ips sexdentatus Borner), the pine shoot beetle (Tomicus piniperda L.), the lesser pine shoot beetle (Tomicus minor Hartig) and the black pine bark beetle (Hylastes ater Paykull) (Georgiev 2006; Rosnev et al. 2006; Tsankov et al. 1997).
To explore the possibilities of using biological agents against forest pests it is necessary to investigate the diversity, distribution, biology and host interactions of their pathogens. Therefore an investigation of pathogens of I. typographus and other bark beetle pests (in total 22 species) was started in Bulgaria in 2003. As a result, Takov et al. (2006, 2007) and Nedelchev et al. (2008) reported 14 pathogen species (four protozoa, two microsporidia, one virus and seven nematodes) of bark beetles from Bulgaria.
The aim of the present study was to obtain new data about the occurrence of pathogens and their prevalence in a variety of bark beetle species from different forest stands in Bulgaria.
Materials and methods
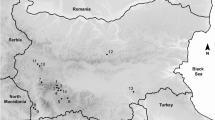
Adult offspring beetles of 21 species were collected from April to September 2009 at 11 sites from various regions in Bulgaria (Fig. 1, Table 1). Beetles were collected from wind-thrown trees by peeling off the bark manually and removing the insects from the maternal galleries and nuptial chambers. In the laboratory, collected beetles were refrigerated at 4°C to reduce movement and prevent horizontal transmission of any pathogens. Bark beetles were dissected and fresh preparations of the gonads, Malpighian tubules, fat body and the entire gut from the host were examined for the presence of pathogens under a light microscope (160–400 x) according to Wegensteiner et al. (1996). Dryocoetes autographus was found mainly in the larval stage and only several adult beetles were observed; therefore mostly larvae of this host were investigated. When pathogens were observed, Giemsa-stained smears were made of the infected tissues (Weiser 1977). Sizes of spores of microsporidia and gregarine trophozoites (protomerite and deutomerite) were measured with an ocular micrometer at 200 and 400 x magnifications.
Collection localities in Bulgaria (data about localities are presented in Table 1). 1—Rila Mt., Skakavitsa, 2—Rhodope Mt., Yundola Vill., 3—Sakar Mt., Zvezdata Place, 4—Maleshevska Mt., 5—Pravets, 6—Lyulin Mt., above Gorna Banya, Monastery St. Cyril and Methodius, 7—Balkan Range, above Gabrovo, 8—Rhodope Mt., Byala cherkva, 9—Rhodope Mt., above Velingrad, 10—Rila Mt., Belmeken Dam, 11—Sofia, Borisova gradina Park
Dead beetles with symptoms of mycosis were placed in a moist chamber at 25°C to allow the development of hyphal and reproductive structures of the fungus. Some parts of cadavers were prepared as permanent preparations using lactophenol with aniline blue; others were prepared as smears stained with methylene-blue. Small amounts of insect cadavers after surface sterilization were used to isolate the fungal pathogens in pure cultures on SDAY (Sabouraud dextrose agar with yeast extract). Morphological characters of the fungal pathogens on the host and on media were studied under a light microscope in order to determine their taxonomic status and were identified according to Samson et al. (1988) and Humber (1997).
Statistical analysis was performed using the computer program STATISTICA, version 7.0 (StatSoft Inc. 1999).
Results
Protozoa, microsporidia, fungi and nematodes were detected in 19 (90.4%) of all investigated bark beetle species (Table 1). Protozoan species (Apicomplexa: Gregarina) were detected in the gut system of eight bark beetle species (Table 1). Trophozoites of Gregarina typographi were observed in the gut lumen of Ips typographus, I. sexdentatus and Pityogenes chalcographus. Other Gregarina species were found in the gut lumen of P. conjunctus, P. bistridentatus, Orthotomicus proximus, O. erosus and Taphrorychus villifrons (Fig. 2). The prevalence of the gregarines varied between 1.5% and 64% but the average prevalence for all investigated host species was 9.5%.
a, Trophoizoites of Gregarina sp. in midgut lumen of Taphrorychus villifrons; b, Trophoizoites of Gregarina sp. in midgut lumen of Orthotomicus erosus; c, Trophoizoites of Gregarina sp. in midgut lumen and nematodes in hemolymph of Pityogenes conjunctus; d, Trophoizoites and syzigia of Gregarina sp. in midgut lumen of Pityogenes bistridentatus; e, Spores of Nosema sp. in gonads and fat body of Taphrorychus villifrons (semithin section stained with Richardson); f, Fresh spores of Nosema sp. from midgut of Hylurgus ligniperda
The systematics and taxonomy of the group are based on morphometric parameters such as the sizes of protomerites and deutomerites which form the gregarine trophozoite and the ratios between them. We measured morphometrical data of gregarine trophozoites found in eight hosts (Table 2). The statistical analysis (Figs. 3, 4 and 5) showed that the sizes of Gregarina spp. from P. chalcographus, P. bistridentatus, O. erosus and T. villifrons are within the limits of the species variability of G. typographi, found in I. typographus and I. sexdentatus. However, the most precise identification of these gregarines would be possible only after a molecular analysis and detailed investigations of life cycle stages.
Microsporidia, which can morphologically be assigned to the genera Nosema and Chytridiopsis, were revealed in three bark beetle species (Hylurgus ligniperda, Ips acuminatus, Taphrorychus villifrons) (Table 1). The prevalence of the microsporidia ranged between 1.5% and 11.8% and the average prevalence for all investigated host species was 1.9%.
Spores and pansporoblasts of Chytridiopsis sp. were found in the gut of I. acuminatus individuals. The size of live pansporoblasts varied from 7.2 to 14.4 μm (n = 30) and up to 30 spores were observed in the gut.
Microsporidia with morphological features typical of the genus Nosema were detected in gonads and fat body tissue of Taphrorychus villifrons (Fig. 2). The Nosema spores were oval and the length of spores stained with Giemsa varied from 2.4 to 3.9 μm and the width from 1.2 to 3 μm (n = 50). Spores of another microsporidium of the genus Nosema were observed in the gut epithelium of Hylurgus ligniperda (Fig. 2). The length of the oval, live spores varied from 4.8 to 7.2 μm and the width from 1.8 to 3.30 μm (n = 30). The length of Giemsa-stained spores varied from 3.6 to 5.1 μm and the width from 1.95 to 3.44 μm (n = 35). Furthermore, the tissue localization of the two Nosema microsporidia, isolated from Hylurgus ligniperda and Taphrorychus villifrons, is different, and therefore we suppose that they belong to two different species. However, for accurate species determination, additional investigations and especially detailed molecular characterization are needed.
The fungus B. bassiana was found in 3.4% of the investigated Hylurgops palliatus specimens (Table 1).
Nematodes were revealed in 19 bark beetle species. The determination of the nematodes in this study was very difficult. In most cases only nematode larvae without a completed sexual system, which is important for species determination, were found in the gut and the hemolymph of the bark beetles. Their prevalence was high and varied between 10% and 98.5% and the average prevalence for all investigated host species was 43% (Table 1).
Individuals of S. rugulosus and P. pityographus were also investigated but no pathogens were revealed.
Discussion
Pathogens (G. typographi, Gregarina spp., Chytridiopsis sp., Nosema spp. and B. bassiana) were found in 19 of the 21 investigated bark beetle species. Four species are new hosts for Gregarina spp., and a Nosema sp. was recorded for the first time in T. villifrons.
Gregarina typographi and Gregarina spp. were the most frequently recovered pathogens. G. typographi is a polymorphic species and was described by Fuchs (1915) in I. typographus and reported in I. sexdentatus by Theodorides (1960). The pathogen was found in Austria, Czech Republic and Bulgaria (Takov et al.2006, 2007; Wegensteiner 1994). The investigations of bark beetle pathogens conducted in Austria by B. Haidler (Diploma thesis,1998, Vienna Univ., Austria) and U. Händel (Ph.D. thesis, 2001, Boku Univ., Vienna, Austria) revealed the presence of G. typographi in Hylastes cunicularius and Dryocoetes autographus and of G. cf. typographi in Hylurgops glabratus, Pityogenes chalcographus and I. amitinus. Takov et al. (2007) conducted a morphometrical characterization of this gregarine and showed its variability.
The Chytridiopsis sp. we recovered in I. acuminatus is possibly Chytridiopsis typographi. It was reported also from Bulgaria by Takov et al. (2007) and from Austria, Czech Republic and Norway by P. M. Zitterer (Diploma thesis, 2002, Boku Univ., Vienna, Austria). The size of the pansporoblasts measured by us corresponds to the size recorded by Zitterer in his thesis, but he did not identify the microsporidium to species level. According to Wegensteiner (2004), C. typographi has a broader specificity and it might be possible that it infects also I. acuminatus. More detailed study of additional material is needed for the precise identification of Chytridiopsis sp. found in this study.
Several microsporidia of the genus Nosema were described in different bark beetles: N. typographi, which infects fat body and Malpighian tubules of I. typographus (Weiser 1955 ) and also Hylurgops palliatus (Purrini 1978). N. curvidens was found in the fat body, hypoderm and connective tissue of Pityokteines curvidens (Weiser 1961 ), N. scolyti in the gut epithelium, Malpighian tubules and hemocytes of Scolytus scolytus, S. multistriatus, S. pygmaeus and S. ensifer; and N. dendroctoni in the fat body of Dendroctonus pseudotsugae (Weiser 1970 ). Händel et al. (2003) discovered a Nosema sp. in the fat body and gut epithelium of H. palliates and Takov et al. (2007) found a Nosema sp. in the gut epithelium of H. ligniperda. Based on the size and tissue localization reported by Takov et al. (2007), we assume that this is the same species we detected during this study. This is the first report of a microsporidium in T. villifrons and gregarines in Pityogenes bistridentatus, P. conjunctus, Orthotomicus proximus, O. erosus and T. villifrons.
Beauverai bassiana was recorded for the first time from H. palliatus by Balazy (1962), but ours is the first record of the fungus in this host in Bulgaria. Its prevalence in this study was low (3.4%). Wegensteiner (1996), Kreutz et al. (2004), Draganova et al. (2007), Sevim et al. (2010) and Steinwender et al. (2010) showed that adults of bark beetles were susceptible to entomopathogenic fungi under laboratory conditions. Bioassays with the entomopathogenic fungus B. bassiana showed high virulence of some isolates, especially of that obtained in pure cultures from dead individuals of the hosts, collected from infected natural populations (Draganova et al.2010). Induced mycoses with a high lethal effect could be a perspective in the use of this pathogen for control of the bark beetles.
The infection rates of nematodes were the highest and we believe that this reflects the low pathogenicity of bark beetle nematodes and the high survival rates of the infected bark beetle hosts.
The development cycle of the pathogens is often synchronized with the host cycle and this assures a successful infection and distribution (Massey 1956; Rühm 1956; Thong and Webster 1973). Pathogens are transmitted via spores (fungi, microsporidia), cysts (protozoa) and invasion of larvae or imago (nematodes) to the next generation. The infection takes place in the beetle galleries via the active penetration of the pathogens into the hosts (nematodes), per os infection (protozoa and microsporidia) or by external contact (fungi). The new generation of the beetles usually feeds additionally in the galleries, where contact with the pathogens occurs. The capability of the pathogens to infect the host is also species-specific. Virulent pathogens kill their host very fast after the infection. The infection has a local character and usually few hosts survive and serve to transmit the infection into new galleries. Therefore, during the investigation of a large number of live hosts, the prevalence of pathogens is low except in the cases when infection foci are found. In this case the infection rate is higher, but because of the mortality the pathogen prevalence in live beetles decreases. Gregarines are less pathogenic (Bjornson and Schütte 2003) and therefore occur more often compared with other pathogens, which is in accord with our findings.
The influence of bark beetle pathogens on the host density is still not sufficiently investigated, but existing studies show that the pathogen prevalence depends on several factors, such as pathogen characteristics, its life cycle, population host density and immunity, host development particularities and other factors (Wegensteiner 2004). Therefore, the investigations of pathogenicity and virulence should be intensified. The findings reported in this study show that bark beetles collected from different Bulgarian forests host a broad range of pathogens from different taxonomic groups, but molecular techniques, inter alia, are needed in order better to characterize bark beetle pathogens.
References
Balazy, S. (1962). Observations on appearing of some entomogenous fungi of fungi imperfecti group of forest insects. Polskie Pismo Entomologiczne (Bulletin Entomologique de Pologne), Ser. B, 3–4, 149–164.
Bjornson, S., & Schütte, C. (2003). Pathogens of mass-produced natural enemies and pollinators. In J. C. van Lenteren (Ed.), Quality control and production of biological control agents. Theory and testing procedures (pp. 133–167). Wallingford, UK: CABI.
Draganova, S., Takov, D., & Doychev, D. (2007). Bioassays with isolates of Beauveria bassiana (Bals.) Vuill. and Paecilomyces farinosus (Holm.) Brown & Smith against Ips sexdentatus Boerner and Ips acuminatus Gyll. (Coleoptera: Scolytidae). Plant Science, 44, 24–28.
Draganova, S., Takov, D., & Doychev, D. (2010). Naturally occurring entomopathogenic fungi on three bark beetle species (Coleoptera: Curculionidae) in Bulgaria. Pesticides & Phytomedicine, 25, 59–63.
Fuchs, G. (1915). Die naturgeschichte der nematoden und einiger anderer parasiten des Ips typographus L. 2. des Hylobius abietis L. Zoologische Jahrbuecher (Syst.), 38, 109–222.
Georgiev, G. (2006). Ips typographus (L.) and drying of the spruce stands in Vitosha. Bulgarian Forest, 1(5), 8 (in Bulgarian).
Händel, U., Wegensteiner, R., Weiser, J., & Zizka, Z. (2003). Occurrence of pathogens in associated living bark beetles (Col., Scolytidae) from different spruce stands in Austria. Anzeiger für Schädlingskunde. /Journal of Pest Science, 76, 22–32.
Holuša, J., Weiser, J., & Zizka, Z. (2009). Pathogens of the spruce bark beetles Ips typographus and Ips duplicatus. Central European Journal of Biology, 4, 567–573.
Humber, R. (1997). Fungi: identification (pp. 153–185). In L. Lacey (Ed.), Manual of techniques in insect pathology. London, UK: Academic.
Kreutz, J., Vaupel, O., & Zimmermann, G. (2004). Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, Ips typographus L., in the laboratory under various conditions. Journal of Applied Entomology, 128, 384–389.
Massey, C. L. (1956). Nematode parasites and associates of the Engelmann spruce beetle (Dendroctonus engelmanni Hopk.). Proceedings of the Helminthological Society of Washington, 23, 14–24.
Nedelchev, S., Takov, D., & Pilarska, D. (2008). Parasitic and associated nematodes of bark beetles in Bulgaria. Acta Zoologica Bulgarica, Suppl., 2, 83–91.
Purrini, K. (1978). Protozoen als krankheitserreger bei einigen borkenkäferarten (Col., Scolytidae) im Königsee-Gebiet, Oberbayern. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz, 51, 171–175.
Rosnev, B., Mirchev, P., Georgiev, G., Petkov, P., Naydenov, Y., Tsankov, G., et al. (2006). Manual of forest protection. Part I. Diseases, insects and other pests and damages on forest trees and shrubs. Sofia, Bulgaria: Obrazovanie i Nauka (in Bulgarian).
Rühm, W. (1956). Die Nematoden der Ipiden. Parasitologische Schriftenreihe, 6, 1–435.
Samson, R. A., Evans, H. C., & Latge, J.-P. (1988). Atlas of entomopathogenic fungi. London, UK: Springer.
Sevim, A., Demir, I., Tanyeli, E., & Demirbag, Z. (2010). Screening of entomopathogenic fungi against European spruce bark beetle, Dendroctonus micans (Coleoptera: Scolytidae). Biocontrol Science and Technology, 20, 3–11.
StatSoft Inc. (1999). STATISTICA for Windows - Computer program manual (http://www.statsoft.com).
Steinwender, B. M., Krenn, H. W., & Wegensteiner, R. (2010). Different effects of the insect pathogenic fungus Beauveria bassiana (Deuteromycota) on the bark beetle Ips sexdentatus (Coleoptera: Curculionidae) and on its predator Thanasimus formicarius (Coleoptera: Cleridae). Journal of Plant Diseases and Protection, 177, 33–38.
Takov, D., Doychev, D., Wegensteiner, R., & Pilarska, D. (2007). Study on the pathogens of bark beetles (Coleoptera, Scolytidae) from different coniferous stands in Bulgaria. Acta Zoologica Bulgarica, 59, 87–96.
Takov, D., Pilarska, D., & Wegensteiner, R. (2006). Occurrence of pathogens in Ips typographus (Coleoptera, Scolytidae) from several Picea abies (L.) (Karst.) stands in Bulgaria. Acta Zoologica Bulgarica, 58, 409–420.
Theodorides, J. (1960). Parasites et phoretiques de coleopteres et de myriapodes de Richelieu (Indre-et-Loire). Annales de Parasitologie, 25, 488–581.
Thong, C. H. S., & Webster, J. M. (1973). Morphology and post-embryonic development of the bark beetle nematode Contortylenchus reversus (Sphaerulariidae). Nematologica, 19, 159–168.
Tsankov, G., Mirchev, P., & Tomovsky, H. (1997). A study on wood eaters and bark beetles in Scots pine ecosystems in Bulgaria. Forest Science, 3(4), 71–82 (in Bulgarian, English summary).
Wegensteiner, R. (1994). Chytridiopsis typographi (Protozoa, Microsporidia) and other pathogens in Ips typographus (Coleoptera, Scolytidae). IOBC/WPRS Bulletin, 17(3), 39–42.
Wegensteiner, R. (1996). Laboratory evaluation of Beauveria bassiana (Bals.) Vuill. against Ips typographus (Coleoptera, Scolytidae). IOBC/WPRS–Bulletin, 19, 186–189.
Wegensteiner, R. (2004). Pathogens in bark beetles. In F. Lieutier, K. Day, A. Battisti, J.-C. Gregoire, & H. Evans (Eds.), Bark and wood boring insects in living trees in Europe, a synthesis. Ch. 12 (pp. 291–313). Dordrecht, the Netherlands: Kluwer Academic Publishers.
Wegensteiner, R., Weiser, J., & Fuhrer, E. (1996). Observations on the occurrence of pathogens in the bark beetle Ips typographus L. (Coleoptera, Scolytidae). Journal of Applied Entomology, 120, 199–204.
Weiser, J. (1955). Prispevek k znalosti cizopasniku kurovce Ips typographus L. II. (Contributions to the knowledge of Ips typographus L. Parasites. II.). Acta Societatis Zoologicae Bohemoslovacae, 9, 374–380.
Weiser, J. (1961). A new microsporidian from the bark beetle Pityokteines curvidens Germar (Col. Scolytidae) in Czechoslovakia. Journal of Insect Pathology, 3, 324–329.
Weiser, J. (1970). Three new pathogens of the Douglas Fir Beetle, Dendroctonus pseudotsugae: Nosema dendroctoni n. sp., Ophryocistis dendroctoni n. sp. and Chytridiopsis typographi n. comb. Journal of Invertebrate Pathology, 16, 436–441.
Weiser, J. (1977). An atlas of insect diseases. Prague, Czechoslovakia: Academia.
Wermelinger, B. (2004). Ecology and management of the spruce bark beetle Ips typographus –a review of recent research. Forest Ecology and Management, 202, 67–82.
Yaman, M. (2007). Gregarina typographi Fuchs, a gregarine pathogen of the six-toothed pine bark beetle, Ips sexdentatus (Boerner) (Coleoptera: Curculionidae, Scolytinae) in Turkey. Turkish Journal of Zoology, 31, 359–363.
Acknowledgments
We are especially indebted to the National Science Fund of Bulgaria project DO-02-251/2008, project BG 051 PO001-3.3.04/41 ESF (European Social Fund) and DFG (German Research Foundation) for their contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takov, D., Doychev, D., Linde, A. et al. Pathogens of bark beetles (Coleoptera: Curculionidae) in Bulgarian forests. Phytoparasitica 39, 343–352 (2011). https://doi.org/10.1007/s12600-011-0167-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-011-0167-3