Abstract
Beetles from Curculionidae and Attelabidae collected from 14 localities (mainly in coniferous stands) in Bulgaria were investigated for the presence of pathogens and nematodes. A microsporidium belonging to genus Nosema in the fat body of Pityogenes chalcographus (prevalence: 0.9%) and the fungus Beauveria bassiana in the oak-leaf roller Attelabus nitens (prevalence: 62%) were detected for the first time. Morphological data and characteristics of Nosema sp. spores and conidia of B. bassiana are presented. Nematode species (Cryptaphelenchus diversispicularis, Parasitorhabditis subelongati and Parasitylenchus dispar), and specimens belonging to other ten nematode genera were found (Bovianema, Bursaphelenchus, Cryptaphelenchus, Neoparasitylenchus, Parasitylenchus, Parasitaphelenchus, Panagrolaimus, Parasitorhabditis, Prothallonema and Sulphuretylenchus). Their prevalences varied from 17.4% to 90%. Nine of the host beetles (Dryocoetes autographus, Ips sexdentatus, I. acuminatus, Orthotomicus laricis, O. erosus, Pityogenes quadridens, P. conjunctus, Hylurgus ligniperda and Taphrorychus villifrons) are reported as vectors of Bursaphelenchus sp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of diversity and occurrence of insect pathogens are related to the development of environmentally friendly methods to control the pest mass outbreaks. Therefore their pathogen complex and parasites are intensively investigated.
Until now at least 16 microsporidian species were revealed in bark beetles (Takov et al. 2010, 2011; Holuša et al. 2016). Hosts of these pathogens were 35 bark beetle species, some of them with seriously economical importance. Beauveria bassiana is the most distributed pathogen causing mycoses in insects. It was found in about 700 arthropod species (Goettel et al. 1990) and is a common fungal pathogen in bark beetle species (Landa et al. 2001; Wegensteiner 1994, 2004; Polovinko et al. 2010). Nematodes of the orders Diplogasterida, Rhabditida and Tylenchida have been reported from bark beetles. Linstow (1890) was the first who observed and described the parasitic nematode Contortylenchus diplogaster (von Linstow, 1890) in Ips typographus from Germany. Data about occurrence and distribution of entomophilic nematodes of Ips spp. were reviewed and summarized by Grucmanová and Holuša (2013). They presented data about 11 nematode species associated with bark beetles (Ips spp.) and 12 endoparasitic nematode species found in the same genus in central Europe (Grucmanová and Holuša 2013).
In Bulgaria, Takov et al. (2006, 2007, 2011, 2012) and Nedelchev et al. (2008, 2011) investigated economically important bark beetle species – Ips typographus, I. sexdentatus, I. acuminatus, Tomicus piniperda, Pityogenes chalcographus etc. They detected viruses, fungi, protozoa, microsporidia and nematodes in these hosts. The parasitic nematodes Contortylenchus diplogaster, Parasitylenchus dispar and the associated nematode Cryptaphelenchoides macrobulbosus (Rühm, 1956) were observed in I. typographus. Parasitorhabditis subelongati, Parasitaphelenchus sexdentati Fuchs, 1937 and Cryptaphelenchus diversispicularis parasitize I. sexdentatus. The nematode Rhodolaimus pini Fuchs 1930 was found also in the same host. Contortylenchus acuminati Rühm, 1956 was detected in I. acuminatus (Nedelchev et al., 2008). Nedelchev et al. (2011) described a new nematode species Prothallonema tomici, parasitizing T. piniperda. However, the leaf-rolling weevils were not studied for the presence of pathogens or parasites in Bulgaria.
The aim of this study is to investigate the presence and the occurrence of pathogens and nematodes of scolytids and a leaf-rolling weevils in Bulgaria and also to obtain additional data on their species composition, distribution and localization in the hosts.
Materials and methods
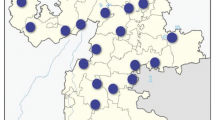
Totally 1112 adult specimens of forest beetles belonging to 12 species of bark beetles and the weevil Attelabus nitens were collected from 14 localities in Bulgaria (Fig. 1).
Map of localities. 1. Rila Mt., Belmeken Dam, 1940 m a.s.l.; 2. Rila Mt., Kurtovo Place, 1760 m a.s.l.; 3. Rila Mt., Yundola Place, 1550 m a.s.l.; 4. Western Rodopes Mts., Pashovi skali Place, 1360 m a.s.l.; 5. Western Rodopes Mts., Kara tepe Hut, 1530 m a.s.l.; 6. Western Rodopes Mts., Beglika Place, 1560 m a.s.l.; 7. Rila Mt., Skakavitsa Hut, 1700 m a.s.l.; 8. Maleshevska planina Mt., W of Tsaparevo Vill., 940 m a.s.l.; 9. Maleshevska planina Mt., S of Tsaparevo Vill., 820 m a.s.l.; 10. Vitosha Mt., Zlatnite mostove Place, 1400 m a.s.l.; 11. Lyulin Mt., Bonsovi polyani Place, 900 m a.s.l.; 12. Balkan Range, near Gabrovo, 660 m a.s.l.; 13. Sakar Mt., Zvezdata Place, 500 m a.s.l.; 14. Western Rodopes Mts., W of Velingrad, 1000 m a.s.l
Bark beetles were collected from bark which was peeled off from infested trees. In one location (Fig. 1; № 14) dead adults of A. nitens were collected from oak leafs. All collected beetles were transferred to the laboratory and kept refrigerated at 1–4 °C to reduce movement and prevent horizontal transmission of infection before investigation.
Bark beetles were dissected in vivo in a drop of water under a stereo microscope as described by Wegensteiner (1996) and were observed for presence of pathogens and nematodes under light microscopes Zeiss and Olympus BX60 DIC at magnifications 100× and 400×. Dead adults of A. nitens were also inspected microscopically. When symptoms of mycosis were found, small pieces of tissue were removed after surface sterilization and placed in a humid chamber to allow the sporulation of the fungal pathogens. Other pieces were placed on SDAY (Sabouraud dextrose agar with yeast extract) plates for isolation of fungi into pure cultures. Fungal conidia and conidiogenous cells from insect cadavers in the humid chamber and from pure cultures were used to prepare smears stained with methylen-blue or slide preparations with lactophenol and aniline blue closed by nail vanish (after Humber 1997). Fungal pathogens were identified according to their morphological characteristics (Samson et al. 1988; Humber 1997).
Nematodes found in bark beetles were isolated, heat-killed (65 °C), fixed in TAF (triethanolamin, destilled water and formalin), processed in glycerin (Seinhorst 1959) and studied in permanent mounts. In most of cases only larval stages of nematodes were observed.
For transmission electron microscopy (TEM) of the found microsporidium infected tissues were fixed in 2.5% glutaraldehyde in 1 M cacodylate buffer (pH 7.2) and postfixed for 2 h in 2% OsO4. The tissues were then dehydrated through an ascending ethanol and acetone series and embedded in Epon-Araldite or in Poly/Bed 812/Araldite 502 (Becnel 2012). Thick sections (1.0 mm), stained according to Richardson et al. (1960) were inspected using light microscopy to locate infected cells. Thin sections were cut on an Ultracut E. Reichert microtome, stained with uranyl acetate and lead citrate, and examined with a Philips EM 208 transmission electron microscope.
The infection levels were calculated as total prevalence of the parasites for each host species.
Results
A microsporidium belonging to genus Nosema Nägeli, 1857 was detected in the fat body of P. chalcographus with a prevalence of only 0.9% (Table 1). The microsporidian spores were oval and measured 2.4 μm (2.0–2.8) х 4.7 μm (3.7–5.6). The ultrastructural investigations of this microsporidium revealed internal structure typical for the genus Nosema, with binuclear sporogonial stages and spores. The nuclei were in diplokaryotic arrangement. The polaroplast was lamellar and the polar filament is isofilar with 15–16 coils, situated in one row (Figs. 2 and 3).
Numerous Nosema sp. spores in fat body of Pityogenes сhalcographus (semithin section, stained after Richardson et al. 1960)
The microsporidium reported in this study is the first Nosema sp. found in P. chalcographus.
Most of the collected dead adults of A. nitens (23 of 37) showed symptoms of mycoses (Table 1). Beetles were covered with a dense whitish mycelium and some of them showed colour changes of the elytra (Fig. 4). The isolates obtained from A. nitens were identified as Beauveria bassiana (Ascomycota anamorph form, Sordariomycetes: Hypocreales, Cordycipitaceae). Morphological characters of the examined fungal pathogen were typical for the species. Isolates from pure cultures on SDAY formed round raised colonies, most of them with powdery surface, with pigmentation from white to cream. Reverse sites of the colonies were with pale cream to pink-tan pigmentation (Fig. 5). Some of the isolates released pink pigment in the media which later paled and gradually disappeared. Conidiogenous cells were densely clustered in whorls, hyaline, smooth and short. They had a globose or elongated base terminated in a narrow extended denticulate apex with a distinctly zig-zag threadlike denticulate rachis with one conidium per denticle (Fig. 5). Conidia were one-celled, hyaline, thin-walled, hydrophobic, subglobose, with average dimensions 1.21 μm, 1.43 μm, 1.60 μm, 1.87 μm etc. for different B. bassiana isolates.
In this study nematodes were isolated from 12 beetle species, and we established 10 nematode genera. Three nematodes were determined to the species level. Infection levels varied from 17.4% to 90%. The nematodes were localized in the gut and haemocoel of the bark beetles.
Nematodes of the genus Bursaphelenchus Fuchs, 1937 were established in 9 of 12 investigated beetle species (Table 1): Dryocoetes autographus, Ips sexdentatus, I. acuminatus, Orthotomicus laricis, Oerosus, Pityogenes quadridens, Hylurgus ligniperda, Tomicus piniperda and Taphrorychus villifrons were identified as vectors for Bursaphelenchus in Bulgaria.
Parasitylenchus dispar was detected in I. typographus and Parasitylenchus sp. in D. autographus. Cryptaphelenchus diversispicularis was found in P. chalcographus and Cryptaphelenchus sp. in P. quadridens and O. erosus. Nematodes of genus Parasitaphelenchus Fuchs 1930 were found in the haemolymph of three bark beetle species – I. acuminatus, I. sexdentatus and T. piniperda. Parasitorhabditis sp. was detected in two hosts – T. piniperda and P. chalcographus. Nematodes of the genus Sulphuretylenchus Rühm, 1956 were found in one host – O. laricis. Another nematode genus, Neoparasitylenchus Nickle, 1967 was detected in O. erosus. Panagrolaimus sp. was found also in O. erosus.
Discussion
A literature review revealed that infections with microsporidia of the genus Nosema have been found in 14 curculionid beetle species todate (Table 2).
In most cases the target tissue of Nosema spp. was the fat body. Some Nosema spp. were also found in the gut tissue (mainly in midgut), Malpigian tubules and gonads. Two Nosema spp. were detected in bark beetles collected in Bulgaria – H. ligniperda (in the midgut) and T. villifrons (in the fat body and gonads) (Takov et al. 2007, 2011; Table 2). The size of the Nosema sp. found in this study was smaller than Nosema sp. from T. villifrons and from H. ligniperda, however, the size is similar to Nosema calcarati, N. typographi and N. hylobii. Nosema calcarati and N. typographi possessed an isofilar polar filament with coils arranged in a single row and 12 coils (N. calcarati), 16–17 coils (N. typographi). Nosema hylobii has an anisofilar polar filament with 9–13 coils. The Nosema sp. in our study has an isofilar filament with 15–16 coils arranged in one row and thus shows similarity to N. typographi. The structure of the polaroplast was lamellar as described for Nosema raphidae Yaman, Radek, Tosun & Unal, 2009 by Yaman et al. (2009). However, for a precise species identification of the microspridium found in this study, molecular analysis is needed.
We also detected a mycosis caused by B. bassiana in the oak-leaf roller A. nitens. Unlike scolytids, A. nitens is not considered as a significant pest species. In Bulgaria its adults make specific and easily recognizable cylindrical rolls on leaves of Quercus spp. predominantly, and occasionaly on Castanea sativa Mill. According to Urbanová and Urban (2016) the young shoots of trees are prefered.
In Bulgaria, fungal infections caused by B. bassiana were detected in populations of different coleopteran forest pests by Markova (1992), Draganova et al. (2007, 2010) and Takov et al. (2011, 2012). However, so far no infection caused by B. bassiana was registerd in A. nitens and thus this leaf roller is a new host for the pathogen.
Nematodes of the genus Bursaphelenchus occur in coniferous forests in Europe, and 28 species (Braasch 2001) were found in more than 15 coniferous tree species in 20 countries of Europe. The list of vectors of this genus includes over 20 beetle species, 16 of which are bark beetles (Linit 1988; Kulinich and Orlinskii 1998). One beetle species can carry several Bursaphelenchus species, and one nematode species can use several beetle species as vectors. So far, only Bursaphelenchus sexdentati Rühm, 1960 has been found in T. piniperda from Bulgaria (Choleva, personal communication). Our study confirms Bursaphelenchus species in nine species of bark beetles. Nematodes of the genus Bursaphelenchus were also found by Burjanadze and Goginashvili (2009) in I. typographus from Georgia.
We recorded Parasitylenchus dispar in I. typograhus. This species was reported for the first time in Germany (Fuchs 1915) and later in Poland and former USSR (Filipjev and Schuurmans Stekhoven 1941), parasitizing I. typographus. In Bulgaria, Nedelchev et al. (2008) also reported this nematode species from I. typographus collected from spruce trees.
The impact of Parasitylenchus spp. on the host was investigated by different researchers (Fuchs 1915; Schvester 1950, 1957; Nickle 1971; Poinar Jr and Caylor 1974). They found changes in the structure of the galleries, shortened lifespan as well as a reduction of the fat body in the infected beetles. Bark beetles infected with Parasitylenchus spp. died shortly after the construction of short horizontal galleries (Nickle 1971; Schvester 1957). Rühm (1956) also investigated the impact of these nematodes and noted that 6% of the studied Pityokteines curvidens population were sterile and had reduced gonads.
Lieutier (1981) reported a delay in the reproduction of Ips sexdentatus after experimental infections with the nematodes Contortylenchus diplogaster, Parasitaphelenchus sexdentati and Parasitaphelenchus sp. in laboratory and field studies. Fat body and ovaries of bark beetles infected with these nematodes were less developed than in healthy beetles and reproduction and egg laying were delayed (Lieutier 1982, 1984).
We recorded Parasitorhabditis sp. in two bark beetle species – P. chalcographus and T. piniperda. Many of the representatives of the genus Parasitorhabditis Fuchs, 1937 are associated with bark beetles and only a few species can cause harm to their hosts. Larvae of Parasitorhabditis reduced the size of the midgut cells of Ips confusus (LeConte, 1876) (Nickle 1963). Parasitorhabditis piniperdae (Fuchs, 1937) may enter the hemocoel of Tomicus piniperda and T. destruens (Wollaston, 1865) (Rhum 1956; Laumond and Carle 1971). Laumond and Carle (1971) reported that Parasitaphelenchus piniperdae and P. papillatus Fuchs, 1937 caused a reduction of fat body size and abnormal development of the gonads in adults of T. destruens.
Other studies confirm the high diversity of nematodes in bark beetles: Tomalak et al. (1989) investigated 31 species of bark beetles collected from nine host plants genera in Canada. Twenty one bark beetle species were infected with 56 nematode species. Among them 30 species were allantonematids, 14 rhabditid and 12 aphelenchoid species. Authors described three new species of Sulphuretylenchus: S. pseudoundulatus Tomalak, Welch & Galloway, 1989 parasitizing Polygraphus rufipennis (Kirby, 1837), S. nopimingi Tomalak, Welch & Galloway, 1989 in Pityokteines sparsus (LeConte, 1868) and S. postuteri Tomalak, Welch & Galloway, 1989 in Ips perroti Swaine, 1915.
According Ashraf and Berryman (1970) and Massey (1964) nematodes of genera Sulphuretylenchus and Neoparasitylenchus could cause sterility and decreased longevity of their hosts. Michalková et al. (2012) showed that Neoparasitylenchus sp. caused massive infections in I. typographus and was found in co-infections with protozoa Gregarina typographi Fuchs 1915.
In our study we found Bovienema sp. in Pityogenes conjunctus. Nematodes of genus Bovienema Nickle, 1963 were found for the first time in this host species. Bovien (1937) described Bovienema tomicis (Bovien, 1937) from the bark beetle Pityogenes bidentatus (Herbst, 1784) in Denmark. Nickle (1963) showed that 4% of Pityogenes fossifrons (LeConte, 1876) individuals collected from lodgepole pine in 1961 were found to harbor body cavity parasites similar to B. tomicis.
The genus Panagrolaimus was described by Fuchs (1930) who discovered the species Panagrolaimus tigrodon Fuchs 1930 in T. destruens. Rühm (1956) investigated T. piniperda and nematodes occurrence in its galleries including also P. tigrodon. Laumond and Carle (1971) found the same nematode species in T. destruens from France. Korentchenko (1992) investigated two bark beetles species, Orthotomicus laricis and O. suturalis (Gyllenhal, 1827), and described Panagrolaimus orthotomici Korentchenko, 1992.
Later Grucmanová et al. (2014) described in Ips duplicatus (Sahlberg, 1836) the nematode species Contortylenchus diplogaster, Parasitylenchus cf. aculeatus (Slankis, 1972), Parasitorhabditis obtusa (Fuchs, 1915), Cryptaphelenchus cf. macrogaster (Fuchs, 1937), Micoletzkya buetschlii (Fuchs, 1915) and Parasitaphelenchus sp., with a prevalence ranging from 1.5 to 74.3%. In Ips cembrae (Heer, 1836), Grucmanová et al. (2016) found C. diplogaster, P. dispar, C. cf. macrogaster, P. obtusa, M. cf. buetschlii, Bursaphelenchus sp. and Laimaphelenchus sp. with a prevalence of 41.7 to 68.2%.
Holuša et al. (2017) studied the occurrence of insect pathogens and nematodes in Orthotomicus laricis, O. erosus and O. nobilis (Wollaston, 1862) in Central-South Europe and found nonspecific gut nematodes and nematodes in the haemolymph, identified as Contortylenchus laricis (Fuchs, 1929). Nematode prevalence in the haemolymph ranged from 3 to 25% in the three Orthotomicus species. Authors concluded that the incidence of pathogens and nematodes in the host species seemed unrelated to the beetle species, the deposition of eggs, the ability to outbreak, or the beetle distribution range.
Conclusion
In this study, we document the diversity of pathogens and nematodes in forest-related beetles in Bulgaria. This is the first report of microsporidiosis caused by Nosema species in the bark beetle P. chalcographus and of mycosis caused by the fungus B. bassiana in the oak-leaf roller A. nitens. The nematode genera Sulphuretylenchus and Neoparasitylenchus are also recorded for the first time in O. laricis and O. erosus, respectively. Dryocoetes autographus, I. sexdentatus, I. acuminatus, O. laricis, O. erosus, P. quadridens, H. ligniperda, P. conjunctus and T. villifrons are confirmed as vectors of Bursaphelenchus sp. in Bulgaria. The influence of the pathogens and nematodes and their effect on the hosts remain to be investigated, however other studies indicate a potential in the biological control of bark beetles. Therefore further laboratory investigations and field experiments about influence and effects of this pathogens and parasites are needed.
References
Ashraf M, Berryman AA (1970) Biology of Sulphuretylenchus elongatus (Nematoda: Sphaerulariidae) and its effect on its host, Scolytus ventralis (Coleoptera Scolytidae). Can Entomol 102:197–213
Becnel JJ (2012) Complementary techniques: preparations of entomopathogens and diseased specimens for more detailed study using microscopy. In: Lacey LA (ed) Manual of techniques in invertebrate pathology. Elsevier, San Diego, pp 451–470. https://doi.org/10.1016/B978-0-12-386899-2.00015-4
Bovien P (1937) Some types of association between nematodes and insects. Vid Meddel Dansk Naturh Forening Kobenhavn 101:1–114
Braasch H (2001) Bursaphelenchus species in conifers in Europe: distribution and morphological relationship between species. EPPO Bulletin 31:127–142
Burjanadze M, Goginashvili N (2009) Occurrence of pathogens and nematodes in the spruce bark beetles, Ips typographus (Col., Scolytidae) in Borjomi Gorge. Bull Georg Natl Acad Sci 3(1):145–150
Draganova S, Takov D, Doychev D (2007) Bioassays with isolates of Beauveria bassiana (Bals.) Vuill. And Paecilomyces farinosus (Holm.) Brown & Smith against Ips sexdentatus Boerner and Ips acuminatus Gyll. (Coleoptera: Scolytidae). Plant Sci 44(1):24–28
Draganova S, Takov D, Doychev D (2010) Naturally occurred entomopathogenic fungi on three bark beetle species (Coleoptera: Curculionidae) in Bulgaria. Pesticides & Phytomedicine 25(1):59–63
Filipjev IN, Schuurmans Stekhoven JN (1941) A manual of agricultural Helmintology. E. J. Brill, Leiden
Fuchs AG (1915) Die Naturgeschichte der Nematoden und einiger anderer Parasiten. 1. Des Ips typographus L. 2. Des Hylobius abietis L. Zoologishe Jahrbucher, Abteilung für Systematik, Oekologie und Geographie der Tiere 38:109–222
Fuchs AG (1930) Neue an Borken und Rüsselkafer gebundene Nematoden, halbparasitische und Wohnungseinmieter. Freilebende Nematoden aus Moos und Walderde in Borken und Rüsselkäfergängen. Zool Jahrb, Jena. Abt Syst 59:505–646
Goettel MS, Poprawski TJ, Vandenberg JD, Li Z, Roberts DW (1990) Safety to nontarget invertebrates of fungal biocontrol agents. In: Laird M, Lacey LA, Davidson EW (eds) Safety of microbial insecticides. CRC Press, Boca Raton, pp 209–231
Grucmanová Š, Holuša J (2013) Nematodes associated with bark beetles, with focus on the genus Ips (Coleoptera: Scolytinae) in Central Europe. Acta zool bulg 65(4):547–556
Grucmanová Š, Holuša J, Nermuť J (2014) Nematodes associated with the double-spined bark beetle Ips duplicatus (Coleoptera: Curculionidae) in Central Europe. J Appl Entomol 138(10):723–732. https://doi.org/10.1111/jen.12142
Grucmanová Š, Holuša J, Čermák V, Nermuť J (2016) Nematodes associated with Ips cembrae (Coleoptera: Curculionidae): comparison of generations, sexes and sampling methods. J Appl Entomol 140(5):395–403. https://doi.org/10.1111/jen.12269
Händel U (2001) Untersuchungen zum Gegenspielerkomplex assoziiert lebender Fichtenborkenkäfer (Col., Scolytidae) aus naturnahen und sekundären Fichtenbeständen unter besonderer Berücksichtigung der Pathogene. Dissertation, BOKU-University, Vienna
Händel U, Wegensteiner R (2003) Zum Auftreten von Borkenkäfern (Col., Scolytidae) an Fichten-Fangbäumen (Picea abies (L.) Karst.) aus sekundären und naturnahen Beständen in Österreich. Centralblatt für das gesamte Forstwesen 120(2):117–136
Holuša J, Lukášová K, Žižka Z, Händel U, Haidler B, Wegensteiner R (2016) Occurrence of Microsporidium sp. and other pathogens in Ips amitinus (Coleoptera: Curculionidae). Acta Parasitol 61(3):621–628. https://doi.org/10.1515/ap-2016-0083
Holuša J, Lukášová K, Hubáčková J, Knížek M, Wegensteiner R (2017) Pathogens and nematodes associated to three bark beetle species of the genus Orthotomicus (Coleoptera Curculionidae) in central-South Europe. Bulletin of Insectology 70(2):291–297
Humber R (1997) Fungi: identification. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, San Diego, pp 153–163. https://doi.org/10.1016/B978-0-12-432555-5.X5000-3
Korentchenko EA (1992) Panagrolaimus orthotomici sp. n. (Cephalobina), a nematode of bark beetles of the genus Panagrolaimus in North-Eastern Asia. Parazitologiya 26:530–534 [In Russian, English summary]
Kulinich OA, Orlinskii PD (1998) Distribution of conifer beetles (Scolytidae, Curculionidae, Cerambycidae) and wood nematodes (Bursaphelenchus spp.) in European and Asian Russia. OEPP/EPPO Bulletin 28:39–52
Landa Z, Hornak P, Bursova E (2001) Entomopatogenni houby asociované s lýkožroutem smrkovým Ips typographus L. (Coleoptera, Scolytidae) v oblasti NP a CHKO Šumava. Aktuality Šumavského Výzkumu 2-4:124–128
Laumond C, Carle P (1971) Nématodes associés et parasites de Blastophagus destruens Woll. [Col. Scolytidae]. Entomophaga 16(1):51–66
Lieutier F (1981) Influence des nématodes parasites sur l’essaimage du scolytide Ips sexdentatus (Boern.) Action régulatrice du froid. Acta Oecol Oecolo Appl 2(4):357–368
Lieutier F (1982) Les variations pondérales du tissue adipeux et des avaires, et les variations de longueur des ovocytes, chez Ips sexdentatus Boern (Coleoptera: Scolytidae); relations avec le parasitisme par les nématodes. Ann Parasitol Hum Comp 57(4):407–418
Lieutier F (1984) Observations histologiques sur les capsules induites par Contortylenchus diplogaster v. Lins. (Nematoda: Allantonematidae) dans le tissu adipeux d’Ips sexdentatus Boern. (Coleoptera: Scolytidae). Ann Parasitol Hum Comp 59(3):245–251
Linit MJ (1988) Nematode-vector relationships in the pine wilt disease system. J Nematol 20(2):227–235
Linstow OFB (1890) Über Allantonema und Diplogaster. Centralbl Bakteriol 8(16):489–493
Lipa JJ (1968) Stempellia scolyti Weiser comb. nov. and Nosema scolyti sp. n. microsporidian parasites of four species of Scolytus (Coleoptera). Acta Protozool 6:69–84
Markova G (1992) Beauveria bassiana (Bals.) Vuillemin as a pathogen of ash weevil, Stereonychus fraxini Deg. (Col., Curculionidae), in Bulgaria. J Appl Entomol 114:275–279
Massey CL (1964) The nematode parasites and associates of the fir engraver beetle, Scolytus ventralis LeConte, in New Mexico. J Insect Physiol 6(2):133–155
Michalková V, Krascsenitsová E, Kozánek M (2012) On the pathogens of the spruce bark beetle Ips typographus (Coleoptera: Scolytinae) in the Western Carpathians. Biologia 67(1):217–221. https://doi.org/10.2478/s11756-011-0154-7
Nedelchev S, Takov D, Pilarska D (2008) Parasitic and associated nematodes of bark beetles in Bulgaria. Acta zool bulg, Suppl 2:83–91
Nedelchev S, Takov D, Pilarska D (2011) Prothallonema tomici n. sp. (Tylenchida: Sphaerulariidae) parasitizing Tomicus piniperda (Coleoptera: Curculionidae: Scolytinae) in Bulgaria. Nematology 13(6):741–746
Nickle WR (1963) Observation on the effect of nematodes on Ips confusus (LeConte) and other bark beetles. J Insect Pathol 5:386–389
Nickle WR (1971) Behavior of the shothole borer, Scolytus rugulosus altered by the nematode parasite Neoparasitylenchus rugulosi. Ann Entomol Soc Am 64(3):751
Poinar GO Jr, Caylor JN (1974) Neoparasitylenchus amvlocercus sp. n. (Tylenchida: Nematodea) from Conophthorus monophyllae (Scolytidae: Coleoptera) in California with a synopsis of the nematode genera found in bark beetles. J Invertebr Pathol 24(1):112–119
Polovinko GP, Yaroslavtseva ON, Teshebaeva ZA, Kryukov VY (2010) Dominating species of Entomophilous ascomycetes anamorphs in West Siberia, Primorsky Krai, and Kyrgyzstan. Contemp Probl Ecol 3(5):515–521
Purrini K (1978) Protozoen als Krankheitserreger bei einigen Borkenkäferarten (Col., Scolytidae) im Königsee-Gebeit, Oberbayern. Anz Schädlingskunde, Pflanzenschutz, Umweltschutz 51:171–175
Purrini K (1981) Nosema hylobii n. sp. (Nosematidae, Microsporida), a new microsporidan parasite of Hylobius abietis L. (Curculionidae, Coleoptera). Z Angew Entomol 92:1–8
Purrini K, Halperin J (1982) Nosema calcarati n. sp. (Microsporidia), a new parasite of Pityogenes calcaratus Eichhof (Col., Scolytidae). Z Angew Entomol 94:87–92
Purrini K, Ormieres R (1981) On three new sporozoan parasites of bark beetle (Col., Scolytidae). Z Angew Entomol 91:67–74
Richardson KC, Jarrett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Rühm W (1956) Die Nematoden der Ipiden. Parasitologische Schriftenreihe 6:1–435
Samson RA, Evans HC, Latgé J-P (1988) Atlas of entomopathogenic fungi. Springer, Berlin - Heidelberg
Schvester D (1950) Sur un nématode du groupe des Parasitylenchus dispar Fuchs, parasite nouveau du Xylebore disparate (Xyleborus dispar F.). Annales de l'Institut national de la recherche agronomique. Serie C. Annales des épiphyties, Paris 1:48–53
Schvester D (1957) Contribution a l’étude de coléoptères scolytides. Annales de l'Institut national de la recherche agronomique. Serie C. Annales des épiphyties, Paris 8:1–162
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydreous glycerin. Nematologica 4:67–69
Takov D, Pilarska D, Wegensteiner R (2006) Оccurrence of pathogens in Ips typographus (Coleoptera, Scolytidae) from several Picea abies (L.) (Karst.) stands in Bulgaria. Acta zool bulg 58(3):409–420
Takov D, Doychev D, Wegensteiner R, Pilarska D (2007) Study on the pathogens of bark beetles (Coleoptera, Scolytidae) from different coniferous stands in Bulgaria. Acta zool bulg 59(1):87–96
Takov D, Pilarska D, Wegensteiner R (2010) List of protozoan and microsporidian pathogens of economically important bark beetle species (Coleoptera, Curculionidae: Scolytinae) in Europe. Acta zool bulg 62(2):201–209
Takov D, Doychev D, Linde A, Draganova S, Pilarska D (2011) Pathogens of bark beetles (Coleoptera: Curculionidae) in Bulgarian forests. Phytoparasitica 39(4):343–352. https://doi.org/10.1007/s12600-011-0167-3
Takov D, Doychev D, Linde A, Draganova S, Pilarska D (2012) Pathogens of bark beetles (Curculionidae: Scolytinae) and other beetles in Bulgaria. Biologia 67(5):966–972. https://doi.org/10.2478/s11756-012-0086-x
Tomalak M, Welch HE, Gallowayt D (1989) Nematode parasites of bark beetles (Scolytidae) in southern Manitoba, with descriptions of three new species of Sulphuretylenchus Rühm (Nematoda: Allantonematidae). Can J Zool 67:2497–2505
Urbanová Z, Urban J (2016) Attelabus nitens (Scop.) – an abundant but biologically little known species from the family of Attelabidae (Coleoptera). Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 64(5):1675–1696. https://doi.org/10.11118/actaun201664051675
Wegensteiner R (1994) Chytridiopsis typographi (protozoa, microsporidia) and other pathogens in Ips typographus (Coleoptera, Scolytidae). IOBC/WPRS Bulletin 17(3):39–42
Wegensteiner R (1996) Laboratory evaluation of Beauveria bassiana (Bals.) Vuill. Against the bark beetle, Ips typographus L. (Coleoptera, Scolytidae). IOBC/WPRS Bulletin 19(9):186–189
Wegensteiner R (2004) Pathogens in bark beetles. In: Lieutier F, Day K, Battisti A, Gregoire J-C, Evans H (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Printed in the Netherlands, pp 291–313
Weiser J (1955) Prispevek k znalosti cizopasniku kurovce Ips typographus, II. (Contributions to the knowledge of Ips typographus parasites, II.). Vestn Ceskoslov Zool Spolec 19:374–380. [in Czech]
Weiser J (1961) A new microsporidian from the bark beetle Pityokteines curvidens Germar (Col. Scolytidae) in Czechoslovakia. J Insect Pathol 3:324–329
Weiser J (1968) Plistophora scolyti sp. n. (protozoa, microsporidia) a new parasite of Scolytus scolytus F. (Col., Scolytidae). Folia Parasitol 15:11–14
Weiser J (1970) Three new pathogens of the Douglas fir beetle, Dendroctonus pseudotsugae: Nosema dendroctoni n. sp., Ophryocystis dendroctoni n. sp., and Chytridiopsis typographi n. comb. J Invertebr Pathol 16(3):436–441
Weiser J, Wegensteiner R, Žižka Z (1997) Ultrastructures of Nosema typographi Weiser 1955 (Microspora: Nosematidae) of the bark beetle Ips typographus L. (Coleoptera; Scolytidae). J Invertebr Pathol 70:156–160. https://doi.org/10.1006/jipa.1997.4677
Yaman M, Radek R, Tosun O, Ünal S (2009) Nosema raphidiae sp. n. (Microsporida, Nosematidae): a microsporidian pathogen of the predatory Snake-fly Raphidia ophiopsis (Raphidioptera: Raphidiidae). Acta Protozool 48:353–358
Acknowledgments
We are especially indebted to the National Science Fund of Bulgaria project DO-02-251/2008 and personally to Prof. Renate Radek for her help for TEM of microsporidia. The present study was implemented also with the support of the National Programme of the Bulgarian Ministry of Education and Science “Young Scientists and Postdoctoral Students”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takov, D., Doychev, D., Pilarska, D. et al. Occurrence of pathogens and nematodes in forest beetles from Curculionidae and Attelabidae in Bulgaria. Biologia 74, 1339–1347 (2019). https://doi.org/10.2478/s11756-019-00250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00250-x