Abstract
The Gd60Co30Fe10 alloy ribbons with different solidification cooling rates were prepared by modifying the melt-spinning speed of 6.0, 12.5, 25.0 and 50.0 m·s−1. With cooling rate decreasing, the (Fe,Co)5Gd and hcp-Gd nanocrystalline was in situ precipitated among the amorphous matrix, which resulted in the composition change of the amorphous phase. Because of the only slight amount of crystalline phase in Gd60Co30Fe10 alloys, the magnetic and magnetocaloric properties mainly depend on the amorphous phase, and all the magnetic entropy change versus temperature (|ΔSM|–T) curves are table-like, indicating the suitability for Ericsson cycle. The magnetic transition temperature of the Gd60Co30Fe10 alloy at a melt-spinning speed of 6.0 m·s−1 shifted obviously to the lower value with the applied magnetic fields increasing. The peak value of magnetic entropy change (|ΔSMpk|) is 2.19 J·kg−1·K−1 at 217 K under the magnetic field change of 0–2 T, and the table-like region is 200–230 K. It was proved that the moderate reduction of the cooling rate will not deteriorate the magnetocaloric performance of the Gd60Co30Fe10 ribbons seriously.
摘要
使用熔体旋淬法, 通过调节辊轮转速为6.0, 12.5, 25.0 和 50.0 m·s−1, 制备了不同冷却速度的Gd60Co30Fe10合金条带。随着冷却速度的降低, (Fe,Co)5Gd和hcp-Gd纳米晶从非晶基体中原位析出, 使非晶相的成分发生变化。由于析出的晶相含量较低, 因此整个样品的磁性能和磁热性能主要取决于非晶相, 磁熵变温变 (|ΔSM|-T) 曲线均呈平台型, 表明其适用于Ericsson循环。辊轮转速为6.0 m·s−1时制备的Gd60Co30Fe10合金的磁转变温度随着外加磁场的增加明显向低温偏移, 在磁场变化为0-2 T的条件下, 217 K时磁熵变峰值 (|ΔSMpk|) 为2.19 J·kg−1·K−1, 平台区为200-230 K。本研究证明适度的降低冷却速度, 不会使Gd60Co30Fe10合金条带的磁热性能表现严重恶化。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic refrigeration is a technology utilizing the magnetic material as a refrigerant based on its intrinsic magnetocaloric effect (MCE), i.e., the alignment of magnetic moments as well as the magnetic entropy increased (or decreased) when the magnetic field is applied (or removed), and the material releases (or absorbs) heat during this process [1,2,3]. The magnetic refrigeration is a prospective replacement for the traditional gas compression refrigeration for its advantages of high efficiency, small occupied area and environmental friendliness [1,2,3,4,5,6]. According to the type of magnetic transition, magnetic refrigeration materials can be roughly divided into the first-order magnetic phase transition (FOMT) and the second-order magnetic phase transition (SOMT) materials [7]. The FOMT materials, e.g., LaFeSi, Mn(Fe,Co)PAs, Ni–Mn–Ga and GdSiGe alloys [5, 8,9,10], have a large MCE due to the simultaneous magnetic-structural transition, which is beneficial for the magnetic refrigerant. However, they usually have a narrow working temperature range. Besides, the accompanied large thermal and magnetic hysteresis as well as the weakened mechanical properties after frequent magnetic cycling impede their practical application [11]. Though the magnetic entropy change value (|ΔSM|) of SOMT materials is much lower than that of FOMT materials, their negligible thermal and magnetic hysteresis indicate better suitability for magnetic refrigeration [5, 12]. The Gd metal is a representative SOMT material and known as the prototype material [13], with the Curie temperature (TC) of 293 K and the maximum magnetic entropy change (|ΔSMpk|) of 5.5 J·kg−1·K−1 (ΔH = 2 T) [14]. Nevertheless, its high cost and poor chemical stability restrict the commercial utilization.
Among SOMT materials, amorphous alloys have the advantages of adjustable Curie temperature (TC), low magnetic hysteresis, low thermal hysteresis, low eddy current loss, high corrosion resistance, high strength, high thermal stability and easy processing and forming due to their unique long-range disordered and short-range ordered atomic structure, attracting more attention as a magnetic refrigerant candidate [6, 15,16,17,18]. Moreover, the amorphous alloy has wide magnetic transition temperature range and large relative cooling power, which is more suitable for the Ericsson thermodynamic cycle working at near room temperature [19]. To achieve ideal Ericsson cycle, the |ΔSM| has to be constant during the hot and cold reservoirs, which means that the table-like |ΔSM|–T curve needs to be configurated. Consequently, the composites consisting of two or more amorphous alloys with similar TC were proposed due to the usually asymmetrical λ-like shape of |ΔSM|–T curve of single-phase amorphous alloy. The different preparation processes, such as bonding, sintering and in situ precipitating methods, have been reported [6, 20,21,22]. For the bonding and sintering method, the heat transfer and the solid phase reaction between the different materials reduce the refrigeration efficiency [23], while it can be avoided through the in situ precipitation method. Mo et al. [24] reported the plateau of |ΔSM| values (~ 2.79 J·kg−1·K−1) in a wide temperature range from 137 to 180 K under the magnetic field change of 0–2 T in the Gd55Co35Mn10 amorphous-nanocrystalline composite manufactured by annealing the fully amorphous precursor at 600 K for 30 min. Wang et al. [25] reported the table-like |ΔSM|–T curve over a wide temperature range as well as a decreased peak value of magnetic entropy change in the Gd48Co52 amorphous-nanocrystalline composite obtained by controlling the cooling rate with copper wheel surface speed of 30 m·s−1.
In this study, in order to investigate the influence of microstructure on magnetocaloric properties of the amorphous-nanocrystalline composite produced by in situ precipitation method, the Gd60Co30Fe10 alloy ribbons with different cooling rates were produced by adjusting the melt-spinning speed. Their phase composition, microstructure and magnetocaloric properties were studied.
2 Experimental
The alloy ingots with nominal composition of Gd60Co30Fe10 were prepared by arc-melting the mixtures of pure Gd (99.9 wt%), Co (99.9 wt%) and Fe (99.8 wt%) under high purity argon atmosphere. The alloy ingots were remelted four times to make sure the homogeneity. As-spun ribbons with 2 mm in width and 20–30 μm in thickness were produced in a high-purified argon atmosphere by melt-spinning method using a single copper wheel with the surface linear rolling speeds of 6.0, 12.5, 25.0 and 50.0 m·s−1.
The phase structure of as-spun ribbons was identified by an X-ray diffractometer (XRD, Bruker D8 Advance) using Cu Kα radiation (λ = 0.15418 nm) at operation conditions of 40 keV and 120 mA in 2θ range of 20°–90°. The thermal properties of ribbons were characterized by a differential scanning calorimetry (DSC, NETZSCH STA 449F3) with a heating rate of 0.33 K·s−1 in the flow of argon. The microstructure analysis was performed using a high-resolution transmission electron microscope (HRTEM, JEOL JEM-2100) at an accelerating voltage of 200 kV, and the HRTEM specimens were prepared by the twin-jet electrochemical polishing with a solution of 4 vol% HClO4 and 96 vol% ethanol at about 250 K. The magnetic properties of as-spun ribbons were detected by the physical property measurement system (PPMS, Quantum Design PPMS-9T system). Concretely, the zero-field cooling (ZFC) temperature dependence of magnetization (M–T) curves was collected during heating process with the temperature range of 150–300 K under the applied magnetic field of 0.001, 0.005 and 0.020 T, while the isothermal initial magnetization (M-H) curves at different selected temperatures around the Curie temperature were measured under field change from 0 to 2 T.
3 Results and discussion
3.1 Characterization of microstructure
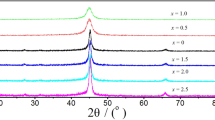
Figure 1a illustrates XRD patterns of Gd60Co30Fe10 alloy ribbons at different surface linear velocities of the copper roller. When the spinning speeds are 50.0 and 25.0 m·s−1, the typical amorphous broad diffuse scattering peak without any obvious diffraction peaks of crystalline phases can be obtained, which indicates the amorphous structure of the as-spun ribbons. However, sharp crystal phase diffraction peaks were detected in the samples with a rolling speed of 12.5 m·s−1, and the identified crystal phase of the peaks is Fe5Gd [PDF No. 00-025-1095]. The average size of the crystallite was calculated to be 23 nm using the Scherrer formula [26]:
where D, K, λ, β and θ are the crystallite size, the Scherrer constant (generally 0.89), the wavelength of the radiation (0.15418 nm), the corrected peak width at half maximum of the intensity and the Bragg angle corresponding to the peak, respectively. In the ribbons spun with the surface linear rotating speed of 6.0 m·s−1, the amorphous phase was accompanied by the hcp-Gd and Fe5Gd type crystalline phases, and the grain size is roughly estimated to be ~ 25 nm. From the above phenomena, it can be observed that the amount of precipitated crystal phase and the crystallite size increases with cooling rate decreasing. In addition, (Co, Fe)5Gd crystals in the samples with spinning speeds of 12.5 and 6.0 m·s−1 present preferential orientation growth along the [101] direction during the rapid solidification. Furthermore, the relative strength of the diffraction peaks of hcp-Gd phase is higher than that of the Fe5Gd phase in the ribbons prepared at 6.0 m·s−1, indicating that the precipitation of hcp-Gd crystalline inhibited the preferential orientation growth of (Co, Fe)5Gd crystalline to some extent.
As shown in Fig. 1b, obvious exothermic peaks can be found in DSC curves of Gd60Co30Fe10 alloy ribbons prepared at different cooling rates, which corresponds to the crystallization process of the amorphous phase in each sample, further demonstrating that the samples with spinning speeds of 12.5 and 6.0 m·s−1 were amorphous-nanocrystalline composites. The glass transition temperature (Tg) and the onset crystallization temperature (Tx) of the samples were determined by intersection of the baseline and extrapolation of the peak. The Tx values of the Gd60Co30Fe10 alloys solidified by cooling rates of 50.0, 25.0, 12.5 and 6.0 m·s−1 are 569, 581, 574 and 569 K, respectively. However, the Tg can only be obtained in the ribbons with spinning speeds of 12.5 and 6.0 m·s−1, and the values are 545 and 540 K. For the amorphous alloys solidified produced at cooling rate of 50.0 and 25.0 m·s−1, there is no evident glass transition in the curves, which is attributed to their lower thermal stability [27]. That is to say, in the Gd60Co30Fe10 alloy quenched at slower speeds of 12.5 and 6.0 m·s−1, the precipitation of the crystalline phases resulted in the composition change of the amorphous matrix with higher glass-forming ability. The Tx discrepancy in the amorphous alloys synthesized at 50.0 and 25.0 m·s−1 is ascribed to the component fluctuation, since the amounts of short-range ordered clusters are influenced by the cooling rate [28]. What should be pointed out is that the Tx of each ribbon is high enough to ensure the stability of the structure during the subsequent magnetic measurement.
From TEM bright-field image of Gd60Co30Fe10 with a cooling rate of 50.0 m·s−1 displayed in Fig. 2, it can be seen the uniform contrast without any spots and streaks, indicating that there is no obvious precipitation of crystal phase in the sample. The inset of Fig. 2b exhibits the fast Fourier transform (FFT) pattern of the selected area, and only a broad diffuse diffraction halo corresponding to the amorphous structure can be observed, which is consistent with the XRD result in Fig. 1 and demonstrates the fully amorphous microstructure in the alloy.
For comparison, HRTEM bright-field image and FFT patterns of the selected areas in Gd60Co30Fe10 with cooling rate of 6.0 m·s−1 are presented in Fig. 3. It is evident that there are crystallites (corresponding to the streak region and FFT patterns with a set of spots) embedded in the amorphous matrix with FFT patterns of a halo in Fig. 3d. The interplanar spacing (d) of the different grains shown in Fig. 3b, c was calculated to be 0.315 and 0.298 nm, respectively, which is coherent with the (100) plane of hcp-Gd (0.3144 nm) and the (101) plane of Fe5Gd (0.2977 nm) structure. Typically, both the morphology and FFT pattern of (Fe,Co)5Gd grains revealed their preferential orientation growth. Besides, it can be seen from Fig. 3a that the crystal grain size is about 5–20 nm. All these results confirm the analysis of XRD pattern and the amorphous-nanocrystalline composite structure of the sample.
3.2 Magnetic and magnetocaloric properties
In order to investigate the influence of partially crystallization on the magnetic and magnetocaloric properties of the Gd60Co30Fe10 alloys, the ribbons prepared at spinning speeds of 50.0, 12.5 and 6.0 m·s−1 with different microstructures were chosen as the representative samples. Figure 4a exhibits the temperature dependence of magnetization (M-T) curves under different magnetic fields of each ribbon. With temperature raising, the magnetization decreases gradually and single distinct transition from ferrimagnetism to paramagnetism occurs in every curve. This magnetic transition correlates with the amorphous matrix phase in the samples, demonstrating that the amount of crystalline phase in the composite samples is slight and their magnetic contribution is neglectable. In this work, the Curie temperature (TC) of the material is determined according to the inflection point method, namely picking the temperature corresponding to the minimum of the dM/dT–T curves, which are plotted in Fig. 4b. Under the applied field of 0.001 T, the TC values of three samples fabricated at cooling rates of 50.0 m·s−1 (243 K), 12.5 m·s−1 (244 K) and 6.0 m·s−1 (245 K) are almost the same. For the ribbon with a cooling rate of 50.0 m·s−1, the TC values detected under different applied magnetic fields are also similar. However, the TC values of the ribbons with cooling rates of 12.5 and 6.0 m·s−1 decrease gradually when the field increases. In addition, the degree of variability is larger for the sample with slower cooling rate. This phenomenon can be ascribed to that the composition change of the amorphous matrix enhances the local composition fluctuation, which results from the precipitation of crystals [29].
The isothermal initial magnetization (M–H) curves of Gd60Co30Fe10 alloys with different spinning speeds under magnetic field from 0 to 2 T are displayed in Fig. 5. Each sample experienced the ferrimagnetic-paramagnetic transition during heating up, and the composite alloy reached magnetic saturation more difficultly than the fully amorphous counterpart because of the complicated microstructure although their magnetizations are similar. To determine the type of magnetic transition in a material, there are several methods supposed [30,31,32,33]. In this work, the nature of magnetic transition in the alloys was researched by the Arrott plot (M2 vs. H/M, converted from the M–H curve), as illustrated in Fig. 6. According to the Banerjee criterion [32, 33], the positive slopes of the curves correlate with the SOMT, and in contrary, the negative slope implies the FOMT. It can be seen that all the slopes of the Arrott plots in Fig. 6 are positive, verifying that the magnetic transition of the Gd60Co30Fe10 alloys is SOMT [34].
The magnetic entropy change (ΔSM) was utilized to characterize the MCE of Gd60Co30Fe10 alloys with different cooling rates, and it can be calculated by the data of M–H isotherms, according to Maxwell relation [33, 35],
where M, T and H are the magnetization, temperature and magnetic field, respectively. The correlation between |ΔSM| and temperature of the ribbons under the applied field of 0–2 T is depicted in Fig. 7, and all the curves are table-like in vicinity of TC (inset of Fig. 7), e.g., the |ΔSM| of the ribbon with 6.0 m·s−1 is almost constant in the temperature range of 200–230 K, which is more suitable for the Ericsson cycle in comparison to the typical λ-type curves of the materials with SOMT [18, 22]. As analyzed above, the magnetic properties are contributed by the amorphous matrix in the Gd60Co30Fe10 alloys with different spinning speeds, resulting in the similar shape of their |ΔSM|–T curve. The maximum values of magnetic entropy change (|ΔSMpk|) of the ribbons with cooling rates of 50.0, 12.5 and 6.0 m·s−1 are 2.08, 1.75 and 2.19 J·kg−1·K−1, respectively. The discrepancy of |ΔSMpk| could be ascribed to the different composition of the amorphous matrix as well as the localized composition fluctuation and heterogeneous microstructure (e.g., cluster and free volume) in the different samples [36, 37].
Table 1 lists the magnetocaloric properties of several representative amorphous and crystalline alloys whose TC is similar to the samples in this study [14, 16, 24, 38,39,40]. Usually, the broadened magnetocaloric effect relates to a higher relative cooling power (RCP, obtained by multiply the value of |ΔSMpk| by full width of half maximum (δTFWHM) in the |ΔSM|–T curve). Despite the |ΔSMpk| values of the Gd60Co30Fe10 alloys with different spinning speeds are smaller than that of the Gd60Co35Fe5 amorphous alloy, their RCP are comparable. Additionally, compared with the Fe89Co1Sc10 amorphous alloy, all the Gd60Co30Fe10 alloys exhibit higher |ΔSMpk| and RCP. Among the three Gd60Co30Fe10 samples, the one with cooling rate of 6.0 m·s−1 possesses the highest |ΔSMpk|. Furthermore, it shows higher RCP than Gd58Co42 amorphous alloy and the Gd55Co35Mn10 (600 K, 20 min) amorphous-nanocrystalline composite. Although the influence of quenching rate on the MCE of the Gd60Co30Fe10 ribbons changes irregularly, it has been demonstrated that the moderate decrease of spinning speed would not deteriorate the magnetocaloric performance of the samples seriously.
4 Conclusion
In summary, the Gd60Co30Fe10 alloy ribbons with different microstructures were fabricated by adjusting the cooling rate at various surface linear velocities of the copper wheel. Fully amorphous alloy can be obtained at spinning speeds of 25.0 and 50.0 m·s−1. However, the (Fe,Co)5Gd nanocrystals were precipitated and embedded in the amorphous matrix with the quenching rate of 12.5 m·s−1, and hcp-Gd nanocrystalline was separated out from the amorphous matrix if the velocity is furtherly reduced to 6.0 m·s−1. With applied magnetic field increasing, the magnetic transition temperatures of all the ribbons shifted to the lower value, and the degree of variability was larger for the sample with slower cooling rate. All the |ΔSM|–T curves of the alloys are table-like in vicinity of TC, which is more suitable for the Ericsson cycle. The |ΔSMpk| of Gd60Co30Fe10 with cooling rates of 6 m·s−1 under the magnetic field change of 0–2 T is 2.19 J·kg−1·K−1 at 217 K and the |ΔSM| is approximately constant in the temperature range of 200–230 K. The discrepancy among the magnetic performance of different ribbons could be attributed to the composition change of the amorphous matrix and local composition fluctuation in the composites due to the slight amount of crystalline phase. In addition, it was revealed that the moderate reduction of quenching speed will not deteriorate the magnetocaloric performance of the Gd60Co30Fe10 ribbons seriously.
References
Tegus O, Brück E, Buschow KHJ, de Boer FR. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature. 2002;415(6868):150.
Pecharsky VK, Gschneidner KA Jr. Giant magnetocaloric effect in Gd5(Si2Ge2). Phys Rev Lett. 1997;78:4494.
de Oliveira NA, von Ranke PJ. Theoretical aspects of the magnetocaloric effect. Phys Rep. 2010;489(4–5):89.
Xia L, Wu C, Chen SH, Chan KC. Magneto-caloric effect of a Gd50Co50 amorphous alloy near the freezing point of water. AIP Adv. 2015;24(9):4494.
Franco V, Blazquez JS, Ingale B, Conde A. The magnetocaloric effect and magnetic refrigeration near room temperature: materials and models. Annu Rev Mater Res. 2012;42:305.
Ma LY, Gan LH, Chan KC, Ding D, Xia L. Achieving a table-like magnetic entropy change across the ice point of water with tailorable temperature range in Gd-Co-based amorphous hybrids. J Alloys Compd. 2017;723:197.
Ram NR, Prakash M, Naresh U, Kumar NS, Sarmash TS, Subbarao T, Kumar RJ, Kumar GR, Naidu KCB. Review on magnetocaloric effect and materials. J Supercond Nov Magn. 2018;31(7):1971.
Miao XF, Hu SY, Xu F, Brück E. Overview of magnetoelastic coupling in (Mn, Fe)2(P, Si)-type magnetocaloric materials. Rare Met. 2018;37(9):723.
Zheng D, Jing C, Lu B, Li Z, Xu K. Martensitic transformation, magnetocaloric effect and phase transition strain in Ni50Mn36–xGexSn14 Heusler alloys. Rare Met. 2016. https://doi.org/10.1007/s12598-016-0820-6.
Wang L, Ye RC, Liu XX, Li JL, Liu PJ, Long Y. Off-STOICHIOMETRIC La1+x(Fe, Si)13 magnetic refrigeration materials prepared by powder metallurgy. Chin J Rare Met. 2019;43(7):774.
Brück E. Developments in magnetocaloric refrigeration. J Phys D: Appl Phys. 2005;38(23):R381.
Wang LC, Shen BG. Magnetic properties and magnetocaloric effects of PrSi. Rare Met. 2014;33(3):239.
Brown GV. Magnetic heat pumping near room temperature. J Appl Phys. 1976;47(8):3673.
Dan’kov SY, Tishin AM, Pecharsky VK, Gschneidner KA Jr. Magnetic phase transitions and the magnetothermal properties of gadolinium. Phys Rev B. 1998;57(6):3478.
Thanveer T, Ramanujan RV, Thomas S. Magnetocaloric effect in amorphous and partially crystallized Fe40Ni38Mo4B18 alloys. AIP Adv. 2016;6(5):055322.
Wu C, Ding D, Xia L, Chan KC. Achieving tailorable magneto-caloric effect in the Gd–Co binary amorphous alloys. AIP Adv. 2016;6(3):035302.
Zhang HH, Cui ZD, Zhu SL, Guo SW, Yang XJ, Inoue A. Microstructure and mechanical properties of TC4 joints brazed with Ti–Zr–Cu–Sn amorphous filler alloy. Rare Met. 2020. https://doi.org/10.1007/s12598-020-01424-2.
Li ZD, Zhang WW, Li GT, Li SS, Ding HS, Zhang T, Song YG. Magnetic field annealing of FeCo-based amorphous alloys to enhance thermal stability and Curie temperature. Rare Met. 2018. https://doi.org/10.1007/s12598-018-1069-z.
Balfour EA, Ma Z, Fu H, Hadimani RL, Jiles DC, Wang L, Luo Y, Wang SF. Table-like magnetocaloric effect in Gd56Ni15Al27Zr2 alloy and its field independence feature. J Appl Phys. 2015;118(12):123903.
Tian HC, Zhong XC, Liu ZW, Zheng ZG, Min JX. Achieving table-like magnetocaloric effect and large refrigerant capacity around room temperature in Fe78-xCexSi4Nb5B12Cu1 (x = 0–10) composite materials. Mater Lett. 2015;138:64.
Álvarez P, Sánchez Llamazares JL, Gorria P, Blanco JA. Enhanced refrigerant capacity and magnetic entropy flattening using a two amorphous FeZrB(Cu) composite. Appl Phys Lett. 2011;99(23):232501.
Zhong XC, Shen XY, Mo HY, Jiao DL, Liu ZW, Qiu WQ, Zhang H, Ramanujan RV. Table-like magnetocaloric effect and large refrigerant capacity in Gd65Mn25Si10–Gd composite materials for near room temperature refrigeration. Mater Today Commun. 2018;14:22.
Liu GL, Zhao DQ, Bai HY, Wang WH, Pan MX. Room temperature table-like magnetocaloric effect in amorphous Gd50Co45Fe5 ribbon. J Phys D Appl Phys. 2016;49(5):055004.
Mo HY, Zhong XC, Jiao DL, Liu ZW, Zhang H, Qiu WQ, Ramanujan RV. Table-like magnetocaloric effect and enhanced refrigerant capacity in crystalline Gd55Co35Mn10 alloy melt spun ribbons. Phys Lett A. 2018;382(25):1679.
Wang ZW, Yu P, Cui YT, Xia L. Near room temperature magneto-caloric effect of a Gd48Co52 amorphous alloy. J Alloys Compd. 2016;658:598.
Ghosh J, Mazumdar S, Das M, Ghatak S, Basu AK. Microstructural characterization of amorphous and nanocrystalline boron nitride prepared by high-energy ball milling. Mater Res Bull. 2008;43(4):1023.
Ichitsubo T, Matsubara E, Numakura H, Tanaka K. Glass-liquid transition in a less-stable metallic glass. Phys Rev B. 2005;72(5):052201.
Xiang S, Li Q, Zuo M, Cao D, Li H, Sun Y. Influence of the preparation cooling rate on crystallization kinetics of Fe74Mo6P13C7 amorphous alloys. J Non-Cryst Solids. 2017;475(1):116.
Schwarz B, Mattern N, Luo Q, Eckert J. Magnetic properties and magnetocaloric effect of rapidly quenched Gd–Co–Fe–Al alloys. J Magn Magn Mater. 2012;324(8):1581.
Yang SX, Zheng XQ, Yang WY, Xu JW, Liu J, Xi L, Zhang H, Wang LC, Xu ZY, Zhang JY, Wu YF, Ma XB, Chen DF, Yang JB, Wang SG, Shen BG. Tunable magnetic properties and magnetocaloric effect of TmGa by Ho substitution. Phys Rev B. 2020;102:174441.
Law JY, Franco V, Moreno-Ramírez LM, Conde A, Karpenkov DY, Radulov I, Skokov KP, Gutfleisch O. A quantitative criterion for determining the order of magnetic phase transitions using the magnetocaloric effect. Nat Commun. 2018;9:2680.
Romero Gómez J, Ferreiro Garcia R, De Miguel CA, Romero Gómez M. Magnetocaloric effect: a review of the thermodynamic cycles in magnetic refrigeration. Renew Sustain Energy Rev. 2013;17:74.
Belo JH, Amaral JS, Pereira AM, Amaral VS, Araújo JP. On the Curie temperature dependency of the magnetocaloric effect. Appl Phys Lett. 2012;101(7):242407.
McMichael RD, Ritter JJ, Shull RD. Enhanced magnetocaloric effect in Gd3Ga5−xFexO12. J Appl Phys. 1993;73(10):6946.
Hashimoto T, Numasawa T, Shino M, Okada T. Magnetic refrigeration in the temperature range from 10 K to room temperature: the ferromagnetic refrigerants. Cryogenics. 1981;21(11):647.
Masumoto T, Egami T. Designing the composition and heat treatment of magnetic amorphous alloys. Mater Sci Eng. 1981;148(2):147.
Miyazaki T, Hayashi K, Yamaguchi S. Magnetization, curie temperature and perpendicular magnetic anisotropy of evaporated Co-rare earth amorphous alloy films. J Magn Magn Mater. 1988;75(3):252.
Zhang HY, Ouyang JT, Ding D, Li HL, Wang JG, Li WH. Influence of Fe substitution on thermal stability and magnetocaloric effect of Gd60Co40−xFex amorphous alloy. J Alloys Compd. 2018;769:186.
Fang Y, Yu Z, Peng G, Feng T. Near room-temperature magnetocaloric effect in amorphous Fe–Sc alloys: the effect of minor Co additions. J Non-Cryst Solids. 2019;505:211.
Fujita A, Fukamichi K. Control of large magnetocaloric effects in metamagnetic La(FexSi1−x)13 compounds by hydrogenation. J Alloys Compd. 2005;404–406:554.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51701003), the Natural Science Foundation of Anhui Province (No. 1908085ME147) and the International Cooperation and Exchanges in Anhui Provincial Key Project of Research (No. 202004b11020010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, HY., Zhang, ZY., Xu, YF. et al. Microstructure and magnetocaloric properties of partially crystallized Gd60Co30Fe10 amorphous alloy prepared by different solidification cooling rates. Rare Met. 41, 246–253 (2022). https://doi.org/10.1007/s12598-021-01745-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01745-w