Abstract
Vanadium nitride was synthesized by one-step method using V2O5 and carbon black as raw materials in nitrogen atmosphere. The phases of different reaction products prepared in different reaction temperatures were analyzed by X-ray diffraction (XRD), and the dynamic behavior of the process of synthesizing vanadium nitride (VN) by one-step method was studied with non-isothermal thermogravimetry. The mechanism function and kinetic parameters of reaction process were calculated by thermal gravimetric analyses (TGA), and the reaction rate equation was established. The XRD results show that for the samples tested with minimal VN after holding for 4 h at 1273 K, the main phase of products is VN at 1476 K, while some vanadium nitrides transform into vanadium carbides again over 1573 K. It is found that N2 is beneficial to stimulate reduction and proceed carbonization reaction, and the reduction and nitridation reaction can occur simultaneously. The activation energy of preparing VN by one-step method is 104.005 kJ·mol−1, and the frequency factor is 470.52 at 1280–1358 K, and 150.052 kJ·mol−1 and 2.35 × 104 at 1358–1426 K, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Vanadium nitrogen alloy is a more effective alloy additive compared with ferrovanadium; while by maintaining the same level of steel strength, the steel adding vanadium nitrogen alloy saves more than 30 % vanadium compared with steel adding ferrovanadium, which reduces the production cost of steel and has obvious economic benefits [1–5]. Currently, the process principle of preparing vanadium nitride (VN) is basically similar at home and abroad, and VN products are synthesized after carbonization and nitriding at high temperature using V2O5, V2O3, or NH4VO3 with carbon or hydrogen as a reducing agent [6–9]. But studies about the reaction kinetics of synthesizing VN by one-step method were rarely reported [10]. In this paper, V2O5 was used as raw material and carbon black was used as the reducing agent. The chemical reaction dynamic behavior, reduction, and nitriding mechanisms of preparing VN were analyzed by thermogravimetric (TG) experiments in order to provide theoretical references for VN production test technology.
2 Experimental
Carbon black and V2O5 powder were put into an oven to dry and then sieved to less than 0.125 mm, respectively, to reserve. The chemical compositions of V2O5 powder and carbon black are shown in Table 1. The addition of carbon black was calculated by C/O molar ratio, which was 1.05. V2O5 powder and carbon black after weighing were placed in an agate mortar to mix uniformly, and then two samples were weighed about 8.06 g, respectively. The TG experiments were carried out by utilizing Germany NETZSCH STA449C with a linear heating rate of 10 K·min−1 from room temperature to 1773 K under high pure Ar and N2, respectively, and the gas flow rate was 30 ml·min−1.
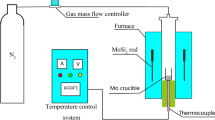
The prepared materials were compressed into Φ15 mm × 30 mm cylindrical blocks, and the blocks were dried in an oven and put into an alumina crucible to carry out reduction and nitridation experiments in a horizontal resistance furnace, and the furnace is shown in Fig. 1. A small amount of N2 was introduced into the furnace to maintain micro-positive pressure firstly, and N2 flow was increased after a period of reaction. The pre-reduction temperature was 923 K for 4 h and then heated to the set temperature, which was 1273, 1473, and 1573 K, respectively, for 4 h. The reaction products were cooled to below 373 K in nitrogen atmosphere after heat preservation and then were pulverized, and their phase compositions were analyzed by X-ray diffraction (XRD).
3 Results and discussion
3.1 XRD analysis
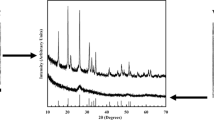
Figure 2 shows the XRD patterns of products at different reaction temperatures. The products are mainly V2O3, some carbides, and minimal VN after holding for 4 h at 1273 K. The main phases of products are VN, a small amount of V6C5, and low-valence vanadium oxides at 1476 K. Reference [11] illustrates that V2O5 is easily reduced to low-valence vanadium oxides; therefore, V2O5 can be reduced to V2O3 at 1273 K. The following reactions will occur in the process of synthesizing VN by one-step method [12]:
Based on Eq. (1), the reaction starting temperature is 1303 K under standard state, and it will accelerate the right of Reaction (1), if CO partial pressure of the system reduces; so, actual reaction starting temperature should be below 1303 K. Therefore, the system can find VN after holding for 4 h at 1273 K. However, VN in Fig. 2a is very little, and only a small amount of N2 participates in nitridation reaction at 1273 K. With temperature increasing, the reduction and nitridation reaction proceed dramatically, so carbides gradually transform into VN under N2; thus, VN content of the reaction products increases and vanadium oxide content continues to decrease, which is shown in Fig. 2b.
Compared with XRD patterns of products at 1473 K, VN content decreases at 1573 K, but V6C5 content increases and the content of V2O3 further reduces, which is shown in Fig. 3. The transformation temperature of VN generating from VC is 1545 K from Eq. (2) under the standard state. It will reverse to the left of Reaction (2) over 1545 K, so some vanadium nitrides transform into vanadium carbides.
3.2 Weight loss analysis
According to TGA experimental curves, the weight loss percentage and weight loss rate can be calculated, respectively, from the following equations [13]:
where a is weight loss percentage, %; t is reaction time, min; w o is initial sample mass, mg; w is sample mass after the reaction time of t, mg; w ∞ is final theoretical sample mass, mg; ∆w is weight loss after the reaction time of t, mg; and ∆w ∞ is final theoretical weight loss of the sample, mg. In the indirect reduction, CO is not considered in the flow of pure Ar and N2 atmosphere, and thus ∆w ∞ can be calculated from the following reactions:
The weight loss of V2O5 reduction is actually caused by the escape of CO from Reaction (5), while that of carbon-burdened V2O5 under N2 results from the escape of CO and the increasing nitrogen content from nitridation reaction by Reaction (6). The weight loss percentage and weight loss rate curves are calculated from Eqs. (3) and (4) with corresponding TGA data, as shown in Fig. 4.
The weight loss percentage and weight loss rate curves of the sample are basically similar under N2 and Ar below 1073 K from Fig. 4a, which shows that N2 only plays a role of protective atmosphere in the early period of reaction. The weight loss increases dramatically at about 946 K. Because some vanadium pentoxides melt, which will increase the contact areas of reactants rapidly nearby V2O5 melting point (948 K), and the carbothermal reduction reaction rate becomes faster and the weight loss is intensified. The weight loss percentage and weight loss rate under N2 are larger than those under Ar over 1073 K, because a small amount of N2 participates in the reaction at high temperature to promote the reduction reaction to proceed. The weight loss percentage under N2 is basically similar at 1473–1573 K, which is caused by the increase of nitrogen content due to the nitridation reaction. The weight loss increases slightly over 1573 K, because VN transforms into VC and releases N2 to make the system lose weight again, which is consistent with previous thermodynamic analysis.
Figure 4b reveals that the weight loss rate begins to accelerate over 1073 K and reach peaks at 1373 K, which is slightly earlier than that under Ar, because the reduction and nitridation reaction occur simultaneously at this temperature range and the nitridation reaction will promote reduction reaction, which accelerates the rate of reduction reaction. However, the system still shows weight loss at 1173–1373 K, which illustrates that the main reaction at this stage is the carbothermal reduction reaction.
The weight loss rate under N2 at 1373–1558 K is less than that under Ar, because as the temperature increases, the nitride reaction rate rises gradually, and the nitrogen content of system increases due to the nitride reaction, and thus, the main reaction at this stage is the nitridation reaction.
4 Kinetics analysis
4.1 Non-isothermal kinetics equation
Heterogeneous reaction kinetics is generally composed of a multi-step process, and the restrictive step can be the diffusion, nucleation, or interfacial reaction; thus, the kinetic rate is different, and the rate equation is [13]:
Substituted into Arrhenius equation: \(k = A{\text{e}}^{{{{ - E} \mathord{\left/ {\vphantom {{ - E} {RT}}} \right. \kern-0pt} {RT}}}} \)
where k is frequency factor; f(a) is a function of reactive mechanism; A is frequency factor; E is activation energy, kJ·mol−1; T is temperature, K; and R is molar gas constant.
The following formula can be obtained by non-isothermal method under a constant heating rate, \(\varPhi = {{{\text{d}}T} \mathord{\left/ {\vphantom {{{\text{d}}T} {{\text{d}}t}}} \right. \kern-0pt} {{\text{d}}t}}\):
There are many non-isothermal model fitting methods [14], and Coats–Redfern method is used in the paper, its integral equation is as follows [15]:
where \(g\left( a \right) = \int {{{{\text{d}}a} \mathord{\left/ {\vphantom {{{\text{d}}a} {f\left( a \right)}}} \right. \kern-0pt} {f\left( a \right)}}}\) is a integral form of reactive mechanism function. When \({{ 2RT} \mathord{\left/ {\vphantom {{ 2RT} E}} \right. \kern-0pt} E}{<<}1\), it can be simplified as follows:
Taking logarithms on both sides:
Different reaction mechanisms have different dynamic mechanism functions [13], and dynamic mechanism function can be obtained by test selection, which is \(g\left( a \right) = - { \ln }\left( { 1- a} \right)\). A straight line with slope of −E/R can be obtained by drawing with \({ \ln }\left[ {{{g\left( a \right)} \mathord{\left/ {\vphantom {{g\left( a \right)} {T^{ 2} }}} \right. \kern-0pt} {T^{ 2} }}} \right]\) and \({ 1\mathord{\left/ {\vphantom { 1T}} \right. \kern-0pt} T}\), and the activation energy and frequency factor of the reaction rate equation also can be obtained by calculation.
4.2 Mechanism of reduction and nitridation
The process of synthesizing VN by one-step method is gas–solid reaction, because the experiments were carried out in the flow of pure argon and nitrogen atmosphere, and the mass of powder samples is very small, so CO reduction and N2 diffusion are ignored. Figure 5 shows that the main process contains: (1) reduction reaction between V2O5 and carbon black; (2) CO from reduction reaction reaching gas–solid boundary layer; (3) CO from reduction reaching gas–solid boundary layer; (4) CO nearby gas–solid boundary layer reaching N2; (5) N2 diffusing to gas–solid boundary layer; (6) N2 nearby gas–solid boundary layer reaching reaction interface; and (7) N2 reacting with carbides. Therefore, carbothermal reduction of vanadium oxides and nitridation reaction experiences different steps, such as mass transfer and chemical reaction. The reaction rate can be determined by different control sections at different stages, resulting in different rates [16]. Non-isothermal kinetics will be discussed as follows when interfacial chemical reaction is used as restrictive step.
4.3 Reduction reaction kinetics
The temperature ranges of faster reaction rate selected by TGA under Ar are 1307–1373 K and 1375–1493 K. The regress equation and dot figure can be calculated from Eq. (12), which is shown in Fig. 6.
Figure 6 illustrates that there is a good linear relationship between \({ \ln }\left[ {{{g\left( a \right)} \mathord{\left/ {\vphantom {{g\left( a \right)} {T^{ 2} }}} \right. \kern-0pt} {T^{ 2} }}} \right]\) and \({ 1\mathord{\left/ {\vphantom { 1T}} \right. \kern-0pt} T}\) in the process of V2O5 carbothermal reduction under Ar, and the correlation coefficients of linear fitting are more than 0.989; thus, it is reasonable to regard \(g\left( a \right) = - { \ln }\left( { 1- a} \right)\) as kinetics mechanism function of first-order reaction, which suggests that the test selection of reaction mechanism is suitable. The activation energy, frequency factor, and correlation coefficient obtained by this function are shown in Table 2.
Table 2 reveals that the activation energy at 1375–1493 K is higher than that at 1307–1373 K, which indicates that the reaction is more difficult to proceed at this temperature, and there is a great difference between two frequency factors, showing that the temperature will have a great influence on reaction rate at later reaction, because vanadium oxides undergo carbonization reaction over 1373 K.
The starting temperature of Reaction (13) is 1377 K calculate from Eq. (12). Low-valence vanadium oxides are reduced to carbides over 1373 K; thus, the carbonization reaction requires higher temperature to proceed. From above analysis, the restrictive step of V2O5 reduction under Ar is the carbonization reaction of low-valence vanadium oxides. The reaction rate equation of preparing VC is as follows:
4.4 Complex reaction kinetics
The temperature ranges selected by TGA of larger reaction rate of the process of synthesizing VN by one-step method are 1270–1358 K and 1358–1426 K. The regress equation and dot figure are shown in Fig. 7.
It is clear that there is a good linear relationship between 1/T and ln[g(a)/T 2], and the correlation coefficients are more than 0.997; thus, it is also reasonable to select the kinetics mechanism function of first-order reaction.
The activation energy, frequency factor, and correlation coefficient are shown in Table 3. It shows that the activation energy at 1270–1358 K is slightly smaller than that of reduction under Ar at the same temperature. The main reaction at this temperature analyzed by TGA is the carbothermal reduction reaction, but a small amount of N2 participates in nitride reaction, which can be proved by previous thermodynamic calculation and a small amount of VN found in Fig. 2a. The nitride reaction accelerates reduction reaction and makes it proceed easier; and thus, the activation energy at this temperature is slightly smaller. With temperature rising, the nitride reaction intensifies and the activation energy and frequency factor of complex reaction at 1358–1426 K are slightly smaller than those of carbonization reaction under Ar at the same temperature, because the reduction and nitridation reaction occur simultaneously, thus reducing the activation energy. Meanwhile, the main reaction analyzed by TGA is the nitride reaction, so the restrictive step of preparing VN by one-step method is the nitride reaction. The reaction rate equation of synthesizing VN is as follows:
5 Conclusion
After tested with minimal VN for 4 h at 1273 K, the main phase of products is VN at 1476 K, while some vanadium nitrides transform into vanadium carbides again over 1573 K. According to TGA results, it can be found that N2 is beneficial to stimulate reduction and carbonization, and the reduction and nitridation reaction can occur simultaneously. The main reaction of preparing VN is the carbothermal reduction reaction at 1173–1373 K and nitride reaction at 1373–1558 K. It is reasonable to select the isothermal thermogravimetry. There is a good linear relationship of fitting line, and the correlation coefficients of linear fitting are more than 0.989. The activation energy of preparing VN by one-step method is 104.005 kJ·mol−1, and frequency factor is 470.52 at 1280–1358 K, and 150.052 kJ·mol−1 and 2.35 × 104 at 1358–1426 K, respectively.
References
Zhou YB. Pan Zhihua vanadium-nitrogen alloy smelting technology. Sichuan Metall. 2012;34(1):13.
Gong DP, He MX, Luo KJ, Zeng G, Mei CY. Application of VN alloy in 400 MPa-grade reinforced bar. Iron Steel Vanadium Titan. 2001;22(1):22.
Han SH, Zhang YM, Bao SX. Effect of high calcium on roasting process of vanadium-bearing stone coal with sodium salt. Chin J Rare Met. 2013;37(6):798.
Li JH, Gao XX, Bao XQ, Cheng L, Xie JX. Wiedemann effect of Fe-Ga based magnetostrictive wires. J Chin Phys B. 2012;21(8):087501.
Wang GH, Chen YM, Zhu YK. Laboratory research on production process conditions of vanadium carbide and vanadium carbonitride. Iron Steel Vanadium Titan. 1988;2:19.
Huang ZS, Chen WL, Luo KJ, Xing JB. Development on research of vanadium nitride. Ferro-alloys. 2008;200(3):20.
Wang X, Chen BZ, Xiao WD, Peng H. Preparation technology of vanadium nitride by microwave heating. Rare Met Mater Eng. 2010;39(5):924.
Ding Y, Fu HH. Application of industrial microwave oven in producing vanadium nitride. Ferro-alloys. 2008;3:16.
Yu SS, Fu NX, Shi LK, Sui ZT. Effect of technical parameters on synthesizing vanadium carbonitride by one-step method. J Northeast Univ (Natural Science). 2006;27(2):11.
Lu ZY. Study on the preparation of high density V-N microalloy additive. Shenyang: Northeast University; 2005. 62.
Liang LK. Thermodynamic analysis of prepetition of metallic vanadium(V), vanadium carbide(VC) and vanadium nitride(VN). Iron Steel Vanadium Titan. 1999;28(3):34.
Deng L, Liu Y, Jiang ZT, Sui ZT. Synthesis of vanadium carbonitride by carbothermal reduction and nitridation method. Funct Mater. 2010;41(5):840.
Wang CZ. Metallurgical Physical Chemistry Research Method. Beijing: Metallurgical Industry Press; 2002. 391.
Coats AW, Redfern JP. Parameters from thermogravimetric data. Nature. 1964;201(4914):68.
Hu RZ, Gao SL, Zhao FQ, Shi QZ. Thermal Analysis Kinetics. Beijing: Science Press; 2008. 54.
Xu XF, Fu HH. Chemical kinetics of preparing VN from reducing and nitriding V2O5. Iron Steel Vanadium Titan. 2004;25(1):1.
Acknowledgments
This study was financially supported by the Twelfth Five-year Scientific Support Plan of the Ministry of Science and Technology of China (No. 2011BAB05B05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, J., Yu, Y. & Xue, ZL. Non-isothermal kinetics of synthesizing vanadium nitride by one-step method. Rare Met. 34, 738–743 (2015). https://doi.org/10.1007/s12598-014-0367-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0367-3