Abstract
In this study, VN was successfully prepared in an ammonia atmosphere using V2O5 as a raw material at 600 °C. The gas composition of the reaction process was analyzed by Factsage 8.0 software. At the same time, based on the first principles of density functional theory, the adsorption model of NH3 on the V2O5 (001) surface is established. Through the analysis of the structure changes of the adsorption model and the size of the adsorption energy, the mechanism of the adsorption reaction of ammonia and V2O5 was revealed. Through thermodynamic calculations and the first-principles calculations method of density functional theory (DFT), the microscopic mechanism of the reaction between ammonia and V2O5 is revealed, which is of great significance to the determination of process parameters in the actual reaction.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
In recent years, transition metal nitrides have received extensive attention due to their superior chemical and physical properties, such as high toughness, high-temperature stability, excellent corrosion resistance, extreme hardness, excellent catalytic performance, and electrical conductivity [1,2,3]. Among transition metal nitrides, vanadium nitride (VN) has attracted much attention due to its excellent physical and chemical properties [4,5,6].Thus, it has many applications in the field of structural materials, electrochemistry, and catalysis [7, 8].
Traditionally, the conventional preparation method of vanadium nitride has high temperature (above 1000 °C) and long reaction time due to the diffusion rate of solid–solid reaction related to temperature, such as carbothermal reduction of vanadium oxide or direct nitridation of pure metal vanadium [5, 9]. In order to find a method of low temperature, short time, and low cost, ammonia reduction-nitridation is widely studied as a low-cost and efficient method for the preparation of vanadium nitride [10,11,12]. Mosavati et al. [10] prepared vanadium-based precursor by hydrothermal method for 24 h, and then synthesized VN nanopowders having an average particle diameter of 47 nm under NH3 atmosphere at 800 °C. Panda et al. [11] reported that nano-sized vanadium nitride was prepared by sol–gel synthesis of V2O5 precursor in the atmosphere of ammonia gas. Qin et al. [12] reported using ultra-fast (within 1 min) solution combustion to synthesize vanadium dioxide (VO2) precursor in an ammonia atmosphere to prepare nano-vanadium nitride powder. The above studies have shown that the nanostructure and high activity of the nanostructure and high activity prepared by the method of hydrothermal method, sol–gel method, and solution combustion synthesis method are used to successfully synthesize the VN nano-powder. During the preparation process of VN, the vanadium-based precursor phase changes to: V2O5 → VO2 → V2O3 → VN. However, there are few theoretical studies on the microscopic mechanism of vanadium nitride formation at the surface of vanadium oxide in ammonia atmosphere.
In this study, VN was successfully prepared by using micron-V2O5 as raw material in ammonia atmosphere at 600 ℃. Through thermodynamic calculation, the variation of gas and solid in gas–solid reaction process was analyzed by FactSage 8.0. In order to further analyze the change of the surface microstructure of V2O5 in the ammonia atmosphere, we use the first-principles calculations method of density functional theory (DFT) to study the structural change of the surface adsorption of V2O5 (001), providing a deeper theoretical basis for the further study of the ammonia reduction-nitridation of V2O5.
Experimental and Computational Methods
Experimental Procedure
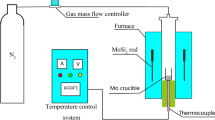
The solid powder raw material for preparing vanadium nitride (VN) is vanadium pentoxide (V2O5, >99.9 wt.%). For preparing vanadium nitride, V2O5 was placed in an alumina crucible and put into the constant-temperature zone of a horizontal resistance furnace. Initially, at the heating stage, a flow of Ar gas (99.999%) was introduced into the furnace tube to get rid of O2 and H2O. The flow rate of Ar gas was kept at about 300 ml/min. When the furnace was heated to the desired temperature (400–600 °C) at the rate of 10 °C min−1, the nitridation reaction of the raw materials was performed in a tube furnace at a constant heating temperature of flowing ammonia (NH3,99.999%) gas at the flow rate of 500 ml/min. After holding at the desired temperature for 1 h, the tube furnace was cooled down under flowing Ar gas. After reaction and nitridation, the obtained products were examined by X-ray powder diffraction (PANalytical D/max 2500). Scanning electron microscope (Quattro S, ThermoFisher) was carried out to monitor overall morphology.
Computational Details
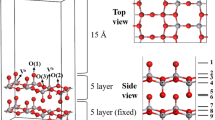
All structural optimizations and corresponding total energy calculations were performed using density functional theory (DFT) with the VASP package by the plane wave basis sets [13]. The exchange correlation function used is the generalized gradient approximation of the Perdew−Burke−Ernzerhof (PBE). The plane wave base cutoff energy of 500 eV is used to ensure good accuracy. The structure of vanadium oxide is geometrically optimized by the conjugate gradient method. The maximum force of each atom in the system is less than 0.03 eV/Å and the total energy is less than 10–5 eV. In order to eliminate the influence of radical-containing systems, we considered spin polarization in our calculations. The V2O5(001) surface was modeled with the slab supercell approach including a 15 Å vacuum region, as shown in Fig. 1. There are two kinds of vanadium (Va and Vb) and three kinds of lattice oxygen active sites on the surface [14, 15]. Among them, Va is vanadium-based oxygen (Va = O) located outside the surface and vanadium-based oxygen (Vb = O) located inside the surface. Lattice oxygen includes single-coordinated terminal oxygen O(1), namely, vanadium-based oxygen (V = O), double-coordinated oxygen O(2), and tri-coordinated oxygen O(3). It is worth noting that from the structural position of O(1), O(1) is the most prone to adsorption reaction site [16], and O(1) is also the research site of this article. We used a (1 × 2) supercell containing a ten-layer slab in which the bottom five layers were fixed and all the other atoms are allowed to relax freely. A 3 × 4 × 1 k-point grid that has been tested for convergence is used to ensure that the interface has sufficient accuracy. Since the zero-point correction has a small effect on the adsorption energy, the relative energy reported does not include the zero-point energy and thermal correction.
The adsorption energy (Ead) is calculated according to Formula (1).
where ENH3+V2O5 is the surface energy of NH3 adsorbed on the V2O5 surface, ENH3 is the energy of NH3, and EV2O5 is the energy of the V2O5 clean surface.
Results and Discussion
Thermodynamic Analysis
As shown in Table 1, the main reactions and their standard Gibbs free energies during reduction-nitridation process were calculated by FactSage 8.0. From the products of Eqs. 3 and 4, it can be seen that there are two possible ways for V2O5 to react with NH3. The products of the two different pathways are N2 and NO, respectively. It can be seen from Eq. 2 that NO easily reacts with NH3, indicating that it is difficult to detect the formation of NO during the actual reaction process. Meanwhile, the reaction pathway of VO2 is similar to that of V2O5. However, it can be seen from Eq. 6 that the initial temperature of NO generation is 966 ℃ under standard condition, indicating that the reaction between VO2 and NH3 is dominated by Eq. 5. In addition, the temperature required to generate VN under standard conditions is 703 ℃, under standard condition by Eq. 7. Equations 8 and 9 show that H2 can reduce V2O5 to V2O3. This indicates that H2 decomposed from ammonia gas also participates in the reaction.
In order to further illustrate the NH3-V2O5 system changes during the reaction, the solid and gas phase composition was calculated as a function of input NH3 under standard condition. Figure 2 shows the equilibrium of the gas–solid composition of the product with the increase of ammonia at 400 and 600 °C. As shown in Fig. 2, the solid phase changes to: V2O5 → VO2 → V3O5 → V2O3. In our previous study, with the further increase of ammonia, V2O3 would eventually change to VN [17]. In the gas product, its main components are H2O and N2. As the reaction progresses, the content of H2 and NH3 will gradually increase. It is worth mentioning that the black line is the change of NO gas. When V2O5 is gradually transformed into V2O3, the NO gas content also drops by orders of magnitudes. Meanwhile, when the temperature increases from 400° to 600° ℃, the content of NO gas increases by several orders of magnitude, while the content of ammonia decreases by several orders of magnitude, indicating that the increase of temperature is beneficial to the reaction. In addition, it can be seen from Eq. 2 that NO and NH3 will react, resulting in a small amount of NO compared to the content of N2.
Phase Transformation Analysis
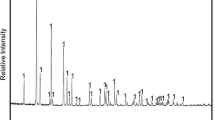
The phase transformation during the preparation of VN was shown in Fig. 3a. As shown in Fig. 3a, after reacting at 400 °C for 1 h, the sample consisted of VO2, V2O3, and VN. It can be concluded that, in this case, the reaction time was not sufficient to completely reduce vanadium oxides. With the increase of temperature to 500 ℃, the diffraction peak of VO2 disappeared leaving only the diffraction peaks of V2O3 and VN. When the temperature was 600 ℃, all diffraction peaks were assigned to VN. In addition, the lattice parameters and structure of VO and VN are similar. Therefore, the VN produced by the reaction is a solid solution of VNxOy (x < 1, y < 1). It is worth noting that the above thermodynamic calculation shows that the initial temperature of VN generation in the standard state is 709 ℃, while the actual experimental reaction temperature is lower than the theoretical temperature, which is due to dynamics and other factors. Figure 3b shows a scanning electron micrograph (SEM) of the product reacted at 600 ℃ for 1 h. The product is micron-sized particles composed of many small cubes.
DFT Analysis
In order to further study the reaction of adsorbed gas on the microscopic surface of V2O5 under ammonia atmosphere, we used DFT to calculate the structural changes and adsorption energy of NH3, H2, N2, H, and N adsorbed on the surface of V2O5 (001), as shown in Fig. 4. On the surface of V2O5, the structural position of O(1) site shows that O(1) is most likely to adsorb gas. Meanwhile, Fu et al. [16] also found that O(1) and H combined to form H2O would leave the solid surface, thus changing the structure of V2O5. From Fig. 4a–c, it can be seen that the adsorption energy (Ead = −0.15 eV) of NH3 adsorbed on the surface of V2O5 (001) is the largest, indicating that in the actual reaction process, ammonia is most easily adsorbed on the surface of the reactant and reacts with V2O5. In addition, NH3 and H2 are easily decomposed to obtain active N atoms and H atoms. In the actual reaction process, there are active N atoms and H atoms in the ammonia atmosphere. Therefore, we calculate the adsorption of H and N atom on the V2O5 (001) surface, as shown in Fig. 4d, e. The adsorption energies of the H and N atom are −2.89 eV and −2.16 eV, respectively, indicating that H and N atoms can react spontaneously with O(1). Meanwhile, the bond length between O(1) and V atom becomes longer, and O(1) has a tendency to desorb on the V2O5 (001) surface.
Conclusions
VN was successfully prepared in ammonia atmosphere using V2O5 as a raw material at 600 °C for 1 h. Through thermodynamic calculation, the gas–solid change in the reaction process was analyzed. The formation of NO gas was found at the initial stage of the reaction. NO gas will react with NH3, resulting in a very low NO content in the product gas. Meanwhile, through DFT study, it was found that NH3 was easily adsorbed on V2O5 (001) surface compared with N2 and H2. The active N and H atoms obtained from the decomposition of NH3 and H2 are easily adsorbed on the surface of V2O5 (001) and bind to O(1). Meanwhile, O(1) has a tendency to detach from the V2O5 (001) surface, which shows the change process of the microscopic reaction process.
References
Chen L, Korányi TI, Hensen EJ (2016) Transition metal (Ti, Mo, Nb, W) nitride catalysts for lignin depolymerisation. Chem Commun 52(60):9375–9378
Huang HH, Hon MH (2002) Effect of N2 addition on growth and properties of titanium nitride films obtained by atmospheric pressure chemical vapor deposition. Thin Solid Films 416(1–2):54–61
Li Q, Chen Y, Zhang J, Tian W, Wang L, Ren Z, Li X, Gao B, Peng X, Chu PK, Huo K (2018) Spatially confined synthesis of vanadium nitride nanodots intercalated carbon nanosheets with ultrahigh volumetric capacitance and long life for flexible supercapacitors. Nano Energy 51:128–136
Jiang X, Lu W, Yu Y, Yang M, Liu X, Xing Y (2019) Ultra-small Ni-VN nanoparticles co-embedded in N-doped carbons as an effective electrode material for energy storage. Electrochim Acta 302:385–393
Roldan MA, Lopez-Flores V, Alcala MD, Ortega A, Real C (2010) Mechanochemical synthesis of vanadium nitride. J Eur Ceram Soc 30(10):2099–2107
Zhang L, Holt CM, Luber EJ, Olsen BC, Wang H, Danaie M, Cui X, Tan X, Lui VW, Kalisvaart P, Mitlin D (2011) High rate electrochemical capacitors from three-dimensional arrays of vanadium nitride functionalized carbon nanotubes. J Phys Chem C 115(49):24381–24393
Zhou P, Xing D, Liu Y, Wang Z, Wang P, Zheng Z, Qin X, Zhang X, Dai Y, Huang B (2019) Accelerated electrocatalytic hydrogen evolution on non-noble metal containing trinickel nitride by introduction of vanadium nitride. J Mater Chem A 7(10):5513–5521
Ningthoujam RS, Gajbhiye NS (2015) Synthesis, electron transport properties of transition metal nitrides and applications. Prog Mater Sci 70:50–154
Sansan YU, Nianxin FU, Feng GAO, Zhitong SUI (2009) Synthesis of vanadium nitride by a one step method. J Mater Sci Technol 23(01):43–46
Mosavati N, Salley SO, Ng KS (2017) Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J Power Sour 340:210–216
Mishra PP, Theerthagiri J, Panda RN (2014) Mesoporous vanadium nitride synthesized by chemical routes. Adsorpt Sci Technol 32(6):465–474
Qin M, Wu H, Cao Z, Zhang D, Jia B, Qu X (2019) A novel method to synthesize vanadium nitride nanopowders by ammonia reduction from combustion precursors. J Alloy Compd 772:808–813
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865
Yao H, Chen Y, Wei Y, Zhao Z, Liu Z, Xu C (2012) A periodic DFT study of ammonia adsorption on the V2O5 (001), V2O5 (010) and V2O5 (100) surfaces: Lewis versus Brönsted acid sites. Surf Sci 606(21–22):1739–1748
Yao H, Chen Y, Zhao Z, Wei Y, Liu Z, Zhai D, Liu B, Xu C (2013) Periodic DFT study on mechanism of selective catalytic reduction of NO via NH3 and O2 over the V2O5 (0 0 1) surface: competitive sites and pathways. J Catal 305:67–75
Fu H, Liu ZP, Li ZH, Wang WN, Fan KN (2006) Periodic density functional theory study of propane oxidative dehydrogenation over V2O5 (001) surface. J Am Chem Soc 128(34):11114–11123
Liu Y, Wang Y, You Z, Lv X (2020) Reduction and nitridation of iron/vanadium oxides by ammonia gas: mechanism and preparation of FeV45N alloy. Metals 10(3):356
Acknowledgements
The authors wish to express their thanks to the National Natural Science Foundation of China (U2003215 and 51974053), the Fundamental and Frontier Research Project of Chongqing, China (cstc2020jcyj–msxmX0515), and the Fundamental Research Funds for the Central Universities (2020CDJ–LHZZ–083) for the financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Liu, Y., Hu, Q., Ma, D., Wang, Y., You, Z. (2022). Low-Temperature Preparation and Mechanism Study of Vanadium Nitride. In: Zhang, M., et al. Characterization of Minerals, Metals, and Materials 2022. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92373-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-92373-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92372-3
Online ISBN: 978-3-030-92373-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)