Abstract
In this paper, the thermodynamics and kinetics of nature rutile carbochlorination in a fluidized-bed were investigated. The thermodynamic calculations of TiO2–C–Cl2 system show that when C is excess in the solid phase, titanium tetrachloride and carbon monoxide can exist stably. At high temperature, the reaction with CO as the product is the dominant reaction. The appropriate reaction conditions are as follows: reaction temperature of 950 °C, reaction time of 40 min, carbon ratio of 30 wt% of rutile, natural rutile particle size of −96 μm, petroleum coke size of −150 μm, and chlorine flow of 0.036 m3·h−1. Under the above conditions, the reaction conversion rate of TiO2 can reach about 95 %. This paper proposed a reaction rate model, and got a rutile chlorination rate formula, which is generally consistent with the experimental data. For the TiO2–C–Cl2 system, the reaction rate is dependent on the initial radius of rutile particle, density, and the partial pressures of Cl2. From 900 to 1,000 °C, the apparent activation energy is 10.569 kJ·mol−1, and the mass diffusion is found to be the main reaction-controlling step. The expression for the chlorine reaction rate in the C–Cl2 system is obtained, and it depends on the degree of reaction, the partial pressure of Cl2, and the size of rutile particle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
By far titanium metal has been still produced commercially by the Kroll process, a complex and expensive batch process. TiCl4 is widely utilized as intermediate materials for producing titanium white (TiO2) and titanium sponge, which are two major products in titanium industry. Because of enhancement of mass transfer, fluidized-bed chlorination of titanium minerals becomes an established technique for titanium tetrachloride production. In the production of TiO2 pigment by the chloride process, TiCl4 is produced by the high temperature fluidized-bed chlorination of TiO2. In China, at least 90.5 wt% of the titanium resource is located in Panzhihua, which all belongs to magnetite with a high content of CaO and MgO. In the temperature range of 1,073–1,273 K, calcium chloride (CaCl2) and magnesium chloride, formed in the chlorination reaction process, are liquid, which can agglomerate the reactant particles and destroy the fluidized chlorination process. Therefore, there is an increasing concern on the importance of importing natural rutile ores with a low content of CaO and MgO. Although a vast amount of titanium tetrachloride are made in fluidized-bed reactors, the mechanism of the reactions is still not completely understood [1–4].
Brain and Schuler [5] investigated the carbochlorination behavior of pure TiO2. The results indicate that the conversion rate of TiO2 with TiO2–C contact was 10 times faster than that without carbon in the reaction system, and the reaction rate decreased with the distance increasing between TiO2 and carbon. Yang and Hlavacek [6] studied the chlorination kinetics of rutile and anatase with C and CO as the reductant in a packed bed reactor at temperature from 1,073 to 1,273 K. When C was used as the reductant, a solid–solid reaction was involved. The reactivity of reactants was highly enhanced by solid carbon.
Youn and Zhou et al. [7–10] developed mathematical models of fluidized chlorination of rutile, respectively. Basic factors that can affect the reaction rate of carbochlorination were analyzed using their developed models. Bergholm [11] chlorinated Australian rutile in the presence of CO and carbon. Bergholm suggested that reduction reaction was a rate-controlling reduction step, followed by rapid chlorination of the reduced rutile. In addition, the reaction rate with carbon as the reductant was dependent on Cl2 concentration, while not dependent on CO. However, kinetic data of carbochlorination of Kenya nature rutile ores were rarely reported in the literatures.
As a part of optimizing operation for a domestic plant, a laboratory study on the carbochlorination rates of Kenya natural rutile ore was carried out with coke as the reducing agent. In the current study, chlorination of rutile ore (powder, −150 μm, 93.29 wt%) was carried out. The suitable range of operating temperatures was 1,073–1,273 K. The total pressure was kept constant at 101.3 kPa in the reaction system. The new developed kinetic model can be used to predict the reaction rate under different operating conditions for a boiling chlorination process.
2 Experimental
A laboratory scale fluidized-bed consists of a quartz reactor, in which the straight tube is 1,000 mm (height) × 60 mm (internal diameter). An electric resistance furnace surrounds the quartz tube to ensure the required reaction temperature. In order to uniformly distribute the fluidizing gas, a porous disk was placed in the lower part of the quartz tube. The gas flow rate was measured using mass flowmeters. Gas mixed, and entered fluidized-bed where they were heated. In order to determine the existence and extent of a volatile species of titanium, the weight loss of titanium oxide was measured. Large amount of excess gaseous chlorine was absorbed by caustic soda.
After temperature got the setting point, the air in the reactor was drove off by pumping nitrogen gas, and petroleum coke (−1,700 to +880 μm) of 6 g was added into the reactor. When the temperature was stable, the mixture of dry natural rutile and calcined petroleum coke was added to reactor. Then chlorine gas was pumped into reactor at a certain flow. After the holding time elapsed, nitrogen was used to drive residual chlorine. The chloride slag was removed and weighed when its temperature fell down.
Kenya nature rutile with particle size of about 70–150 μm, as described in Table 1, was used as starting material. The particles of natural rutile can be fluidized using a small flow rate of gaseous chlorine. The coke was a kind of calcined petroleum coke with carbon content 94.94 wt%. In order to make the materials mix completely and temperature be uniform within the bed, and not cause pneumatic conveying of fine particles, the total gas flow rate was determined to be 4 times as much as the minimum fluidizing velocity. The flow rate was approximately 0.036 m3·h−1, and the initial weight of ore was 200 g.
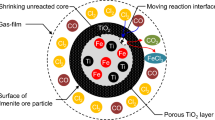
By scanning electron microscope (SEM, Model JSM-6360LA, NEC), morphology and particle size of rutile ore are observed in Fig. 1. The results, which were confirmed in the previous study, indicate that Kenya rutile is a nonporous solid which does not undergo internal chlorination. Therefore, the reaction occurs exclusively at the surface of the particles.
Chemical compositions are determined by titration and element compositions by plasma emission spectrometer (ICP, Model Profile LEEMAN).
The conversion rate of TiO2 χ was calculated by the following equation:
where W slag is the weight of the chloride slag, \( W_{{{\text{TiO}}_{ 2} - {\text{slag}}}} \) is the TiO2 content in slag, W rutile is the weight of natural rutile, and \( W_{{{\text{TiO}}_{ 2} - {\text{rutile}}}} \) is the TiO2 content in rutile.
3 Results and discussion
3.1 Theoretic analysis
3.1.1 Chemical equilibrium composition for reaction system
The reaction thermodynamics has a guiding role for studying natural rutile carbochlorination [6, 12, 13]. Based on the minimum free energy principle, the chemical equilibrium composition in the research system where rutile and coke were used as the reductants at different temperatures under the atmospheric pressure was calculated.
The carbochlorination process of rutile may be described as follows:
Figure 2 shows the calculated chemical equilibrium compositions for different ratios of reactants. Chlorination of TiO2 with different amounts of carbon was performed. The four ore/carbon ratios (molar ratio) calculated are TiO2/C = 1:1.336, 1:1.670, 1:2.000, and 1:2.330. Owing to that, the theoretical TiO2/C should be between 1:1 (according to Eq. (2)) and 1:2 (according to Eq. (3)), the ratios of TiO2/C = 1:2.000 and 1:2.330 mean that C is in excess in the system.
The calculation of the equilibrium compositions in the TiO2–C–Cl2 system indicates that within the temperature range of this research, the carbochlorination reaction of TiO2 occurs completely. CO2 is produced including outer TiCl4 below 673 K, while CO is one of the stable products at temperatures above 1,173 K.
Figure 2d shows chemical equilibrium compositions in the system with carbon in excess. At higher temperatures (>1,173 K), titanium tetrachloride and carbon monoxide can exist stably, and the appropriate addition of petroleum coke should be about 30 wt% of the rutile. Too much carbon cannot change equilibrium composition.
3.1.2 Ratios of CO/CO2
Thermodynamic analysis indicates that the ratios of CO/CO2 under the reaction equilibrium at 800 and at 1,040 °C are 58 and 340, respectively. This illustrates that Reaction (3) is the dominant reaction.
Supposed that the probability of generating CO reaction is η, the above Reactions (2) and (3) can be jointly expressed as follows:
where η = [p(CO)/2]/[p(CO)/2 + p(CO2)], p(CO) and p(CO2) are the partial pressures of carbon monoxide and carbon dioxide in furnace gas, respectively.
When calculating the Gibbs free energies of the nature rutile carbochlorination reactions, it is known from Reaction (5) that, the value range of η for thermodynamic calculation can be determined from \( p_{{{\text{TiCl}}_{ 4} }} \) + (1 + η) \( p_{{{\text{TiCl}}_{ 4} }} \) ≤ 101.325 kPa under a certain TiCl4 partial pressure, \( p_{{{\text{TiCl}}_{ 4} }}. \)The results are shown in Fig. 2, with η values of 0.2, 0.4, 0.5, and 0.7, and temperature ranges from 900 to 1,400 K, \( p_{{{\text{TiCl}}_{ 4} }} \)/p is 0.10, 0.20, 0.30, 0.35, and 0.40, and exhaust pressure p is 101.325 kPa.
Thermodynamic computation is described in Fig. 3a–d under different TiCl4 partial pressures. Reaction (3) is dominant at a high temperature.
3.2 Experimental
3.2.1 Effects of reaction temperature
The effect of temperature on reaction conversion rate at different temperatures is shown in Fig. 4.
The experimental results indicate that when the temperature is below 900 °C, the reaction conversion rate of rutile improves significantly with an increase of temperature. When the reaction temperature is higher than 950 °C, the conversion rate just increases slightly with a further increase of temperature. Chlorination results of other high content oxides show that their possibility for the chlorination reaction is Fe2O3 > TiO2 > SiO2.
3.2.2 Effect of rutile particle diameter
The laboratory studies show (in Fig. 5) that the particle size has a significant effect on the conversion rate of natural rutile. When the particle size is smaller than 96 μm, the conversion efficiency of TiO2 can reach more than 92 % at 950 °C. A further decrease in particle size of natural rutile only has a slight influence on the chlorination efficiency. The reasons for this result might be that the finer the particle of rutile is, the greater the specific surface area is, and the better the kinetic conditions of chlorination reaction are. However, when ore particle size is too small, it is very difficult to establish a good fluidization situation, and prone to occur channeling, slugging, etc. This destroys a good gas–solid contact situation. Natural rutile has smooth surfaces and rare pores, and rarely occurs internal interfacial chemical reaction. This is one of the main reasons for a slow chlorination reaction rate of natural rutile. Therefore, raw materials with small sizes can increase the reaction interface; thereby enhance the rate of reaction. Taking the above factors into account, the appropriate particle size range should be smaller than 96 μm.
3.2.3 Effects of petroleum coke particle size
Figure 6 shows the effect of coke size on conversion rate. The experimental results show that when the calcined petroleum coke size is about 1.4 times that of natural rutile, the mixing of the two kinds of materials is better. Fluidization gas flow rate is generally very large. Therefore, turbulent mixing is strong, and there is no obvious stratification phenomenon. In a certain range of particle size, petroleum coke and natural rutile can mix very well, with only a little effect on the chlorination experiments.
3.2.4 Effect of gas flow rate
The reaction conversion rate falls with a decrease of fluidization gas velocity, as shown in Fig. 7. But a high-speed gas flow is easy to bring powder outside the furnace which increases the amount of dust, and reduces the residence time of gaseous chlorine in the furnace. The conversion rate does not change significantly with a further increase of gas flow. According to the experimental results, gas flow rate of 0.036 m3·h−1 (reactor Φ26 cm) can establish a good fluidization and achieve a good conversion rate.
3.2.5 Effect of carbon/rutile (mass ratio)
Figure 8 shows that the appropriate addition of petroleum coke should be about 30 wt% of the rutile. Too much carbon not only cannot increase the reaction rate, but also lead to a waste. However, too small carbon ratio results in natural rutile chlorination incomplete, thereafter TiO2 will enter the furnace slag.
3.2.6 Effect of chlorination time on reaction conversion rate
Figure 9 shows that the conversion of natural rutile increases with the increase of reaction time. From 950 to 1,000 °C, reaction conversion rate changes a little. Under the conditions of the reaction temperature of 950 °C, reaction time of 40 min, the conversion rate reaches more than 95 %, and the reaction is close to complete.
3.2.7 Effect of diluted gaseous chlorine on reaction conversion rate
Figure 10 shows the experimental results of conversion rate under the conditions that the ratios of Cl2:O2 are equal to 1:0, 85:15, and 70:30, respectively. In the case of sufficient carbon, low concentration chlorine is also useful for the chlorination reaction of rutile. However, more reaction time is required for achieving a higher conversion rate. With the chlorination time increasing, the effect of chlorine concentration on conversion rate becomes smaller. Conversion rate of TiO2 reaches 95 % at chlorination time of 50 min using the gas with 85 % chlorine. However, concentration of chlorine should not be too low, because low temperature is not beneficial for high production efficiency. Compared dilution composition between oxygen and air, it can be concluded that the effects of diluted chlorine on the conversion rate of rutile are only time-relating, with little relation with dilution composition.
For fluid-bed chlorination reaction, the appropriate reaction conditions are reaction temperature of 950 °C, reaction time of 40 min, carbon ratio of 30 wt% rutile, natural rutile particle size of −96 μm, petroleum coke size of −150 μm, chlorine flow of 0.036 m3·h−1, gas pressure 0.14 MPa, and concentration of chlorine 100 %. Under such conditions, the reaction conversion rate can reach about 95 %.
3.3 Carbochlorination kinetics of rutile
Kenya rutile is a nonporous solid which does not undergo internal chlorination. Therefore, the reaction occurs exclusively at the surface of the particles at a rate proportional to the receding surface area [14, 15].
where m is the weight of rutile particle, g; t is reaction time, k is the reaction rate constant, g·s−1·m−2 ; S is the surface area of particle ore, m 2
A good approximation of particle shape is a sphere, so that substituting for m and S gives the following equations:
where r is the mean radius of rutile particle, m; ρ s is the density of rutile particle, g·cm−3.
Thus, the radial penetration rate is constant. Integrating between definite limits gives:
where r 0 is the initial mean radius of rutile particle, m is the weight of rutile particle, g; θ is the ending time of reaction, s.
The fractional conversion of the rutile particle X B is defined by:
where m 0 is the initial weight of rutile particle, g.
Substituting this value of r into Eq. (10) gives
Microscopic examination of the rutile particles solids shows that at high conversions (X B > 0.9), particle shape has a significant deviation from a spherical shape, and the particles become ellipsoidal. Therefore, the above rate expression should be used with correction under the conditions of X B > 0.9.
For the rutile-coke-chlorine system, a total of 21 runs were carried out to analyze the influential factors. Temperature has an appreciable influence on the reaction rate (Fig. 10). At the temperature range of 900–1,000 °C, chlorination kinetic analysis was carried out. Experimental conditions: gas flow of 0.036 m3·h−1, inlet gas pressure of 0.14 MPa, natural rutile particle size of −96 μm, carbon addition 30 % of rutile for 200 g Kenya rutile charge at the flow rate equal to 4 times of the minimum fluidization velocity.
According to Arrhenius equation:
where k is the apparent rate constant which can be obtained by fitting the slopes of three lines in Fig. 11, E a is the apparent activation energy, R is the gas constant, 8.314 kJ·mol−1, T is absolute temperature with K as the unit, A is a constant.
According to the change of lgk with 1,000/T, the slope can be obtained. The apparent activation energy of chlorination process (E a) is calculated using Arrhenius equation. From 900 to 1,000 °C, the apparent activation energy is 10.569 kJ·mol−1. In this temperature range, diffusion is the main reaction controlling step.
By the use of multiple linear regression techniques, the following empirical equation for the conversion rate of Kenya rutile in the C–Cl2 system is obtained, which fits with the experimental data fairly well (Fig. 12).
where \( p_{{{\text{Cl}}_{ 2} }} \)is the partial pressure of Cl2, Pa; d is the harmonic mean diameter of rutile particle, m
Replacing the right side of Eq. (13) to Eq. (15), k is calculated using the following equation:
Therefore, k is proportional to the radius and the density of rutile.
The fractional order kinetics implied by the fractional partial pressure exponents is common in reaction systems. It suggests that the TiO2 surface is covered with relatively stable primary reaction products, which limits the access of CO and Cl2 to the surface, thereby reducing the apparent order of the reaction.
In commercial chlorinators, it is more important to know the rate of chlorine removal on passing through the fluidized-bed. Accordingly,
where n(Cl2) is the content of Cl2, mol; \( \sigma_{{{\text{Cl}}_{ 2} }} \) is reaction rate of the gaseous chlorine, g·mol·(min·g)−1; M is molecular weight of TiO2.
For the C–Cl2 systems, chlorine reaction rate is defined as follows:
Returning to the expression for the chlorine reaction rate in the coke system, it can be noticed that the rate increases with a decrease in rutile particle diameter and it depends on the degree of reaction, partial pressures of Cl2, and the size of petroleum coke particle.
4 Conclusion
The appropriate reaction conditions are reaction temperature of 950 °C, natural rutile particle size of −96 μm, reaction time of 40 min, carbon ratio of 30 % rutile, petroleum coke size of −150 μm, and chlorine flow of 0.036 m3·h−1, the reaction conversion rate can reach about 95 %.
A reaction rate model was proposed. The expression for the rutile chlorination rate constant was derived, which is dependent on both the initial particle radius and the density of rutile. From 900 to 1,000 °C, the apparent activation energy is 10.569 kJ·mol−1. In this temperature range, mass diffusion is the main reaction controlling step. The empirical equation for the rate of Kenya rutile chlorination in the C–Cl2 system is obtained, and fits with the experimental data fairly well. In fluidized-bed, the chlorine reaction rate depends on the degree of reaction, the size of rutile particle, and the Cl2 partial pressure.
References
Ding BF. Investigation of structural and magnetic properties of Ni implanted rutile. Sci China Phys Mech Astron. 2012;55(2):247.
Yuan ZF, Zhu YQ, Xi L, Xiong SF, Xu BS. Experiment of TiCl4 preparation with multistage series combined fluidized bed. Trans Nonferr Metal Soc. 2013;23(1):283.
Ye WJ, Mi XJ, Song XY. Martensitic transformation of Ti–18Nb (at%) alloy with zirconium. Rare Met. 2012;31(3):227.
Hou QL, Duan HT, Yang S, Liu YJ, Chen JH. Process of rutile titanium dioxide coated with ZrO2. Chin J Rare Met. 2013;37(3):411.
Brain I, Schuler W. On the kinetics of the chlorination of titanium dioxide in the presence of solid carbon. Metall Trans B. 1980;11(2):199.
Yang F, Hlavacek V. Carbochlorination kinetics of titanium dioxide with carbon and carbon monoxide as reductant. Metall Trans B. 1998;29(6):1297.
Youn IJ, Park KY. Modeling of fluidized bed chlorination of rutile. Metall Trans B. 1989;20(6):959.
Zhou L, Sohn HY. Mathematical modeling of fluidized-bed chlorination of rutile. AIChE J. 1996;42(11):3102.
Sohn HY, Zhou L, Cho K. Intrinsic kinetics and mechanism of rutile chlorination by CO + Cl2 mixtures. Ind Eng Chem Res. 1998;37(10):3800.
Zhou L, Sohn HY, Whiting GK, Leary KJ. Microstructural changes in several titaniferous materials during chlorination reaction. Ind Eng Chem Res. 1996;35(3):954.
Bergholm A. Chlorination of rutile. Trans Am Inst Min Metall Pet Eng. 1961;221(121):1121.
Yuan ZF, Wang XQ, Xu C, Li WB, Mooson K. A new process for comprehensive utilization of the complex titania ore. Miner Eng. 2006;19(9):975.
Yang F, Hlavacek V. Carbochlorination of tantalum and niobium oxides: thermodynamic simulation and kinetic modeling. AIChE J. 1999;45(3):581.
Morris AJ, Jensen RF. Fluidized-bed chlorination rates of Australian rutile. Metall Trans B. 1976;7(1):89.
Chen JM, Chang FW, Chang CY. Chlorination kinetics of silicon dioxide in the presence of carbon. Ind Eng Chem Res. 1990;29(6):778.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Nos. 51374064, 51004033, and 51074044), the National High-Tech Research and Development Program of China (No.2012AA062303), the National Key Technology Support Program during the 12th Five-Year Plan Period (No. 2012BAE01B02), and the Fundamental Research Funds for the Central Universities (Nos.N130402012 and N130702001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, LP., Ni, PY., Zhang, TA. et al. Mechanism of fluidized chlorination reaction of Kenya natural rutile ore. Rare Met. 33, 485–492 (2014). https://doi.org/10.1007/s12598-014-0281-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0281-8