Abstract
Reaction mechanism involved in mostly general titanium metallurgy books and literature differs from some basics and is difficult to be understood for researchers and engineers. Although various authors have proposed different types of theories to recognize this reduction process, there is still no consensus on the reaction pathways and kinetic characteristics of magnesiothermic reduction of TiCl4 in the semi-batch reactor, and the theoretical innovations are required for further research of today’s titanium sponge metallurgy. To develop a better understanding of the reduction mechanism of magnesiothermic reduction of TiCl4 in a semi-batch reactor, the present study defined a minimum set of independent reactions via generalized stoichiometry methodology, and proposed an innovative kinetic modeling approach via the net chemical reaction rate models of the main components and the reaction rate models of the independent reactions near the gas–liquid interfaces of magnesiothermic reduction of TiCl4. We also established the overall chemical reaction kinetics model of magnesiothermic reduction of TiCl4 via the isothermal reduction experiment in a prototype 12 tons Kroll reactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to their excellent properties, titanium alloys are widely applied to aerospace, national defense, and marine applications.[1,2] As the main material of the titanium products value chain, the titanium sponge is produced by magnesiothermic reduction of titanium tetrachloride (TiCl4), known as the Kroll process.[3,4] The magnesiothermic reduction of TiCl4 is a high-exothermic reaction that sustains the overheating near the reaction area,[5,6,7] and it causes rapid pressurization and thermal runaway in a semi-batch reactor.[8,9,10] Thus, the reaction mechanism involved in mostly general titanium metallurgy books and literature,[11,12] considering that the refined TiCl4 is reduced by pre-added liquid magnesium (Mg) at around 1073.0 K to 1123.0 K, differs from some basics and is difficult to be understood for researchers and engineers.[5,13] The insufficient theoretical understanding of the pressure behaviors, reaction pathways, and kinetic characteristics of magnesiothermic reduction of TiCl4 may be a principal reason.
Although various authors have proposed different types of theories to recognize this reduction process,[14,15,16,17] there is still no consensus on the reaction pathways and kinetic characteristics of magnesiothermic reduction of TiCl4 in the semi-batch reactor. In the actual reduction process, the gas phase temperature in the semi-batch reactor is about 873.0 K to 973.0 K,[18] which is higher than the supercritical temperature of TiCl4.[19] The liquid TiCl4 would be heated to vaporize completely and then reacted with Mg along the reaction pathways: TiCl4(gas) → TiCl3(gas) → TiCl2(solid) → Ti(metal),[20] and the temperature in this reactor would continuously increase with exothermic reactions and pressure raised due to the various non-condensable gaseous reactants, intermediate products, and by-products.[21,22] Meanwhile, the by-product magnesium chloride (MgCl2) would temporarily cover the free surface of the liquid Mg and act as a barrier for the reactions of this magnesiothermic reduction system. The formation of the product Ti sponge should take place in stages through multiphase reactions.[10,22] Thus, it is necessary to understand comprehensively the pressure behaviours, thermal hazard, reaction pathways, and kinetic characteristics of magnesiothermic reduction of TiCl4 in a semi-batch reactor, and the theoretical innovations are required for further research of today’s titanium sponge metallurgy by the Kroll process.
In this work, we defined a minimum set of independent reactions via generalized stoichiometry methodology and proposed an innovative kinetic modeling approach via the isothermal reduction experiment in a prototype 12 tons Kroll reduction reactor, to develop a better understanding of the reduction mechanism of titanium tetra-, tri-, and dichloride with Mg near the gas–liquid interfaces of magnesiothermic reduction of TiCl4 in a semi-batch reactor.
Technical Background
Independent Reactions and Pathways
Near the gas–liquid interfaces of magnesiothermic reduction of TiCl4 in the prototype Kroll reduction reactor, there are six different chemicals presented as eight different chemical species are considered in this close-reacting system, regarding the Mg and MgCl2 as two distinguishable different species (see Table S1 in the Section S.1.1 in the electronic Supplementary Material). Except for chemical reactions that cannot take place from viewpoints of kinetics, there are still many linearly related non-independent reactions in this multi-phase system. The minimum set of five independent reactions via generalized stoichiometry methodology,[23] which established in the Section S.1.2 in the electronic Supplementary Material, is necessary and sufficient to model the possible and efficient reaction pathways of magnesiothermic reduction of TiCl4 near the gas–liquid interfaces, which is detail described in Figure 1.
Step 1: Phase change near the gas–liquid interfaces
The latent heat of the exothermic reactions and the electrical resistance furnace increases the temperature of the gas–liquid interfaces of magnesiothermic reduction of TiCl4 in the semi-batch reactor. The molten Mg near the interfaces is heated and partially vaporized (Chosen independent pathway 1 in the Figure 1). And so did the MgCl2 (Chosen non-independent pathway 7). In addition, the liquid TiCl4 fed into the reactor, and it vaporizes completely.
Step 2: Homogeneous reaction in the gas phase
The gaseous TiCl4 and gaseous Mg would be fully mixing in the upper space of the semi-batch reactor. Gaseous TiCl4 is reduced to gaseous TiCl3 by Mg in the homogeneous phase (Chosen independent pathway 2). Of course, the gaseous TiCl4 is not fully reduced in the gas phase, and the unreduced gaseous TiCl4 and the intermediate product of the homogeneous reaction, gaseous TiCl3, transport to the gas–liquid interfaces.
Step 3: Heterogeneous reduction near the gas–liquid interfaces
The gaseous TiCl4 and TiCl3 transport to the free surface of liquid Mg, and the multi-step reduction pathways, chosen independent pathways 3 and 4, occur via magnesiothermic reduction and lead to the formation of TiCl2. The reduction reaction of gaseous TiCl4 with free liquid Mg also gives rise to the formation of TiCl2, chosen non-independent pathway 6. Because the solubility of TiCl2 in liquid MgCl2 is very large, about 40 pct at 1175.0 K,[24] a solution of TiCl2/MgCl2 salt is formed near the gas–liquid interfaces. The TiCl2/MgCl2 salt may be continuously swept toward the wall of the reactor with the liquid MgCl2 due to the convection driven by the buoyancy and surface-tension forces.[13,22]
Step 4: Like dissolves like of TiCl2 and new-formed Ti in the liquid phase
When the solution of TiCl2/MgCl2 salt reaches an active site where the nucleation and growth of Ti sponge are favored, the geometric interfaces between the TiCl2 and new-formed Ti disappears due to their same lattice and the similar lattice parameters, and the like dissolves like of TiCl2 and new-formed Ti occurs. Chosen independent pathway 5 leads to the formation of Ti sponge from TiCl2/MgCl2 salt at any active site where the supply of free liquid Mg can be ensured. Thus, the new-formed sponge structure provides a react place for TiCl2 and Mg, not for TiCl4 and Mg, and provides an active site for nucleation and growth of the subsequent Ti sponge.
Isothermal Kinetic Modeling
An innovative kinetic modeling approach for magnesiothermic reduction of TiCl4 will be proposed via the net chemical reaction rate models of the main components and the reaction rate models of the independent reactions near the gas–liquid interfaces of magnesiothermic reduction of TiCl4, which are established in the Sections S.2.2.1 and S.2.2.2 in the electronic Supplementary Material.
Net chemical reaction rate of Ti sponge
According to the net chemical reaction rate models for the main components and the reaction rate models of the independent reactions, the net chemical reaction rate of Ti sponge is equivalent to
where r(Ji) is the net chemical reaction rate of chemical species Ji, in unit of kg m−2 h−1, M(Ji) is the relative molecular mass of chemical species Ji, in unit of kg mol−1, ri is the reaction rate of independent reactions IR-i, in unit of mol m−2 h−1, m(Ji) is the total amount of chemical species Ji, in unit of kg m−2, qm is the feeding rate of TiCl4, in unit of kg m−2 h−1, respectively.
Thus, we calculated the overall chemical reaction rate of Ti sponge by the implied mathematical relations of the total amount of gaseous TiCl4 and gaseous TiCl3 near the gas–liquid interfaces of magnesiothermic reduction of TiCl4 in the prototype Kroll reduction reactor, which derived in the Section S.2.4 and experimented in the Section S.3 in the electronic Supplementary Material.
Overall chemical reaction kinetics modeling
We, therefore, conclude that for this complex reaction system of magnesiothermic reduction of TiCl4, the overall chemical reaction kinetics model cannot be inferred from the stoichiometric equation, but must be determined experimentally. Let the overall chemical reaction rate be the functional relationship between the net chemical reaction rate of the Ti sponge product r(Ti) and the feeding rate of TiCl4 at a constant temperature and a constant pressure, which is defined as
where m(Ti) is the total amount of new-formed solid Ti sponge, in unit of kg m−2, k(Tin) and f(Δp) are the reaction rate constants that, respectively, be related to the temperature and pressure of the gas–liquid interfaces in the prototype Kroll reduction reactor, Tin is the temperature of the gas–liquid interfaces in unit of K, and Δp is the gauge pressure of the gas–liquid interfaces in the prototype reactor, in unit of kPa. The power α is called the overall reaction order of magnesiothermic reduction of TiCl4.
Experimental Works
Kinetics experiments by other researchers were usually carried out in a lab-scale batch reduction reactor, consisting of the temperatures and pressure measurements. The measuring equipment transferred most of the latent heat released by exothermic reactions to the environment. Thus, it is difficult to determine the influences of the pressure behaviors and thermal hazard on the kinetic modeling of magnesiothermic reduction of TiCl4, with low reliability of the experimental results. The present isothermal experiment carried out in a full-scale Kroll reduction reactor of a prestigious Ti sponge plant in Zunyi City, Guizhou, China.
Feedstocks and Reactants
The feedstocks used in this isothermal experiment, refined liquid TiCl4 with the minimum mass fraction purity of 99.96 pct, Mg ingots with the minimum mass fraction purity of 99.95 pct, and argon with the minimum mass fraction purity of 99.99 pct, are purchased by the Ti sponge plant, and the quality of the reactants should meet the requirements of Chinese standards, YS/T 655 for titanium tetrachloride[25] and GB/T 3499 for magnesium ingots,[26] while the chemical properties are presented in Tables S4 and S5 in the Section S.3.1 in the electronic Supplementary Material.
Isothermal Experiment and Experimental Procedure
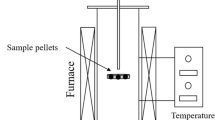
A prototype 12 tons Kroll reduction reactor system, with a capacity to produce 12 tons of Ti sponge per batch, is used to explore the pressure behaviors and kinetic characteristics, and to establish the overall chemical reaction kinetics model of magnesiothermic reduction of TiCl4, as shown in our previous work[27,28,29] or in Figure S1a in the Section S.3.3 in the electronic Supplementary Material.
The isothermal experimental system consists of a heat-resistant steel reduction reactor, 2200 mm in internal diameter and 5200 mm in height, and an electrical resistance furnace, measuring 3300 mm in internal diameter and 6100 mm in height, with a maximum power of 1050.00 kW. Titanium tetrachloride is fed into the reactor by nozzles on the lid of the reactor, and there are several other nozzles for argon injection and pressure control. The molten MgCl2 is periodically drained by a drain pipe at the bottom of the reactor. The gauge pressure inside the reduction reactor is maintained with the ranges of 9.00 to 15.00 kPa with the help of the injected argon and a bleeder valve on the lid of the reactor.[22]
The temperature of the gas–liquid interfaces is measured by the inner thermocouples (Typed K, class II, ranges 298.0 K to 1273.0 K) with an accuracy of 0.01 pct full scale that calibrated by the plant. The signals of the inner thermocouples, flow transmitter of the feeding rate of TiCl4, and the differential pressure transmitter of the gauge pressure are transferred to and recorded by the DCS system of the plant. The experimental data could be directly derived from the DSC system.
After each tapping of the molten MgCl2, the height level of the liquid Mg surface is measured and recorded. The gas–liquid interfaces of magnesiothermic reduction of TiCl4 is heated to the predetermined temperature quickly by the increased feeding rate of TiCl4. We begin the experiment. The initial conditions of the isothermal experiment are listed in Table I.
Due to the high exothermicity of magnesiothermic reduction of TiCl4, its feeding rate is decreasing to maintain the temperature Tin at each predetermined temperature. During the isothermal experiments, the periodic bleed-off and tapping of molten MgCl2 are not observed, therefore, the gauge pressure inside the reduction reactor is increasing. We terminate the isothermal experiment when the gauge pressure inside the reactor exceeded the threshold value, about 15.00 kPa, or the tapping of molten MgCl2 is needed.
Results and Discussion
Preliminary Experimental Results
Product (Ti sponge)
The isothermal experiments are carried out on a prototype 12 tons Kroll reduction reactor, while the quality of Ti sponge product can meet the requirements of the Chinese standards, GB/T 2524.[30] The main element analysis of Ti sponge product is obtained in percentage by ICP-ASE, and the details of Ti sponge product could be found in Table S7 in the Section S.4 in the electronic Supplementary Material.
Pressure behaviors
The isothermal experiments are carried out at different temperatures of the gas–liquid interfaces in the Kroll reduction reactor, ranging from 1053.0 K to 1123.0 K. Since all the chemical reactions of magnesiothermic reduction of TiCl4, TiCl3, and TiCl2 are highly exothermic, the highest temperature is always close to the gas–liquid interfaces that measured by the inner thermocouples. There would be an obvious increase in the temperature of the gas–liquid interfaces at an increased or fixed feeding rate of TiCl4, within a poor heat transfer in the Kroll reactor and weak heat dissipation of the air-cooling system. Meanwhile, a rapid rise in gauge pressure inside the semi-batch reactor may be caused either by the escaping of argon from the fed TiCl4, or the progressively rising of the height level of the gas–liquid interfaces due to the accumulation of the agglomerated products, or the accumulation of the unutilized gaseous TiCl4 and TiCl3 in the gas phase, showing in Figure 2(a). To stabilize the temperature and pressure in the semi-batch reactor, we gradually reduce the feeding rate of TiCl4 to slow down the chemical reaction rate, which could be found in Figure 2(b). The feeding rate of TiCl4 limits the chemical reaction process in appearance, but the heat and mass transfer of the TiCl4–Mg system limits that actually.[28]
Isothermal Kinetic Characteristics
It is necessary to establish the kinetic model of magnesiothermic reduction of TiCl4, basing on the elucidated reaction pathways and estimated kinetic parameters, to recognize the reaction mechanism of titanium tetra-, tri, and dichloride with Mg near the gas–liquid interfaces. All the individual confidence intervals of the estimated parameters are fitted at a confidence level of 0.95 (k = 2) in this section.
Net chemical reaction rate of Ti sponge
The calculated results of the net chemical reaction rate of Ti sponge at different temperatures of the gas–liquid interfaces are provided in Figure 3. It could be found that there is also a decreasing trend in the net chemical reaction rate of Ti sponge, which is similar to but differ from the trend of the correspondent feeding rate of TiCl4. The trend of the net chemical reaction rate of Ti sponge indicates that it also affected by the gauge pressure inside the reactor. It may be caused by the fact that the molar fraction of the gaseous components affected by the gauge pressure. This result also proves that the overall chemical reaction kinetic model of magnesiothermic reduction of TiCl4, Eq. [2], is reasonable and acceptable.
Reaction rate constant related to pressure f(Δp)
The chemical reaction rate constant related to temperature k(Tin) and the reaction order α could be regarded as constants at a defined temperature of the gas–liquid interfaces. The net reaction rate of Ti sponge r(Ti) is equivalent to
We calculate the chemical reaction constant related to gauge pressure f(Δp), and plot the results in Figure 4.
To effectively evaluate the kinetic parameters and model, we determine a function for the chemical reaction constant related to gauge pressure, based on the square root relationship between pressure and feeding rate of TiCl4 in the Bernoulli equation, with the brief format and a good fitness:
where pa is the ambient pressure and equal to 92.26 kPa in this experimental work.
Reaction rate constant related to temperature k(T in)
Based on the net chemical reaction rate of Ti sponge r(Ti) and the chemical reaction constant related to gauge pressure f(Δp), we estimate an exponential function, r(Ti)·f(Δp)−1, with a feeding rate of TiCl4 qm as an independent variable at each temperature of the gas–liquid interfaces, to obtain the reaction rate constant of k(Tin) values. Figure 5 provides the values of the exponential function, the reaction rate constant related to temperature, and the reaction order at each temperature. For each temperature, the experimental results are fitted quite well, suggesting that the chemical reaction kinetics model of magnesiothermic reduction of TiCl4 is reasonable and acceptable and describes well the concentration dependence of the net chemical reaction rate of Ti sponge and feeding rate of TiCl4. Thus, we have demonstrated that the established chemical reaction kinetics model, Eq. [2], could explain the reaction mechanism of magnesiothermic reduction of TiCl4 well at each temperature and gauge pressure.
The chemical reaction rate constants for the temperature range of 1053.0 K to 1073.0 K are much higher than ones for the temperature range of 1073.0 K to 1123.0 K. A similar conclusion that the exothermic reactions are more favorable in lower temperatures. Figure 5 also shows that the reaction order is α = 1.30 at temperature range of 1053.0 K to 1073.0 K, and the reaction order is α = 1.50 at temperature range of 1073.0 K to 1123.0 K. By comparing the reaction order at different temperature ranges, it could be considered that the exothermic reactions in magnesiothermic reduction of TiCl4 promote the temperature rising near the gas–liquid interfaces, which accelerates the chemical reaction and enhances its own exothermic effect.
Arrhenius equation and overall chemical reaction kinetics modeling
A large portion of the field of the chemical reaction kinetic modeling can be displayed by, or investigated in terms of the Arrhenius equation that related the reaction rate constant k(Tin) to the temperature Tin, and we have
where R is the gas constant, Ea is the activation energy with dimensions of kJ mol−1, and Λ is the pre-exponential factor, which has unit of k(Tin).
The goal of isothermal kinetic modeling is to calculate the reaction rate constants or to establish the overall chemical reaction kinetics equation, this is equivalent to determine Ea and Λ, which can yield from the slope and the intercept of the straight line in an Arrhenius plot of ln[k(Tin)] against 1/Tin, and are presented in Figure 6. The activation energies, Ea, and the pre-exponential factors, Λ, are also summarized in Table II.
The activation energy for the temperature range of 1053.0 K to 1073.0 K is a little larger than that for the temperature range of 1073.0 K to 1123.0 K. The energy barrier is lower on the magnesiothermic reduction of TiCl4 within homogeneous reaction in higher temperatures than that without homogeneous reaction. The autocatalytic effect of the sponge structure of the new-formed that provides more active sites for nucleation and growth of the subsequent Ti sponge may play an irreplaceable role.
Thus, the overall chemical reaction kinetic model of magnesiothermic reduction of TiCl4 can be expressed as:
For temperature range of 1053.0 K to 1073.0 K,
For temperature range of 1073.0 K to 1123.0 K,
Conclusions
To develop a better understanding of the pressure behaviors, thermal hazard, reaction pathways, and reaction mechanism of magnesiothermic reduction of TiCl4 in the semi-batch reactor for today’s Ti sponge metallurgy, we determined efficient and possible reaction pathways firstly based on a minimum set of independent reactions via generalized stoichiometry methodology, which consisted of the phase change near the gas–liquid interfaces, the homogeneous reaction in the gas phase, the heterogeneous reduction near the gas–liquid interfaces, and the like dissolves like of TiCl2 and new-formed Ti in the liquid phase.
Afterward, we carried out the isothermal experiments at different temperatures of the gas–liquid interfaces, from 1053.0 K to 1123.0 K, in a prototype 12 tons Kroll reduction reactor. The feeding rate of TiCl4 was gradually reducing to stabilize the temperature in that semi-batch reactor, and the gauge pressure inside the Kroll reactor had a rapid rising. There is also a decreasing trend in the net chemical reaction rate of Ti sponge, which indicates that it also affected by the gauge pressure inside the reactor.
Finally, we proposed an isothermal kinetic modeling approach via the net chemical reaction rate models of the main components and the reaction rate models of the independent reactions near the gas–liquid interfaces of magnesiothermic reduction of TiCl4. The reaction order and activation energy were α = 1.30 and Ea = 60.85 ± 4.58 kJ mol−1 at temperature range of 1053.0 K to 1073.0 K, respectively. The reaction order and activation energy were α = 1.50 and Ea = 56.46 ± 1.31 kJ mol−1 at temperature range of 1073.0 K to 1123.0 K, respectively. The self-heating of magnesiothermic reduction of TiCl4 causes its self-acceleration. The autocatalytic effect of the sponge structure of the new-formed that provides more active sites for nucleation and growth of the subsequent Ti sponge may play an irreplaceable role.
Supporting Information
The details of possible reaction and independent reactions via generalized stoichiometry methodology, net chemical reaction rate modelling of main components, reaction rate modelling pf independent reactions, experiment of gaseous component near the gas–liquid interfaces of magnesiothermic reduction of TiCl4, and the quality of Ti sponge product can be found in the Supporting Information.
References
O. Takeda and T.H. Okabe: JOM, 2019, vol. 71, pp. 1981–90.
D. Raabe, C. Cem Tasan, and E.A. Olivetti: Nature, 2019, vol. 575, pp. 64–74.
E. Platacis, I. Kaldre, E. Blumbergs, L. Goldsteins, and V. Serga: Sci. Rep., 2019, vol. 9, p. 17566.
F. Gao, Z. Nie, D. Yang, B. Sun, Y. Liu, X. Gong, and Z. Wang: J. Clean. Prod., 2018, vol. 174, pp. 771–79.
O. Takeda, T. Ouchi, and T.H. Okabe: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 1315–28.
N. Krauter, S. Eckert, T. Gundrum, F. Stefani, T. Wondrak, P. Frick, R. Khalilov, and A. Teimurazov: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2089–96.
Y. Kado, A. Kishimoto, and T. Uda: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 57–61.
W.H. Wang and F.Z. Wu: Front. Energy Res., 2021, vol. 9, p. 751781.
W.H. Wang, F.Z. Wu, Q.B. Yu, and H.X. Jin: Int. J. Heat Mass Transf., 2018, vol. 122, pp. 1308–12.
J.T. Jung and H.S. Sohn: J Korean Inst. Res. Recycling, 2017, vol. 26, pp. 54–60.
W.S. Zhang, Z.W. Zhu, and C.Y. Cheng: Hydrometallurgy, 2011, vol. 108, pp. 177–88.
R.N. Roux, E. Van der Lingen, and A.P. Botha: S. Afr. J. Ind. Eng., 2019, vol. 30, pp. 115–33.
A.I. Putilin: JOM, 2011, vol. 63, pp. 66–68.
Ch.R.V.S. Nagesh, T.S. Sitaraman, C.S. Ramachandran, and R.B. Subramanyam: Mater. Sci., 1994, vol. 17, pp. 1167–79.
J.C. Lee, H.S. Sohn, and J.Y. Jung: Korean J. Met. Mater., 2012, vol. 50, pp. 745–51.
O. Takeda and T.H. Okabe: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 823–30.
O. Takeda and T.H. Okabe: J. Alloy Compd., 2008, vol. 457, pp. 376–83.
Ch.R.V.S. Nagesh, C.S. Ramachandran, and R.B. Subramanyam: Trans. Indian Inst. Met., 2008, vol. 61, pp. 341–48.
W.H. Wang, F.Z. Wu, and H.X. Jin: ACS Omega, 2020, vol. 5, pp. 18573–78.
T.H. Okabe, T. Uda, E. Kasai, and Y. Waseda: J. Jpn. I, 1997, vol. 61, pp. 610–18.
Rafael Bolivar and Bernd Friedrich: J. Sustain. Metall., 2019, vol. 5, pp. 219–29.
Ch.R.V.S. Nagesh, Ch.S. Rao, N.B. Ballal, and P.K. Rao: Metall. Mater. Trans. B, 2004, vol. 35B, pp. 65–74.
J.-C. Paniagua-Rodríguez, G. Jiménez-García, and R. Maya-Yescas: Energy Fuel, 2011, vol. 25, pp. 4070–76.
A. Fuwa and S. Takaya: JOM, 2005, vol. 57, pp. 56–60.
Ministry of Industry and Information Technology of China, Titanium tetrachloride. http://hbba.sacinfo.org.cn/stdDetail/97d9063e42ae3f82764f164cb8fc5280
State Administration for Market Regulation, Standardization Administration, Magnesium ingots. https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=4C3816A5D3559B0DD4981B47D3A11E9B
W.H. Wang and F.Z. Wu: Int. J. Exergy, 2017, vol. 22, pp. 89–101.
W.H. Wang, F.Z. Wu, and H.X. Jin: Heat Mass Transf., 2017, vol. 53, pp. 465–73.
W.H. Wang, F.Z. Wu, and Q.B. Yu: Russ. J. Non-ferrous Met., 2017, vol. 58, pp. 258–68.
State Administration for Market Regulation, Standardization Administration, Titanium Sponge. http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=50E68BAFD235A21C7FBD0F84B6963E42
Acknowledgments
The author acknowledges the financial support received from the Innovative Development Institute for Carbon Peak & Neutrality and Renewable Energy Technology of Guizhou Province [Grant No. DCRE-2023-07]. The authors are also grateful to senior engineers Qiang Liang, Jinze Li and Lvguo Zhang of Zunyi Titanium Co., Ltd for their valuable discussions and advice.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Wu, F. Pressure Behaviors and Isothermal Kinetics of Magnesiothermic Reduction of Titanium Tetrachloride in a Semi-batch Reactor. Metall Mater Trans B 55, 1167–1175 (2024). https://doi.org/10.1007/s11663-024-03043-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-024-03043-z