Abstract
In this study, a novel, simple method has been used for fabricated of Au:TiO2 nanoparticles. The manufacture consisted of two steps: first, ablating a gold (Au) target immersed in CTAB solution to produce colloidal Au NPs and then inserting a titanium (Ti) target in the solution to prepare Au:TiO2 NPs via laser ablation in liquid (LAL) at various laser energies. Then, it was placed on porous-Si (PS). PS is made by etching n-type crystalline c-Si wafers by photo-electrochemical etching (PECE). The XRD, TEM, AFM, PL analyses were employed to characterize the samples. Lastly, the impact of varying operation temperature of hydrogen sulfide (H2S) and nitrogen dioxide (NO2) gas sensors fabricated from prepared specimens on the sensors sensitivity, response time, and time to recover was explored. We found the greatest sensitivity of Au:TiO2 NPs/PS when ablated at 1000 mJ. The synthesized Au/TiO2 NPs thin films show high sensitivity 94.12% and 42.69% with fast response and recovery of H2S and NO2 gas at for low concentration 12.6 and 64.5 ppm, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nano-materials are used to manufacture devices such as gas sensors, photo-detector, and solar cell, due to the quantum confinement effect, low cost, easy fabrication, large active area, and charge transport [1,2,3,4].
Gas sensor is an instrument that detects the presence of various gases in an area, particularly those that are potentially dangerous to humans and animals. In recent years, the development of gas sensor technologies for monitoring environmental contamination has gotten a lot of attention [5].
The characteristics of the sensing materials used are well recognized to influence chemical gas sensor performance parameters such as selectivity, temporal response, sensitivity, stability, durability, and repeatability [6]. The specific surface of sensing materials has a significant impact on chemical gas sensor sensitivity. The sensor sensitivity increases as the specific surface of the detecting material increases [7,8,9]. Semiconductor and metallic nanoparticles gas sensors continue to play an important part in their applications [10]. Because of their low cost, distinctive structure, ease of production, and outstanding physicochemical characteristics, transition metal oxide semiconductor substances like TiO2, ZnO, and CuO are a potential class of sensors [11,12,13,14].
TiO2 is an n-type semi-material with a high resistance and a band gap of roughly 3.2 eV. It has gained a lot of attention for its use in gas sensors, photo-catalysis, and solar cells [10, 15]. This semiconductor of n-type has been investigated to employ in the sensing of H2S; it is produced in significant amount from both human and natural processes, particularly in crude oil refineries with the extraction of acid natural gas [17,18,19].
Inhaling H2S has been demonstrated to have significant health consequences on the respiratory system; also, H2S poisons the human body and can cause death at concentrations greater than 250 ppm [20, 21].
Other desirable characteristics of TiO2 include its strong photocatalytic activity, superior chemical and physical stability, ease of oxygen adsorption on its surface, ease of preparation, and low cost. Because of its excellent application stability, TiO2 offers a lot of potential for NO2 gas sensing [22].
NO2 is a harmful air pollutant for plants and the respiratory systems of humans and animals. Furthermore, NO2 emissions have resulted in major environmental issues such as acid rain and photochemical smog [23, 24]. The development of inexpensive, small, sensitive, and reliable gas sensors to monitor and manage NO2 gas concentrations from automobiles and industrial processes is critical to preserve human life [25, 26].
Laser ablation of large materials in a liquid medium is a common, simple, and cost-effective method for formation nanoparticles [27, 28]. Throughout the laser ablation in solution approach, a high-power laser pulse was focused on the face of a bulk object submerged in a solution. Ionization, atomization, and decomposition of the target are all caused by irradiation [27, 29].
In this study, Au:TiO2 NPs can be combined using laser ablation in a liquid medium, after that deposited on porous-Si, employed for gas sensor applications.
Experimental details
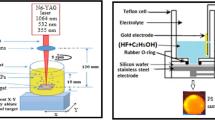
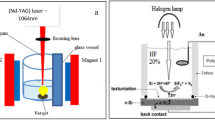
The Au:TiO2 NPs were made using the laser ablation process: firstly we put the plate of gold in the bottom of a glass container which filled by 3 mL of CTAB solution. Ablation of plate was carried out by a single 100 pulse at 1064 nm wave length with laser energy 600, 800 and 1000 mJ. Following the ablation technique for validating Au NPs generation, a Ti target was placed in a glass vial containing an Au NPs solution and ablated by the same condition of preparation Au NPs, after that the Au:TiO2 NPs colloids solution was obtained.
Secondly, we formed PS by using the PECE method [30] from n-type silicon wafer with resistivity of 1.5–4 Ω.cm. PEACE has been obtained via etching a silicon plate in 16 percent HF (hydrofluoric acid) as the electrolyte for 15 min at a current density of 12 mA/cm2 and illuminating with a halogen beam. In the last step, Au:TiO2 suspension dropped on this PS.
Results and discussion
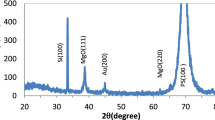
The phases and grain size are determined via XRD analysis. XRD pattern for the examined Au:TiO2 specimen, which was generated via PLAL in CTAB solution at 800 mJ laser energy and then deposited on porous-Si substrate, is illustrated in Fig. 1. The XRD structure of the sample shows a strong peak of x-ray diffracted from the Si substrate at 2θ = 69◦. The XRD peaks for Au:TiO2 NPs can be identified to (fcc) Au (JCPDS card No. 002–1095) and anatase TiO2 (JCPDS card No. 21–1272).
The peaks were observed at 2θ = 34.05°, 44.4° correspond to the (110) and (200) planes of the cubic crystal of Au NPs, respectively. The TiO2 NPs' XRD shows two distinct peaks at 37.28° and 62. 9°, which correspond to planes (004) and (204), respectively.
The structural characterization of the PS, Au:TiO2 NPs/PS samples was analyzed using AFM as illustrated in Fig. 2. The surface of PS has a sponge-like structure with average diameter of 40.33 nm and average roughness of 24 nm as shown in Fig. 2A.
Figure 2B depicts Au:TiO2 NPs completely filling or entirely covering PS pores. This is due to the surface PS layer's like-sponge morphology with a large surface region and a pores, which makes PS an adhesive substrate for allowing Au:TiO2 NPs to enter its pores. As a result, the Au:TiO2 NPs behaved like a transparent capping, which also given good coverage of a PS substrate, potentially improving the PS substrate's structural strength. The average roughness and diameter for Au:TiO2 NPs/PS particles are shown in Table 1.
Figure 3 shows TEM images of Au:TiO2NPs. Laser ablation with a laser energy of 800 mJ/pulse was used to generate Au:TiO2 NPs. Au:TiO2NPs, which are virtually spherical shape, with different in size from 7 to 55 nm, as can be observed. The creation of the core shell structures is confirmed by complementary contrast in TEM images. The Au NPs were responsible for the black core, whereas the TiO2 shell was responsible for the grey color.
Photoluminescence (PL) studies provide knowledge on distinct energy states available between valence band and conduction band responsible for irradiative recombination. The PL spectra of Au:TiO2 NPs prepared by laser ablation in ethanol solution deposited on PS substrate are shown in Fig. 4. The intensity of the photoluminescence spectra illumination of 602 nm is shown in Fig. 4, whereas the blue shift in a band gap depending on the Si wafer has been seen, because the last comes from quantum confinement effects (QCEs). The PL spectrum at room temperature for specimens Au:TiO2 NPs/PS prepared PL bands at 350 – 550 nm on PS. The PL gave three peaks that were observed after the deposition of Au:TiO2 NPs as compared to PS. Photoluminescence emission peaks at 417 nm (2.97 eV) which matched to the an anatase TiO2 NPs at 497 nm corresponding to band edge of 2.5 eV for Au NPs PL spectral locations.
The quantum size effects from the Au:TiO2 NPs are responsible for the significant blue-shift in the sharp peaks of plasmon absorption.
The sensor sensitivity is stated as (S = (Ro-Rg) / Rg), where Rg represents the sensor resistance when exposed to a test gas and Ro denotes the sensor resistance while exposed to air. Figure 5 displays the sensitivity of Au:TiO2 NPs/PS thin prepared with the previously mentioned conditions by using the LAL technique for NO2 and H2S gases as a function of operating temperature. The figure illustrates that as the working temperature increases in the scope 30 – 300 °C, the sensitivity of H2S and NO2 gases increases. The Au:TiO2/PS thin film at 1000 mJ have greater sensitivity for H2S and NO2 with temperature of 250 – 300 °C. The Au:TiO2/PS films exhibit gradual raise in gas sensitivity, reaching a maximum sensitivity about 42.69% at 300 °C of 64.5 ppm NO2 gas responsivity.
Similar results are achieved when H2S is employed as the investigating gas: the optimum sensitivity of the Au:TiO2/PS film at 1000 mJ gas sensor to 12.6 ppm of H2S may achieve at 94.12%, and the optimal sensor temperature of the Au:TiO2/PS sensing is around 250° C. Tables 2 and 3.
There are difference in reaction times and recovery period for various laser ablation energy as a function of working temperature. The time recovery is the time that it takes for the specimen to back to its initial state, in other words the specimen state before pumping the gas, and the response time seems to be the time it takes for the specimen to respond to the gas.
The response and recovery cycles of Au:TiO2 NPs toward 64.5 ppm NO2 and 12.6 ppm H2S, air mixed ratio have been explored. The results are shown in Fig. 6. Response and recovery times of devices were calculated and are indicated in Tables 2 and 3.
Conclusions
In this works, laser ablation of Au:Ti target immersed in (CTAB) solution is a promising and environmentally friendly method for preparing Au:TiO2NPs. As deduced by their XRD and TEM analysis and AFM properties were employed to characterize the samples. The enhancement in sensitivity of gas sensor increases, with increases the laser energy used to ablate an Au:TiO2 nanoparticles deposited on PS. The gas sensor should be highly selective when it comes to analytic gas. As a result, we evaluated an Au:TiO2 NPs/PS thin film gas sensor for various gases at various concentrations, including H2S and NO2. The Au:TiO2 NPs/PS thin film sensor has a better response to H2S gas, with a response of 94.12% when exposed to 12.6 ppm H2S.
References
N. Abdulkhaleq, U. Nayef, A. Albarazanchi, MgO nanoparticles synthesis via laser ablation stationed on porous silicon for photoconversion application. Optik 212, 164793 (2020)
H. Hussein, U. Nayef, A. Abdul Hussien, Synthesis of graphene on porous silicon for vapor organic sensor by using photoluminescence. Optik 180, 61–70 (2019)
N. Abdulkhaleqa, A. Hasan, U. Nayef, Enhancement of photodetectors devices for silicon nanostructure from study effect of etching time by photoelectrochemical etching Technique. Optik 206, 164325 (2020)
R. Jamal, F. Mutlak, F. Ibrahim, U. Nayef, Synthesis of Ag2O films by pulsed laser deposited on porous silicon as gas sensor application. Optik 218, 164971 (2020)
D. Jwied, U. Nayef, F. Mutlak, Synthesis of C: Se (core:shell) nanoparticles via laser ablation on porous silicon for photodetector application. Optik 231, 166493 (2021)
U. Nayef, I. Khudhair, Study of porous silicon humidity sensor vapors by photoluminescence quenching for organic solvents. Optik 135, 169–173 (2017)
N. Abdulkhaleqa, A. Hasan, U. Nayef, Enhancement of photodetectors devices for silicon nanostructure from study effect of etching time by photoelectrochemical etching Technique. Optik 206, 163 (2020)
H. Abid, U. Nayef, F. Mutlak, Preparation and characterization Co3O4 nanoparticles on porous silicon for humidity sensor by photoluminescence. Optik 178, 379–383 (2019)
Z. Jin, H. Zhou, Z. Jin, R. Savinell, C. Liu, Application of nano-crystalline porous tin oxide thin film for CO sensing. Sens Actuat B-Chem 52(1–2), 188–194 (1998)
X. Tian, X. Cui, T. Lai, J. Ren, Z. Yang, M. Xi, B. Wang, X. Xiao, Y. Wang, (2021). Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater Sci, 5
U. Nayef, K. Hubeatir, Z. Abdulkareem, Ultraviolet photodetector based on TiO2 nanoparticles/porous silicon hetrojunction. Optik 12(5), 2806–2810 (2016)
S. Khudiar, U. Nayef, F. Mutlak, Preparation and characterization of ZnO nanoparticles via laser ablation for sensing NO2 gas. Optik 246, 167762 (2021)
P. Samarasekara, N. Kumara, N. Yapa, Sputtered copper oxide (CuO) thin films for gas sensor devices. J Phys :Condens Matter 18(8), 2417 (2006)
J. Bai, B. Zhou, Titanium dioxide nanomaterials for sensor applications. Chem Rev 114(19), 10131–10176 (2014)
X. He et al., Metal-organic frameworks derived C/TiO2 for visible light photocatalysis: simple synthesis and contribution of carbon species. J Hazard Mater 403, 124048 (2020)
T. Li et al., Anatase TiO2 nanorod arrays as high-performance electron transport layers for perovskite solar cells. J Alloys Compd 849, 156629 (2020)
N. Chinh, C. Kim, D. Kim, UV-light-activated H2S gas sensing by a TiO2 nanoparticulate thin film at room temperature. J Alloy Compound 778, 247–255 (2018)
A. Davoodi, M. Babaiee, M. Pakshir, Imitating seasonal temperature fluctuations for the H2S corrosion of 304L and 316L austenitic stainless steels. Metal Mater Int 19(4), 731–740 (2013)
A. Alonso-Tellez, D. Robert, N. Keller, V. Keller, A parametric study of the UV-A photocatalytic oxidation of H2S over TiO2. Appl Catal B Environ 115, 209–218 (2012)
S.K. Pandey, K.-H. Kim, K.-T. Tang, A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal Chemi 32, 87–99 (2012)
G.N. Chaudhari, D.R. Bambole, A.B. Bodade, P.R. Padole, Characterization of nanosized TiO2 basedH2S gas sensor. J Mater Sci 41(15), 4860–4864 (2006)
Z. Zhu, S.-J. Lin, C.-H. Wu, R.-J. Wu, Synthesis of TiO2 nanowires for rapid NO2 detection. Sens Actuat: A Phys 272, 288–294 (2010)
U.M. Nayef, R.I. Kamel, Bi2O3 nanoparticles ablated on porous silicon for sensing NO2 gas. Optik 208, 164146 (2020)
D. Jwied, U. Nayef, F. Mutlak, Synthesis of C: Se nanoparticles ablated on porous silicon for sensing NO2 and NH3 gases. Optik 241, 167013 (2021)
F.M. Liu, Y.H. Guan, H.B. Sun, X.M. Xu, R.Z. Sun, X.S. Liang, P. Sun, Y. Gao, G.Y. Lu, YSZ-based NO2 sensor utilizing hierarchical In2O3 electrode. Sens Actuator B: Chem 222, 698–706 (2016)
S.S. Shendage, V.L. Patil, S.A. Vanalakar, S.P. Patil, N.S. Harale, J.L. Bhosale, J.H. Kim, P.S. Patil, Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens Actuator B: Chem 240, 426–433 (2017)
B. Feizi Mohazzab, B. Jaleh, O. Kakuee, A. Fattah-alhosseini, Formation of titanium carbide on the titanium surface using laser ablation in n-heptane and investigating its corrosion resistance. Appl Surf Sci 478, 623–635 (2019)
M. Jabir, U. Nayef, W. Abdulkadhim, Z. Taqi, G. Sulaiman, U. Sahib, A. Al-Shammari, Y. Wu, M. El-Shazly, C. Su, Fe3O4 nanoparticles capped with PEG induce apoptosis in breast cancer AMJ13 cells via mitochondrial damage and reduction of NF-κB translocation. J Inorg Organomet Polym Mater 313, 1241–1259 (2021)
A. De Giacomo, M. Dell’Aglio, A. Santagata, R. Gaudiuso, O. De Pascale, P. Wagener, G.C. Messina, G. Compagnini, S. Barcikowski, Cavitation dynamics of laser ablation of bulk and wire shaped metals in water during nanoparticles production. Phys Chem Chem Phys 15(9), 3083–3092 (2013)
R. Kamel, D. Ahmed, U. Nayef, Synthesis of Bi2O3 nanoparticles by laser ablation on porous silicon for photoconversion application. Optik 193, 163013 (2019)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sulaiman, E.M., Nayef, U.M. & Mutlak, F.A.H. Structural, Morphological, Photoluminescence, and sensitivity of Au:TiO2 nanoparticles via laser ablation on porous silicon. J Opt 52, 1621–1627 (2023). https://doi.org/10.1007/s12596-022-00930-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-022-00930-z