Abstract
This article describes the synthesis of 4,4′-methylenediphenyl diisocyanate (MDI) based polyurethane-ureas-amide using diamine-diamide chain extenders and dihydroxy polystyrene (PSt) having a weight average molecular weight of 2000 g/mol. The weight average molecular weight of the soft segment is varied from 2000 to 10,000 g/mol using MDI as a chain extender and thereby changing the hard segment content from 30 to 17 wt% in the copolymer. The inherent viscosity of the polymer is found to be in the range of 1.6–3.4 dL/g is suggesting that the polymer is of high molecular weight. FT-IR results conclude that the urea groups form monodentate assemblies. DSC data show the peaks for Tg of soft and Tm of hard segments. Depending on the amide concentration, the melting temperature of the polymer varies from 189 to 249 °C. Also, at high concentration of the hard segment, the cooling curve shows crystallization exotherm peak. The entire series of polymer shows single stage decomposition temperature centered around 400 °C shown by the TGA measurement. The solubility of the polymer in chloroform and swelling ratio is depending on the concentration of the hard segment. The studied polymer shows excellent solvent resistivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane (PUs) comes under thermoplastic elastomers consists of hard and soft segments. In general, the polyols forms the soft segment and the isocyanate along with the chain extender will form the hard segment [1, 2]. The hard and the soft segments are distributed alternating in the polymer chain. Among different types of polyurethanes, linear segmented polyurethanes are of importance since their physical and chemical properties can be easily tailored by changing the chemical composition, segment molecular weight, etc. [1]. The segmental structure of these polyurethanes, with alternating hard and soft segments along the backbone, offers control of unique morphologies and properties. The hard segments, which are of relatively low content, can organize to form domains. These hard domains are dispersed in the soft matrix and act as physical or virtual crosslinks. The soft segment regions can benefit mechanical properties at low temperature [2]. Despite numerous studies focusing on preparation and characterization of new polyurethanes, improvements in polyurethane properties, such as mechanical properties, still receive considerable attention due to their scientific implications and commercial applications are kept on expanding [3]. The most common approach to prepare high performance polyurethanes is by incorporating unique chemical structures into their polymer chains to tailor various properties for specific applications [4].
Polystyrenes are come under high Tg (100 °C) materials which finds lots of applications in electrical, electronics, medical, home application, packaging industry, etc. The main disadvantage of the amorphous polystyrene is due to poor solvent resistance and environmental stress cracking. This disadvantage is often eliminated by blending polystyrene with a high melting semi-crystalline material at a higher quantity (>40 wt%) [5, 6]. Apart from this, the amorphous polystyrene also modified by reacting with diisocyanates to form semi-crystalline polyurethane. This modification will lead to a semi-crystalline copolymer and it found to have both amorphous soft segment (SS) and crystalline hard segment (HS) phases with different chemical entities. However, the resultant polyurethane often has a low crystallization window which severely hampered the crystallization rate of the HS and there crystallinity [7].
PUs derived from various diisocyanates and chain extenders can result in different morphological structures and physical properties. Chu and coworkers investigated the dependence of microphase separation on the flexibility of diisocyanates [8, 9]. They observed that the 4,4′-methylenebis(phenyl isocyanate) (MDI) gave rise to a partial microphase-mixed morphology, while 1,6-hexamethylene diisocyanate (HDI) resulted in increased microphase separation. Due to the larger solubility parameter difference between MDI and soft segments such as poly(tetramethylene glycol) (PTMG) or polycaprolactone (PCL), thermodynamic effects suggest a more distinct microphase separation for the MDI based polyurethane. However, greater mobility, introduced by the incorporation of flexible HDI can enhance microphase separation, which suggests that microphase separation is at least also greatly influenced by kinetic factors such as hard segment mobility rather than purely thermodynamic factors. Lee et al. [10] studied the comparison among polyurethanes based on a series of diisocyanates. They found that the hard segments containing aromatic diisocyanates such as toluene diisocyanate (TDI) and MDI have been normally more miscible with the PTMG soft segments than the aliphatic diisocyanates such as isophorone diisocyanate (IPDI) and HDI. It is also reported that chain extenders can be specially designed to impart particular chemical and physical properties to the polyurethanes [11, 12].
Changa et al. synthesized segmented polyurethanes based on polytetramethylene glycol (PTMG) of 1000 g/mol by adopting a two-step polymerization procedure. In his study, the polyurethane is synthesized using HDI or MDI as a diisocyanates part along with the chain extenders like hydroquinone bis(2-hydroxyethyl)ether (HQEE) or triptycene-1,4-hydroquinone bis(2-hydroxyethyl)ether (TD) [13]. He et al. [14] studied the polyureas prepared using three different diisocyanates units. From his studies, he concluded, is that the symmetric diisocyanate structures facilitated much better self assembled ribbon-like structure of hard segments and which is driven by strong bidentate hydrogen bonding. A recent investigation of our group reveals that the polyurethane urea made from ethylene diamine as a chain extender showed much better crystallinity than the hexamethylen diamine counterpart. This shows that as the chain length increases, the crystallinity of the PUU decreases [15]. Also, the synthesized PUUs are showing moderate solvent resistivity [16].
In general, introducing the amide groups into the PUs will lead to increase in the crystallinity due to high thermal stability of amide than urethane groups and therefore the amide-interchange reactions hardly occurs at high temperature in the melt polymerization reactions [17]. van der Schuur et al. studied segmented polyurethane amide (PUA) based on poly(propylene oxide) end capped with 2,4-toluene diisocyanate (TDI2,4) and diamine-amide extenders (nylon-6,T). These PUAs block copolymers showed similar results with the previously studied polyether amides with uniform amide segments based on nylon-6,T. Also, in his paper he compared the properties of the PUAs copolymers with a commercially available polyether-urethane (TPU) [18].
Therefore, it would be interesting to analyze that whether the high melting and fast crystallizing uniform di-amide units will be able to crystallize in a high Tg PUUAs system. The present work deals with the synthesis and characterization of poly(urethane-urea-amide) prepared using MDI and PSt with two different diamine-diamide chain extenders namely, 6A6 or 6T6. The overall goal of this work is to make a semi-crystalline copolymer using polystyrene and crystallizable hard segments. Amorphous polystyrene is known for their poor solvent resistivity but the synthesized PUUAs with MDI are expected to show excellent solvent resistivity. Also, this paper compares the properties of the PUUAs copolymers derived from 6A6 and 6T6 are compared.
Experimental
Materials
4,4′-methylenediphenyl diisocyanate (MDI), phenol and 1,1,2,2-tetrachloroethane, dibutyltindilaurate (DBTDL) and dimethylsulfoxide-d6 are purchased from Aldrich Chemicals Co (USA). Tetrahydrofuran, toluene, methanol, dimethylacetamide (DMAc) are purchased from S.D fine chemicals (India) and used after distillation. The functionalized hydroxy terminated polystyrene (OH–PSt–OH) is synthesized using the known route [19]. The 6T6 and 6A6 diamine-diamide are synthesized using the known route [20].
General procedure for the synthesis of block copolymer (scheme 1)
PUUAs are synthesized using various molar ratios of soft and hard segments. The two different chain extenders used in this study are 6A6 or 6T6 (Fig. 1; Table 1). The generalised synthetic routes of the PUUAs are presented in the scheme 1. The preparation procedure for the copolymer A1 is given here as an example. For other polymer systems, a similar procedure is adopted. The reaction is carried out in a 250 ml stainless steel vessel fitted with a nitrogen inlet and a mechanical stirrer. 6 g of PSt2000 (0.003 mol) and 1.5 g (0.006 mol) of MDI dissolves in 20 ml of DMAc were charged into the reactor. 2–3 drops of DBTDL are added as a catalyst and the content was heated to 80 °C for 3 h. After 3 h, 1.03 g (0.003 mol) of 6A6 dissolved in 20 ml of DMAc is added and the temperature was subsequently raised to 150 °C over a time period of 2 h. The solvent DMAc is removed by vacuum distillation and the resultant copolymer is dried overnight in vacuum at 70 °C for lateral use. All the copolymers are transparent. A similar procedure is adopted for the synthesis of polymer series A1 to A4 and T1 to T4. Yield: 9.1 g (88 %). 1H NMR (DMSO-d6, δ, ppm): 6.5–7.5 (b, aromatic proton), 5.1 (b, >NH), 3.4–3.7 (m, –CH 2 – connected to urea and amide group), 3.1 (b, –CH 2 – connected to urethane group) 1.1–1.8 (b, aliphatic proton).
For T series polymers: 1H NMR (DMSO-d6,δ, ppm): 6.3–8.0 (b, aromatic proton), 5.2 (b, > NH), 3.3–3.7 (m, –CH 2 – connected to urea and amide group), 3.0 (b, –CH 2 – connected to urethane group) 0.9–1.7 (b, aliphatic proton).
Fourier-transform infrared spectroscopy
Alpha Bruker FT-IR (Germany) instrument with a resolution of 2 cm−1 is used for IR measurements. Samples were prepared by adding a droplet of a polymer solution [HFIP (1 g/L)] on a pressed KBr pellet and the measurements was carried out at room temperature. These conditions allow the polymer films to have a stable morphology with desirable properties.
Inherent viscosity measurements
The inherent viscosity of the copolymers at a concentration of 0.1 g dL−1 in a 1:1 (molar ratio) mixture of phenol/1,1,2,2-tetrachloroethane is determined at 25 °C by using a capillary Ubbelohde.
where t elution time of the polymer solution, t0 elution time of the solvent, c concentration of the polymer solution (g/dL).
Nuclear magnetic resonance
1H NMR spectra of the copolymers are run on a Bruker FT-NMR spectrophotometer (Germany) operating at 320 MHz at room temperature using DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal reference.
Differential scanning calorimetry
Perkin Elmer DSC 7 apparatus (USA) equipped with a PE 7770 computer and TAS-7 software is used for the DSC measurements. The polymers second heating and first heating curves were used to evaluate the Tm and Tc of hard segment respectively. About 10–15 mg of dried copolymer sample is heated at a rate of 20 °C/min in the nitrogen atmosphere for recording DSC spectra.
Thermo gravimetric analysis
Dupont 951 thermo gravimetric analyser (USA) is used for measuring the thermal stability of the synthesized polymers. About 8–10 mg of the sample is heated from 30 to 600 °C at the rate of 10 °C/min in a nitrogen atmosphere.
Swelling ratio of polymer
The swelling behavior of the polymer is estimated using the equilibrium water absorption (WA) method. Pieces of injection-moulded polymer bars are placed in desiccators with a layer of demineralised water for 4 weeks at room temperature. The water absorption is defined as the weight gain of the polymer according to Eq. 2.
where, m is the weight of the dry sample and m0 the weight of the sample after conditioning to equilibrium.
Solvent resistivity
Previously weighed bar samples of diamensions 10 × 10 × 2 mm are dipped in 50 ml of organic solvents taken in a flask and shaken for 1 h. After that, the solvent is decanted and the flask is dried for 24 h at 70 °C and weighed. From the weight loss, the weight of polymer sample dissolved in that solvent is calculated.
where, mo and m is the weight of dry the substance (mg) before solvent treatment and the weight of the substance (mg) after solvent treatment respectively.
Results and discussion
Synthesis of poly (urethane-urea-amide)
A two-step poly addition reaction is used for the preparation of PUUAs. The first step of the reaction involves the prepolymer synthesis. One mole of hydroxyl terminated polystyrene [soft segment (SS)] and two moles of aromatic diisocyanate (MDI) were reacted to yield MDI endcapped polystyrene. This first step reaction produces urethane linkage. The isocyanate endcapped polymer is further reacted in the second step with different diamine-diamide chain extender [6A6 (A series) or 6T6 (T series)] to yield PUUAs. The molecular weight of the polymer is thereby increased in the second step. The chemical structures of the two diamine- diamide extender used in the PUUAs preparation are given in the Fig. 1. The synthetic route of the PUUAs is shown in the Scheme 1. The synthesized compounds are characterized by FT-IR and NMR techniques. The NMR data confirm that the presence styrene, urea and urethane linkage. The appearance of the peaks around 3.3–3.6 ppm, which is due to the CH2 group connected to the amide group clearly proves that the hard segment, i.e., diamine -diamide (6A6 or 6T6) is present in the polymer chain and thereby increases the molecular weight of the PUUAs. However, the NMR spectrum of the PUUAs are not a conclusive method for determining the constituents of the polymer chain due to the fact that it gives only the presence of protons in different environments. Therefore the combination of different technique like, NMR, inherent viscosity measurement, IR, etc. will give a fair idea about the structure of the PUUAs.
Inherent viscosity measurements
The molecular weight of the polymer is determined by the inherent viscosity measurement using an equimolar solution of phenol/1,1,2,2-tetrachloroethane. The hydroxyl terminated polystyrene showed the inherent viscosity of 0.1dL/g. Table 1 shows the inherent viscosity data of the polymer and Fig. 2 shows the correlation between SS molecular weight to the inherent viscosity of the polymers. In both the series, the inherent viscosity of the polymer is linearly increasing with the increase in the repeating unit molecular weight. The inherent viscosity of A and T series polymer is about 1.6–3.2 and 1.8–3.4 dL/g respectively. This increase in inherent viscosity along the series is due to increase in the molecular weight polymer.
FT-IR
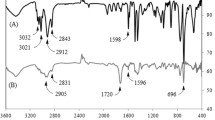
FTIR spectroscopy is a well-known tool for investigating the state of hydrogen bonding in polyurethanes and polyureas. The NH groups of the urea serve as proton donors while the acceptor groups include the carbonyl group of urea and ether oxygen present in the soft segment. Four types of NH absorbance generally appear namely, free NH, ordered hydrogen bonds (some degree of order or regularity), disordered hydrogen bonds and ether oxygen hydrogen bonded NH groups [21]. The FTIR spectrum of the polymers A1 and T1 is shown in Fig. 3. The FTIR data are shown in the Table 2. The amide (NH) structure in urethane/urea linkages was observed at 3288–3295 cm−1 and the peaks between 1726 and 1732 cm−1 correspond to the free urethane carbonyl groups. The peak around 1632–1645 cm−1 correspond to the H-bonded urea carbonyl groups. C–N–H bending in the urethane linkages is observed around 1545 cm−1. The 2200–2400 cm−1 region remained clear, demonstrating that no unreacted isocyanate groups present in the PUUAs. The NH group in polyurethane could form hydrogen bonding with the carbonyl oxygen group. The presence of hydrogen bond acts as physical cross-links leading to restricted segmental motion and as a result, it shows an excellent phase separation between the hard and soft segments. The phase separation improves the mechanical properties of polyurethanes but reduces the flexibility and solubility [22]. But it was very challenging to determine the crystallinity of the synthesized PUUAs using FTIR spectroscopy due to the overlap of the peaks of crystalline urethane and amorphous urea.
DSC
The structures of the PUUAs further confirms through the DSC technique. Figure 4 shows the heating and cooling curve DSC curve of the sample A1 and T1. The data are summarized in the Table 3. The heating curve of the PUUAs shows Tg of soft and Tm of hard segment. Remarkably, the cooling curve of PUUAs, T1 shows crystalline exotherms which is due to the presence of uniform crystallisable amide segments incorporated in the polymer chain. This uniform crystallisable amide segment crystallises so well and prevents trans urethane reaction at elevated temperatures. The glass transition temperature (Tg) of the T series polymer series starts at 62 °C (for T1) and ends at 59 °C (for T4). Similarly, the Tg value of A series polymers starts at 64 °C (for A1) and ends at 61 °C (forA4). The Tg value of the aromatic diisocyanate (MDI) based PUUAs is lower than the PUUAs based on aliphatic diisocyanate like HMDI [23]. Also, the Tg value of the MDI based PUUAs is very close to the Tg value of the pure polystyrene (PSt) (58 °C) which is clearly shown that the MDI present in the hard segment will facilitate better phase separation than the HMDI counterpart. The relationship between the Tg to that of the repeating length of the soft segment is shown in the Fig. 5 and it shows that the Tg decreases as the length of hard segment decreases.
Melt transition (Tm) of the PUUAs of T series is started at 249 (for T1) and ends at 240 °C (for T4). The decrease in the melting temperature is due to decrease in the hard segment content as you move on from T1 to T4 in the series. Interestingly, the A series PUUAs showed a low melting temperature ranged between 189 (for A1) to 183 °C (for A4). The presence of aromatic ring (see the Fig. 1) in the T series PUUAs provides much better thermal stability to the polymers. The cooling curve of the DSC showed the crystallization exotherm (Tc) value of T1 at 215 °C. Similar to the Tm (melting of hard segments) peak, the Tc peak for the T1 is sharp and showed a low Tm–Tc value, suggesting that these copolymers have low under-cooling values and this intern suggests that the monodispersed crystallizable rigid segments crystallized very fast. However, the cooling curves of the A1 polymer not shown any cooling exotherms.
TGA
Figure 6 shows the TGA curves of the polymer samples A1 and T1 and the data are presented in the Table 3. The initial decomposition temperature is taken as the onset point. The decomposition temperature of copolymer based on 6A6 and 6T6 is centered around 405 °C and 395 respectively. In general, the synthesized PUUAs showed single stage decomposing temperature centered around 400 °C, which clearly shows a high thermal stability of the synthesized block copolymers. The thermal stability of the PUUAs in a series slightly increases with increase in hard segment content which is due increase in the rigidity of the system. MDI-based systems are found to have good thermal stability due to the highly rigid structure of MDI [24].
Swelling ratio of polymer
The effect of hard segment content on the water absorption is presented in the Fig. 7 and the data are presented in the Table 3. PUUAs are semi crystalline and therefore physical crosslinks present between the chains. The physical crosslinking density depends on the hard segment content. This physical crosslinking point prevents the water molecule to penetrate through the soft matrix. Therefore, we can expect the decreases in swelling ratio upon an increase in hard segment. However, these water molecules can also interact with functional groups of material thereby increasing swelling behaviour. This type of functional group interaction can occur when the PUs are exposed to water extensive moisture atmosphere [25]. The penetrated water molecules can involve in the hydrogen bonding and thereby increasing the chain mobility. And therefore the water absorption of the PUs is a very complicated system. The water absorption value of the A series polymers is higher than the T series due to low crystallinity or high hydrophilic character of A series polymers. Comparatively the swelling ratio value is less in the case of aromatic diisocyanate (MDI) based PUUA than to aliphatic diisocyanate (HMDI) [23].
Solvent resistivity
The crystalline regions are not easily dissolved and are physical network points that give the dimensional stability. The PSt modified with hard segments is semi-crystalline materials. The solvent resistivity data are presented in the Table 3. The amorphous PSt dissolved in chloroform within 1 h to a large extent, whereas the PUUAs showed a dramatic lower weight loss in chloroform. The solubility decreases with increasing HS concentration which is presented in the Fig. 8. The phase separation between the soft and hard segment improves the mechanical properties of polyurethanes but reduces the chain flexibility and solubility [22]. Even with very small amounts of HS, the solvent resistance of the copolymer are improved strongly due to the monodispersed character of the HS which is crystallizes so good. PUUAs prepared using aromatic diisocyanate (MDI) showed a better solvent resistance compare to aliphatic diisocyanate (HMDI) [23].
Conclusion
Diamine-diamide based PUUAs with different concentrations of hard segment and different chain extender of 6A6 and 6T6 have been prepared and characterized by NMR, FTIR, DSC and TGA techniques. The weight average molecular weight of the soft segment (polystyrene) is increased from 2000 to 10,000 g/mol using MDI as a chain extender and in turn the hard segment concentration is changed from 30 to 17 wt%. The heating curve of the DSC showed two transitions, Tg of SS and the melting of HS as expected for semi crystalline materials. Due to high crystallinity of the 6T6 based PUUAs, Tm value is higher than the 6A6 based PUUAs. The decomposition temperature of the PUUAs is centered on 400 °C, which clearly shows a high thermal stability of the synthesized block copolymers. The swelling and the solubility of the polymer (in chloroform) is found to be depends on the type of the chain extender and the concentration of the hard segment. Overall, the synthesized segmented copolymer is transparent, high molecular weight materials and excellent solvent resistivity.
References
Król P (2007) Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog Mater Sci 52:915–1015
Wang CB, Cooper SL (1983) Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 16:775–786
Rogers ME, Long TE (2003) Synthetic methods in step-growth polymers, vol 4. Wiley-Interscience, Hoboken
Zhang Q, He H, Xi K, Huang X, Yu X, Jia X (2011) Synthesis of N-Phenylaminomethyl POSS and Its Utilization in Polyurethane. Macromolecules 44:550–557
Bates FS, Hillmyer MA, Lodge TP, Bates CM, Delaney KT, Fredrickson GH (2012) Multiblock polymers: panacea or Pandora’s box? Science 336:434–440
Russell TP, Karis TE, Gallot Y, Mayes AM (1994) A lower critical ordering transition in a diblock copolymer melt. Nature 368:729–731
Zhang L, Eisenberg A (1995) Chemically induced supramolecular reorganization of triblock copolymer assemblies: trapping of intermediate states via a shell-crosslinking methodology. Science 268:1728–1731
Li Y, Ren Z, Zhao M, Yang H, Chu B (1993) Multiphase structure of segmented polyurethanes: effects of hard-segment flexibility. Macromolecules 26:612–622
Li Y, Kang W, Stoffer JO, Chu B (1994) Effect of hard-segment flexibility on phase separation of segmented polyurethanes. Macromolecules 27:612–614
Lee DK, Tsai HB (2000) Properties of segmented polyurethanes derived from different diisocyanate. J Appl Polym Sci 75:167–174
Gao R, Zhang M, Wang SW, Moore RB, Colby RH, Long TE (2013) Polyurethanes containing an imidazolium diol-based ionic-liquid chain extender for incorporation of ionic-liquid electrolytes. Macromol Chem Phys 214:1027–1036
Biemond GJE, Brasspenning K, Gaymans RJ (2012) Synthesis and selected properties of polyurethanes with monodisperse hard segments based on hexane diisocyanate and three types of chain extender. J Appl Polym Sci 124:1302–1315
Chang Z, Zhang M, Amanda G, Hudson E, Orler B, Robert B, Moore GL, Wilkes S, Turner R (2013) Synthesis and properties of segmented polyurethanes with triptycene units in the hard segment. Polymer 54:6910–6917
He Y, Zhang X, Runt J (2014) The role of diisocyanate structure on microphase separation of solution polymerized polyureas. Polymer 55:906–913
Kayalvizhi M, Vakees E, Suresh J, Nagarajan S, Arun A (2014) Spacer length controlled hghly thermo reversible polyurethane-urea based on polystyrene: synthesis and crystallization studies. Polym Adv Technol 26:160–166
Kayalvizhi M, Vakees E, Suresh J, Arun A (2015) Poly(urethane-urea) based on functionalized polystyrene with HMDI: synthesis and charecterisation. Arab J Chem. doi:10.1016/j.arbjc.2015.04.005
Van der Schuur M, Feijen J, Gaymans RJ (2005) Synthesis and characterization of bisester-amide segments of uniform and random length. Polymer 46:4584–4595
Van der Schuur M, Noordover B, Gaymans RJ (2006) Polyurethane elastomers with amide chain extenders of uniform length. Polymer 47:1091–1100
Vakees E, Suresh J, Kayalvizhi M, Srinivasan VV, Arun A (2013) Synthesis and characterization of functionalized polystyrene using ATRP with strong base technique. IOSR-J Appl Chem 7(4):32–38
Krijgsman J, Husken D, Gaymans RJ (2003) Synthesis and characterisation of uniform bisester tetra-amide segments. Polymer 44:7043–7053
Yilgör E, Burgaz E, Yurtsever E, Yilgör L (2000) Comparison of hydrogen bonding in polydimethylsiloxane and polyether based urethane and urea copolymers. Polymer 41:849–857
Oertel G (1985) Polyurethane Handbook. Carl Hanser, Munich, p 31
Kayalvizhi M, Vakees E, Suresh J, Arun A (2015) Manuscript send to Journal
Desai S, Thakore IM, Sarawade BD, Devi S (2000) Effect of polyols and diisocyanates on thermo-mechanical and morphological properties of polyurethanes. Eur Polym J 36:711–725
Ya-Jen Yu (2011) The effect of moisture absorption on the physical properties of polyurethane shape memory polymer foams. National Taiwan University, Taiwan
Acknowledgments
We wish to thank the Department of Science and Technology (DST), India for their financial support under Fast Track Scheme for Young Scientists to Dr.A.Arun (No.SR/FT/CS-018/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kayalvizhi, M., Suresh, J., Karthik, S. et al. Synthesis and characterization of MDI and functionalized polystyrene based poly(urethane-urea-amide). Int J Plast Technol 20, 128–142 (2016). https://doi.org/10.1007/s12588-016-9145-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-016-9145-4