Abstract
PVC—PEMA polymer blend based electrolyte films complexed with NaIO4 were prepared by solution—cast technique. The complexation of the blend with the salt was confirmed from XRD and SEM studies. DC conductivity was measured in the temperature range 303–398 K. The electrical conductivity increased with increasing dopant concentration. Which is attributed to the increase in amorphous nature of the complexes on dopining.. Transference number measurements were carried out to investigate the nature of charge transport. The discharge characteristics of the cells were studied under a constant load.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes have been extensively studied in the past decades due to their excellent mechanical and thermal stability and high ionic conductivity. To achieve high ionic conduction, flexibility and good mechanical strength, polymer blends are most promising as reported by many research groups [1–7]. A few attempts have tried electrolytes based on sodium complexed films. Apart from the scientific interest, the use of sodium has several advantages over their lithium counterparts. Sodium has available in abundance at a cheaper cost than lithium. It may be possible to obtain solid electrolyte of sufficiently high conductivity because sodium does not form an alloy with aluminium, making it possible to use this metal as current collector instead of the costlier and heavier nickel. Furthermore, the softness of these materials makes it easier to achieve and maintain contact with other components in the battery. Investigations have also been made on sodium ion conducting polymer electrolytes based on PEO, PPO, PVP, PVA and PVC complexed with NaI, NaBr, NaCl, NaNO3, NaClO4, and NaSCN [8–14].
In the present study, the authors have directed their attention to polyvinyl chloride (PVC) as a host polymer as it is commercially available, inexpensive and is compatible with a large number of plasticizers such as ethylene carbonate (EC), propylene carbonate (PC), dibutyl phthalate (DBP) and dioctyl adipate (DOA). The second host polymer polyethyl methacrylate (PEMA) also has excellent chemical resistance, high surface resistivity and mechanical strength. Its high elongation strength makes it an important candidate for the formation of blends with other polymers [15, 16]. In this paper we report detailed studies on the synthesis and characterization of a new SPE consisting of PVC, PEMA and NaIO4 using XRD, SEM, and ionic conductivity techniques.
Experimental
Films of (thickness 100–200 μm) PVC + PEMA and (PVC + PEMA + NaIO4) (50:50), (47.5:47.5:5), (45.0:45.0:10); (42.5:42.5:15) and (40:40:20) were prepared by solution-cast technique using tetrahydrofuran (THF) as a solvent. The mixture of these solutions was stirred for 12 h, cast onto polypropylene dishes and evaporated slowly at room temperature. The final product was vacuum dried thoroughly at 10−3 mbar.
X-ray diffraction studies were performed by means of SEIFERT X-ray diffractometer. The surface morphology of these polymer blend films was observed using JEOL JSM 840A scanning electron microscope (SEM). The DC conductivity was studied as a function of temperature using a conductivity cell designed in our laboratory. The total ionic transport number (tion) was measured using Wagner’s polarization technique [17]. In this technique the freshly prepared polymer electrolyte films were polarized in the configuration C/polymer electrolyte/C under a dc bias (step potential of 1.5 V). The resulting current was monitored as a function of time time using Keithly programmable electrometer (Model 617) and the transference numbers were evaluated. Electrochemical cells were fabricated with a configuration Na/ (PVC +PEMA +NaIO4)/(I2 +C). Details regarding the electrochemical cell are given in ref [18]. The discharge characteristics of these cells were monitored for a constant load of 100 kΩ.
Results and discussion
XRD measurements
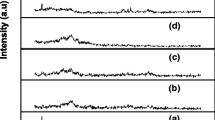
To investigate the complexation of sodium periodate salt with the polymer blend, XRD studies were performed. Figure 1(a–h) shows the XRD patterns of pure PVC, PEMA and PVC/PEMA-based composite polymer electrolytes and NaIO4 salt respectively. Comparison of the XRD patterns of complexed polymer blend films with those of pure PVC, PEMA, and NaIO4 reveals the following differences. The amorphous nature of PVC and PEMA and crystalline nature of NaIO4 salt were observed. Peaks observed for 2θ values around 21° are less intense and slightly displaced in complexed polymer blend films compared to those in pure PVC film. This indicates that the addition of NaIO4 salt causes a decrease in the degree of crystallinity and a simultaneous increase in the amorphicity of the complexed films. The crystalline peaks for 2θ values at 14°, 32°,43 and 61° corresponding to NaIO4 (Fig. 1h) are absent in polymer complexes. This indicates the absence of any excess (uncomplexed) salt in the complexed polymer blend electrolyte films. Absence of sharp peaks at higher concentrations of NaIO4 salt indicates the dominant presence of amorphous phase. This amorphous nature is responsible for greater ionic diffusivity resulting in high ionic conductivity. This observation confirms that complexation has taken place in the amorphous phase.
Salt concentration and temperature dependence of d.c. conductivity
The variation in dc conductivity as a function of the NaIO4 salt composition in PVC+PEMA blend at various temperatures is given in Fig. 2. The conductivity of polymer blend (PVC+PEMA) is ~ 10-9 Scm−1 at room temperature and its value increased to ~10-8 Scm−1 on complexing it with 5 wt.% of NaIO4. The increase in conductivity becomes slower on further addition of NaIO4 salt to the polymer which may be explained on the basis of ion association and charge multiplets formation [19, 20]. In general, it is believed that the conductivity increases as the degree of amorphous phase increases. In the present study the continuous increase in conductivity with increasing NaIO4 salt concentration is attributed to the increase in amorphous phase as supported by XRD and SEM analysis.
The variation of conductivity as a function of temperature for pure PVC+PEMA and for different compositions of (PVC+PEMA+NaIO4) polymer electrolyte over the temperature range 303–398 K is shown in Fig. 3. The conductivity increases with temperature in pure PVC+PEMA and also in all the compositions of the (PVC+PEMA +NaIO4) polymer electrolyte system. The increase in ionic conductivity may be interpreted on the basis of a hopping mechanism between coordinating sites, local structural relaxations and segmental motions of the polymer chains, which are essential to ensure high conductivity to the electrolytes [21, 22]. The variation of conductivity was found to follow the Arrhenius relation
where σ 0 is a pre-exponential factor, E a , the activation energy of the system and k, the Boltzmann’s constant.
As temperature increases, the polymer expands and produces free volume in which ions, solvated molecules or polymer segments can move easily. The increase in free volume leads to an increase in ion mobility. This assists ion transport and hence increases the conductivity of the system [23–25]. The activation energy (a combination of the energy of defect formation and the energy for ion migration) of the system, determined from the slopes of log σ versus 1000/T plots, was found to be 0.29 eV for pure (PVC+PEMA) and in the range 0.27–0.20 eV for complexed (PVC+PEMA). The activation energies for both the regions are given in the Table 1.
SEM Analysis
Figure 4 shows SEM photographs of polymer electrolytes at different blending ratios. From the figure, it is clear that the morphology of the polymer electrolytes is uniform but with different degrees of roughness. The micrograph of pure PVC +PEMA films exhibited no features attributable to any crystalline morphology. The surface morphology of (PVC +PEMA + NaIO4) system showed salt aggregates or chunks on the top surface. But as a whole, the compatibility of PVC +PEMA polymer matrix with NaIO4 was still uniform and homogeneous. The appearance of several pores or craters on the surface is due to the rapid evaporation of the solvent (THF). The difference in the pore size is related to the difference in the driving force for phase separation [26]. When phase separation is less, more homogeneous solutions tend to form because of which the conductivity increases [Fig. 4(e)].
Transference numbers
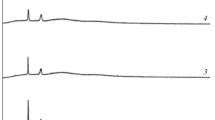
The transference numbers, both ionic (tion) and electronic (tele) of PVC+PEMA and various compositions of complexed films with NaIO4 salt with different weight ratios were evaluated using the Wagner’s polarization technique. In this technique, the dc current was monitored as a function of time on application of fixed dc voltage of 1.5 V across the Na / polymer electrolyte / C cell. Figure 5 shows the current versus time plots for different compositions of NaIO4 doped polymer blend electrolyte films. The transference numbers were calculated using the relation
where It is the total current (ionic + electronic) at the start of the time (i.e., when t = 0) and Is is the saturation current (electronic current only). The calculated transference numbers are given in Table 2. The ionic transference numbers are in the range 0.94–0.97. This suggests that the charge transport in these polymer blend electrolyte films is predominantly ionic with negligible contribution from electrons.
Solid state sodium battery
Using this polymer electrolyte, electrochemical cells were fabricated in the configuration Na / (PVC+PEMA+NaIO4) / (I2 +C electrolyte). Their discharge profiles are shown in Fig. 6 at ambient temperature for a constant load of 100 kΩ. The effective ara of the cell is 1.32cm2. The initial sharp decrease in the voltage and current may be because of the polarization and/or formation of a thin layer of sodium salt at the electrode-electrolyte interface. Various cell parameters like open circuit voltage (OCV), short circuit current (SCC), power density, current density etc. were evaluated and are presented in Table 3. The cells parameters were found to be comparable to those reported earlier [27–30]. On the basis of these study it may be said that (PVC + PEMA + NaIO4) polymer electrolytes are promising materials for solid state batteries. Further work, in the direction of obtaining higher cell capacities and energy densites, is in progress.
Conclusions
Free-standing, flexible polymer electrolyte films based on different compositions of (PVC + PEMA + NaIO4) were prepared. The complexation behavior of polymer salt matrices was confirmed from XRD and SEM studies. The increase in conductivity with increasing dopant concentration is attributed to the increase in amorphous phase of the complex. The Arrhenius plots show two regions having different activation energies. Transference number data showed that the conductivity is mainly due to ions rather than electrons. Cell parameters, evaluated for (PVC + PEMA + NaIO4) polymer electrolyte cells, exhibited better performance, which indicates that these cells are suitable for fabricating solid-state batteries.
References
Scrosati B (2000). Poly(methyl methacrylate)-based protonic gel electrolytes: a spectroscopic study Electrochim Acta 45:246
Berthier C, Gorecki W, Miner M, Armand MB, Chabagno JM, Rigand D (1989). Increasing the conductivity of polymer solid : A review. Solid State Ionics 36:165
Scrosati B (1992). Lithium rocking chair batteries: an old concept? J Electrochem Soc 139:2776
Khan MS, Abdul S, Jan N (2010). Conductance study of poly(ethylene oxide)-and poly(propylene oxide)-based Polyelectrolytes. Ionics 16:539
Luo Y, Guo J, Wang C, Chu D (2010). Quaternizedpoly(methyl methacrylate-cobutyl acrylate-co-vinylbenzyl chloride) membrane for alkaline fuel cells. J Power Sources 195:3765
Chandra A, Agrawal RC, Mahipal YK (2009). Ion transport property studies on PEO–PVP blended solid polymer electrolyte membranes. J Phys D Appl Phys 42:135107
Reddeppa N, Reddy TJR, Achari VBS, Rao VVRN, Sharma AK (2009). Electrical and optical characterization of (PEO+PVAc) polyblend films. Ionics 15:255
Alamgir M, Abraham KM (1993). Li Ion Conductive Electrolytes Based on Poly(vinyl chloride). J Electrochem Soc 140:L96
Han HS, Kang HR, Kim SW, Kim HT (2002). Phase-separated polymer electrolyte based on poly(vinyl chloride)/poly(ethyl methacrylate) blend. J Power Sources 122:461
Buraidah MH, Teo LP, Majid SR, Yahya R, Taha RM, Arof AK (2010). Characterizations of chitosan-based polymer electrolyte photovoltaic cells. Int J Photoenergy 1–7. doi: 10.1155/2010/805836
Jaipal Reddy M, Sreekanth T, Subba Rao UV (1999). Study of the plasticizer effect on a (peo + nayf4) polymer. Solid State Ionics 126:55
Sreepathi Rao S, Jaipal Reddy M, Laxmi Narsaiah E, Subba Rao UV (1995). Development of electrochemical cells based on (PEO+NaYF4) and (PEO+KYF4) polymer electrolytes. Mater Sci Eng B 33:173
Hashmi SA, Chandra A, Chandra S et al (1992) In: Chowdari BVR (ed) Solid State Ionics: Materials and 178 Applications. Singapore, World Scientific, p 567
Vijaya Kumar K, Sunita Sundari G, Chandra Sekhar M, Srinivasa Rao A (2012). Effect of plasticizer on a new (PEO+NaHCO3) solid polymer electrolyte system for battery characterization studies. IJCBS 1:59
Al-Saigh ZY, Chen P (1991). Characterization of semicrystalline polymers by inverse gas chromatography. 2. A blend of poly(vinylidene fluoride) and poly(ethyl methacrylate). Macromolecules 24:3788
Kwei TK, Patterson GD, Wang TT (1976) Macromolecules 9:780
Wagner JB, Wagner C (1957). Electrical Conductivity Measurements on Cuprous Halides. J Chem Phys 26:1597
Reddy JR, Janaki Rami Reddy VBS, Achari AK, Sharma VV R, Narasimha Rao CV, Reddy S (2008). Charge transport and discharge mechanism in (PVC+KClO3) polymer electrolyte films. J Phys Chem Solids 69:1033
Maurya KK, Bhattacharya B, Chandra S (1995). Hydrogen Ion Transport Studies in PEO:NH4HSO4 Polymer Electrolyte. Phys Stat Sol (a) 147:347
Ramya CS, Selvasekarapandian S, Savitha T, Hirankumar G, Baskaran R, Bhuvaneswari MS, Angelo PC (2006). Conductivity and thermal behavior of proton conducting polymer electrolyte based on poly (N-vinyl pyrrolidone). Eur Polym J 42:2672
Reddy TJR, Achari VBS, Rao VVRN, Sharma AK (2007). Preparation and electrical characterization of (PVC+KBrO3) polymer electrolytes for solid state battery applications. Ionics 13:435
Vickraman P, Aravindan V, Lee YS (2010). Lithium ion transport in PVC/PEG 2000 blend polymer electrolytes complexed with LiX (X=ClO4, BF4,and CF3SO3). Ionics 16:263
Dillip KP, Samantaray BK, Choudhary RNP, Awalendra Thakur K, Katiyar RS (2007). Effect of Plasticizer on Structural and Electrical Properties of Polymer Nanocompsoite Electrolytes. Int J Electrochem Sci 2:861
Hema M, Selvasekerapandian S, Sakunthala A, Arunkumar D, Nithya H (2008). Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system. Physica B 403:2740
Druger SD, Itzan A, Ratner MA (1983). Dynamic bond percolation theory: A microscopic model for diffusion in dynamically disordered systems. I. Definition and onedimensional case. J Chem Phys 79:3133
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997). Ionic conduction in plasticized PVC/PMMA blend polymer electrolytes. Electrochim Acta 42:15
Bhide A, Hariharan K (2007). Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur Polym J 43:4253
Ramya CS, Selvasekarapandian S, Savitha T (2008). Proton-conducting membranes: poly (N-vinyl pyrrolidone) complexes with various ammonium salts. J Solid State Electrochem 12:807
Chandrasekaran R, Selladurai S (2000). A New Type of Electrochemical Cell Based on the Configuration NaI /PEG/ I2. Int J Ionics 6:218
Jaipal Reddy M, Sreekanth T, Subba Rao UV (1998). Conductivity and parametric studies of a (PEO+(glass)(15Na2 O–15NaF–70B2 O3)) cell. J Power Sources 76:30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramamohan, K., Umadevi, C., Achari, V.B.S. et al. Conductivity studies on (PVC/PEMA) solid polymer blend electrolyte films complexed with NaIO4 . Int J Plast Technol 17, 139–148 (2013). https://doi.org/10.1007/s12588-013-9054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-013-9054-8