Abstract
Heart failure is one of the major cardiovascular complications in patients with type 2 diabetes mellitus (T2DM) and increases the risk of morbidity and mortality. Although active management for heart failure is needed in patients with T2DM, traditional treatment and some new class of antihyperglycemic drugs, such as glucagon-like peptide-1 receptor agonists or dipeptidyl peptidase-4 inhibitors, could not reduce the risk of heart failure. Recent major trials demonstrated sodium–glucose co-transporter-2 (SGLT2) inhibitors improve prognosis of T2DM patients through prevention of heart failure. Both heart failure with reduced ejection fraction and that with preserved ejection fraction (HFpEF) is observed in T2DM patients, and HFpEF is often overlooked and misdiagnosed in these population. Left ventricular hypertrophy, left atrial dilatation, diastolic dysfunction, and subclinical systolic dysfunction indicated as reduced global longitudinal strain are major abnormalities on echocardiography in patients with diabetic cardiomyopathy. These structural and functional changes are also prevalent in the general patients with T2DM, and those with these abnormalities have higher incidence of heart failure than those without them. Glycemic control might improve some of these abnormalities on echocardiography, but it is still unclear whether their improvement could be associated with risk reduction for heart failure. At now, there are only limited data on the effects of DPP-4 inhibitors or SGLT2 inhibitors on echocardiography in T2DM patients. Large-scale trials are needed to clarify how antihyperglycemic drugs affect echocardiographic parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heart failure as a complication of diabetes mellitus
Diabetes mellitus is one of the major risk factors of cardiovascular (CV) events and its risk is considered as equivalent to that of previous coronary artery disease (CAD). The prevalence of diabetes in Japan has been steadily and sharply increasing along with worldwide “diabetes pandemic”. Japanese Ministry of Health and Welfare reported that it increased to 3.29 million in 2017 from 2.47 million in 2005. The increase in diabetic population would result in the increase in CV events and it will be one of the biggest burden in Japanese healthcare system, though the incidence of CV complications in Japanese patients is lower than that in western ones.
Poor glycemic control is associated with increased incidence of CV events and mortality. Each 1% increase in the level of glycated hemoglobin (HbA1c) was associated with an 8% increase in MI and a 9% increase in stroke during a period of 2.4 years in diabetic patients without known CV disease [1]. However, intensive glycemic control, which successfully reduces the microvascular complications, does not always reduce the CV risk, or even might have detrimental effects. The UKPDS (United Kingdom Prospective Diabetes Study) demonstrated that intensive therapy (target HbA1c < 7.0%) was associated with a 25% lower risk of developing microvascular complications comparing to conventional therapy (< 8.0%), but it failed significant reduction of MI or stroke in patients with type 2 diabetes (T2DM) [2]. In the subsequent large-scale trials, the ACCORD (Action to Control Cardiovascular Risk in Diabetes), the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation), and the Veterans Affairs Diabetes Trial (VADT), intensive therapy (target HbA1c ≈ 6.0%) failed to demonstrate significant reduction in the incidence of major CV events (MACE) compared with conventional therapy (≈ 7.0%) [2]. Intensive therapy was associated with increased mortality in the ACCORD trial [2], which might be explained by increased incidence of severe hypoglycemia. Thus, strict glycemic control is not sufficient for prevention of macrovascular complications in patients with T2DM.
Dipeptidyl peptidase-4 (DPP-4) is an enzyme expressed in various cell types such as T cells, macrophages, adipocytes, hepatocytes and endothelial cells. DPP-4 cleaves N-terminal dipeptides from several proteins including incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The incretin hormones are secreted by the gut in response to nutrients, and are released into circulation within minutes of meal ingestion, and promote insulin secretion by activating their receptors on the pancreatic β cells. Active forms of GLP-1 and GIP are rapidly degraded by DPP-4, resulting in a half-life of approximately 1 min in the circulation. DPP-4 inhibition decreases the degradation of endogenous GLP-1 and increased their concentrations, leading to lowering of postprandial glucose concentrations. Incretin hormones are mostly secreted after meal digestion, and DPP-4 inhibitors are less likely to induce hypoglycemia.
DPP-4 inhibition not only increases GLP-1 concentration but also cardioprotective peptides like neuropeptide Y and stromal cell derived factor-1 (SDF-1). GLP-1 receptors are widely expressed in cardiovascular system such as endothelium, vascular smooth muscle, and cardiac atrium, and their activation on endothelial cells activates endothelial nitric oxide synthase. With low incidence of hypoglycemia and pleiotropic cardioprotective effects, DPP-4 was expected to have more cardioprotective action than other glucose-lowering medications. However, large-scale clinical trials with DPP-4 inhibitors in T2DM patients demonstrated the cardiovascular safety about MACE, but superiority was not indicated compared with placebo. Moreover, SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus)-TIMI 53 trial demonstrated hospitalization for heart failure (HHF) was significantly increased in patients receiving saxagliptin [3]. Alogliptin also tended to increase in HHF whereas sitagliptin or linagliptin did not increase HHF incidence [4, 5].
New-class of antihyperglycemic drugs has made a significant breakthrough in prevention of CV events in T2DM patients. Sodium-glucose co-transporter 2 (SGLT2) is a member of the sodium-glucose co-transporter family which transport sodium and glucose into cells. Under physiological conditions, about 180 g of glucose is filtered by the glomeruli in a day, and it is completely reabsorbed by SGLTs of kidney. SGLT2 is expressed almost exclusively in the initial convoluted portion (S1 segment) of the proximal tubule, and 90% of glucose reabsorption is mediated by SGLT2. Patients with T2DM had higher number of SGLT2 in the proximal tubule than healthy individuals, and glucose reabsorption is greatly increased. Inhibition of glucose reabsorption by SGLT2 lowers blood glucose through increased urinary excretion of glucose. SGLT2 inhibitors cause insulin-independent HbA1c reduction of ≈ 0.7% to 1.0% and body weight loss of ≈ 2–3 kg in T2DM patients. SGLT-2 inhibitors also increase fractional excretion of sodium and have modest diuretic and natriuretic effects.
The EMPA-REG OUTCOME trial (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) demonstrated that a SGLT2-inhibitor, empagliflozin, reduced MACE (CV death, MI and stroke) by 14% and CV death by 38% in patients with T2DM and cardiovascular disease. Empagliflozin reduced HHF by 35%, while no significant difference was observed in the rate of nonfatal MI and stroke (Fig. 1) [6]. The CANVAS (Canagliflozin Cardiovascular Assessment Study) Program demonstrated that canagliflozin reduced MACE by 14% and HHF by 35% compared with placebo [7]. DECLARE-TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events) trial enrolled 17,160 T2DM patients including 10,186 (59.4%) without atherosclerotic cardiovascular disease (ASCVD), and dapagliflozin treatment was associated with a lower rate of CV death or HHF [8]. All these studies showed significant reduction of renal hard end points, while no reduction was observed in the rate of MI and stroke. Thus, SGLT2 inhibitors could reduce the rate of HHF and possibly CV death in T2DM patients as a class effect.
Cardiovascular outcomes in randomized control trials with a DPP-4 inhibitor and an SGLT-2 inhibitor. The cumulative incidence of the primary outcomes (a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal ischemic stroke) and that of hospitalization for heart failure in SAVOR (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus)-TIMI 53 [3], using a DPP-4 inhibitor saxagliptin, and in EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) [6], using an SGLT-2 inhibitor empagliflozin are shown
GLP-1 receptor antagonist (GLP-1RA) is another antihyperglycemic drug which could have CV benefits in T2DM patients. The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial demonstrated that liraglutide reduced MACE by 13% and all-cause mortality was reduced by 15% compared with placebo [4]. In the SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes), semaglutide reduced MACE by 26% with a consistent magnitude and direction of effect for the key components of nonfatal stroke and nonfatal MI [4]. No reduction in HHF was observed in both studies [4, 5]. Other GLP-1RAs such as exenatide and lixisenatide failed to show evidence for a reduction in MACE outcomes in large-scale trials [4, 5]. These results imply that some of GLP-1RAs could reduce MACE probably through reduction in ASCVD, but not HHF.
The successful results of SGLT2 inhibitor trials boldly demonstrated the importance of heart failure (HF) in the prognosis of patients with T2DM. The intact relation between HF and T2DM has been established by many cohort studies and clinical trials. T2DM is an independent risk factor for HF, and patients with T2DM have four times higher risk to develop HF than those without T2DM [4]. Among patients with T2DM patients, higher HbA1c level is associated with more incidental HF [4]. Patients with T2DM who developed HF had a 10 to 12 times greater mortality than those who did not develop HF [5]. On the other hand, T2DM is observed in about 30% of patients with chronic HF irrespective of HF phenotype (heart failure with reduced ejection fraction (HFrEF) or heart failure with preserved ejection fraction (HFpEF)) [5]. High HbA1c is associated with increased all cause- and CV mortality in patients with T2DM and HF, and 1% increase in HbA1c is associated with an 18–19% increased risk of HF [9]. Thus, T2DM and HF are highly interacted not only in their occurrences but also in clinical outcomes.
CAD is the most common concomitant condition that causes HF in patients with T2DM, along with hypertension. However, the majority of the available data suggest that T2DM is associated with higher risk of mortality in both patients of ischemic and non-ischemic HF [5], indicating that CAD is not the only cause of HF in T2DM patients. Some diabetic patients develop HF without involvement of CAD or hypertension, and the condition is called “diabetic cardiomyopathy”. There is no universal definition of diabetic cardiomyopathy, and the most commonly accepted definition refers to a myocardial dysfunction which occurs in the absence of all other CV disease. Both HFrEF and HFpEF are present as the phenotype of HF related with diabetic cardiomyopathy. Diabetic cardiomyopathy with systolic dysfunction is usually observed in patients with long-standing type 1 diabetes. Most of diabetic cardiomyopathy in T2DM have a phenotype of HFpEF.
Left ventricular (LV) diastolic dysfunction can be detected in 75% of T2DM patients [10], and almost half of HF patients with T2DM have HFpEF. HFpEF is more frequent in older, hypertensive and female patients with T2DM. The degree of glycemic control correlates with LV diastolic dysfunction severity and with increased risk of incident HF and CV mortality in T2DM patients [11]. HFpEF is usually associated with mild T2DM complications in the early stages of T2DM, whereas HFrEF is associated with more severe T2DM complications. HFpEF in T2DM patients is difficult to diagnose because the symptoms are often mild and could be frequently misdiagnosed as other conditions such as chronic obstructive pulmonary disease. Echocardiography plays an important role in the diagnosis of HF in patients with T2DM, and the characteristic echocardiographic findings in patients with T2DM should be shared with echocardiographers.
Functional and structural changes on echocardiography in T2DM patients
Many of the T2DM patients have concomitant conditions relevant to heart failure such as CAD and hypertension, and it is not easy to determine the disease-specific echocardiographic findings. LV hypertrophy (LVH) is the major morphological change in diabetic cardiomyopathy associated with HFpEF. Increased LV mass is independently associated with diabetes in echocardiographic studies [12, 13] and is observed even in the pre-diabetic stage such as impaired glucose tolerance [13]. Increased LV mass is a recognized predictor of cardiovascular morbidity and mortality, and is likely to be a key contributor to HF development in T2DM patients. LV mass/volume ratio [14] and relative wall thickness [12, 15] are also increased in diabetes, and concentric LVH represents the main structural characteristic of diabetic cardiomyopathy. Along with underlying myocardial fibrosis in diabetic hearts, LVH would lead to LV diastolic dysfunction; echocardiography studies have demonstrated that T2DM patients have lower transmitral E/A ratio [10], lower mitral annular early diastolic velocity (e′), greater E/e′ [15], and larger left atrial (LA) volume [12].
Subclinical impairment in systolic function is also found in T2DM patients with normal LVEF. Tissue Doppler imaging and 2D-speckle tracking echocardiography studies [12, 16] demonstrated that systolic LV global longitudinal strain (GLS) is reduced in T2DM patients than in those without it irrespective of concomitant hypertension. These subclinical abnormalities in systolic function could be a precursor to the onset of clinical HF in diabetes.
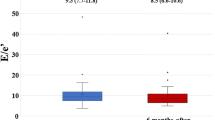
ARIC (the Atherosclerosis Risk In the Community) study is a prospective observational cohort study of the natural history of ASCVD and CV risk factors with 15,744 participants. Echocardiography was performed in 4419 participants without prevalent CAD or HF to determine the association between glycemic status and cardiac structure and function. It was found that worsening glycemic control was associated with increased LV mass, worse diastolic function, and reduction in LV systolic function (Fig. 2). Every 1% increase in HbA1c was associated with 3.0 g increase of LV mass, 0.5 increase of E/e′, and 0.3% worsening of GLS [12]. Thus, echocardiographic changes observed in diabetic cardiomyopathy such as LVH, diastolic dysfunction and impaired GLS, could be observed in patients with dysglycemia irrespective of the presence or absence of HF symptoms, and these changes might be affected by glycemic status.
Association between HbA1c and echocardiography parameters in a community-based cohort study. Association between HbA1c and left ventricular (LV) mass, global longitudinal strain, septal E/e′ ratio and right ventricular (RV) systolic function in 4419 participants without prevalent coronary heart disease or heart failure in the ARIC (Atherosclerosis Risk in Communities) study, a prospective epidemiologic study conducted in four US communities. Long denotes longitudinal; LV, left ventricle; and RV FAC, right ventricular fractional area change [12]
An echocardiography study was performed in asymptomatic, older than 65 years T2DM patients with preserved LVEF and no CAD to determine the ability of structural and functional changes described above for detection of HF [17]. LVH (LV mass index > 115 g/m2 for men and > 95 g/m2 for women) was observed in 23% of the 290 study patients, abnormal E/e′ (> 13) in 10%, left atrial (LA) enlargement (> 34 ml/m2) in 35%, and impaired GLS (cutoff 16%) in 23%. HF and death were more frequent in patients with any of these abnormalities than those without them over a median follow-up of 1.5 years, and a higher number of abnormalities was associated with higher incidence of HF or death. Cox regression analysis revealed that LVH, LA enlargement and impaired GLS were associated with increased risk of the composite end point of death and HF independent of clinical risk score and HbA1c, whereas abnormal E/e′ was not [17].
In another study, T2DM patients without previous diagnosis of HF underwent a standard diagnostic procedure including echocardiography and examination by cardiologists. HF was newly diagnosed in 27.7% of these patients, and most of them had HFpEF. Diabetic cardiomyopathy was the possible cause of HF in 30% of them. Patients with newly diagnosed HF had significantly higher E/e′ and larger LA volume index than those without HF [18]. These results demonstrated that echocardiographic abnormalities have the potential to predict the development of HF in patients with T2DM.
A cluster analysis was performed on echocardiographic variables in asymptomatic T2DM patients with overt heart disease and found that three different types of patients were present. Patients who had the lowest LV mass index (LVMi) and E/e′ ratio had the highest LVEF and were predominantly male with the lowest rate of obesity or hypertension. Those with the highest GLS and highest E/e′ ratio were the oldest, were predominantly female, and had the lowest rate of T2DM without hypertension or obesity. Those who had the highest LV mass index and the lowest LVEF and GLS were predominantly male. The second and third groups had higher all-cause mortality and hospitalization than the first group. The first and the third group had similar age and rate of obesity and hypertension, which implied that the echocardiographic changes had prognostic value irrespective of obesity and hypertension [19].
In summary, LVH, diastolic dysfunction (indicated as increased E/e′ or LA enlargement) and subclinical systolic dysfunction (impaired GLS) are major changes observed in T2DM independent of CAD or hypertension. These echocardiographic changes have predictive values for the development of HF in T2DM patients. These points should be carefully observed when echocardiography is performed in a T2DM patient.
Glycemic control and changes in echocardiographic parameters
The echocardiographic abnormalities in T2DM patients seem to be related with glycemic control [12]. It is unclear whether improvement of glycemic control could reverse these structural and functional changes. The effects of a multifactorial intervention including targeting of glycemic control, cholesterol, and blood pressure on echocardiographic parameters were investigated in T2DM patients with poor glycemic control [20]. They had impaired GLS and e′ at baseline despite a normal LVEF. With reduction in both HbA1c and low-density cholesterol, relative improvement in GLS, septal e′ and E/e′ was observed after 12 months of intervention (Fig. 3). Those who had the largest decrease in HbA1c had the greatest improvement in LV systolic and diastolic function, and patients with the lowest HbA1c at follow-up had the largest improvement in GLS. No significant change was observed in LV mass index, LA volume and E/A ratio. No change was obtained in body mass index or systolic blood pressure, suggesting that glycemic control might mostly contribute to these echocardiographic changes. Although these results suggest that optimization of glycemic control improves LV systolic and diastolic function, it was a non-randomized study without placebo and had limitations. Moreover, it is unclear whether the functional improvement would lead to prevention of HF in T2DM patients.
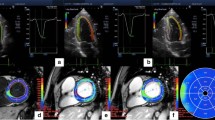
Improvement of global longitudinal strain (GLS) after intensive glycemic control in a patients with type 2 diabetes mellitus (T2DM). Longitudinal strain images by 2-dimensional speckle tracking for the apical 4-, 2-, and 3-chamber views before (a) and after 12 months of intensive glycemic control (b) in a patient with poorly controlled T2DM. The strain throughout 1 cardiac cycle can be seen for each of the color-coded left ventricular (LV) segments (mean strain shown in white). A, The mean peak strains at baseline of − 13.3%, − 12.2%, and − 13.1%, which occur during LV ejection. The global longitudinal strain (GLS) improved from − 12.8 to − 18.2% after treatment [20]
Intensive glycemic control in diabetic patients does not reduce the risk of HF [4]; major randomized controlled trials (RCTs) such as UKPDS, ADVANCE, ACCORD and VADT found no difference in the incidence of HF development between intensive and standard glycemic control arms. A meta-analysis of eight RCTs with 37 229 patients, including the above major RCTs, found no significant difference in the risk of HF between intensive and standard treatment arms [2]. Thus, the improvement of echocardiographic parameters by glycemic control might not be always associated with prevention of HF development.
Not only the degree of glycemic control, but also agents used for it affect the development of HF. Thiazolidinediones are associated with fluid retention and exacerbation of symptomatic HF and are not recommended in patients with established HF. Insulin therapy is reported to increase HHF in observational studies but not in RCTs [4], Metformin and sulfonylurea treatment seem equivocal for HF development [4]. As described above, recent RCTs demonstrated that SGLT2 inhibitors prevent T2DM patients from HHF [6,7,8]. The different classes of antihyperglycemic drugs may have different effects on HF development in T2DM patients. How about their effects on echocardiographic parameters?
To answer this question, the literal search is performed for studies with DPP-4 inhibitors and SGLT2 inhibitors. These two agents were selected because SGLT2 inhibitors have beneficial effects on HF, while the effects of DPP-4 inhibitors are neutral or somehow detrimental. Peer-reviewed articles investigating the effects of DPP-4 inhibitors and SGLT2 inhibitors on echocardiography in patients with T2DM were identified by using PubMed up to March 2019. The search strategy is demonstrated in Appendix. Ten publications describing the effects of DPP-4 inhibitors and three of SGLT2 inhibitors were identified. One article on DPP-4 inhibitors might be a sub-study of another one, and it was excluded from the present analysis. Three out of nine studies on DPP-4 inhibitors were single-arm studies and excluded. The remaining six studies are 2-arm studies [21,22,23,24,25,26], and only one of them is a RCT [26]. The effects of DPP-4 inhibitors on LV hypertrophy, LA dilatation, systolic and diastolic function in these studies are demonstrated in Table 1.
The only RCT study with DPP-4 inhibitors examined the effects of vildagliptin treatment in T2DM patients with HFrEF. There was no difference in changes in LVEF during treatment between the vildagliptin and the placebo arm (Fig. 4). Interestingly, LVEDV increased significantly with vildagliptin compared with placebo. LVESV also tended to increase more in the vildagliptin arm, and there was a significant increase in stroke volume but no change in left ventricular wall thickness or mass (Fig. 4) [26]. In other prospective open-label or retrospective studies, no changes were observed in LVEF or in LA size. LVMi was reduced in only one [24] out of three studies, and two studies showed decrease in E/e′ ratio [22, 25] but the other two did not.
Changes in left ventricular (LV) volumes and systolic function after treatment with a DPP-4 inhibitor. Change from baseline in a LVEF (LV ejection fraction), b LVEDV (LV end-diastolic volume) c LVESV (LV end-systolic volume), and LVSV (LV stroke volume) in patients receiving vildagliptin, a DPP-4 inhibitor, and in those with placebo for 52 weeks in VIVIDD (Vildagliptin in Ventricular Dysfunction Diabetes) trial. There was no significant difference in changes in LVEF between the two arms. LVEDV increased significantly with vildagliptin compared with placebo, and there was a trend in the same direction for LVESV. There was a significant increase in LVSV in the vildagliptin arm, but no change in left ventricular wall thickness or mass [26]
Only three studies were found to investigate the changes in echocardiography by SGLT2 inhibitor treatment, all of which were single-arm studies [27,28,29]. No two-arm echocardiography study was found at the time of literature search. The results of these studies with SGLT2-inhibitors are listed in Table 2. LVMi was significantly reduced and diastolic function improved during treatment in all studies (Fig. 5), whereas LVEF was improved in only one study [29]. Similar reduction of LVMi was reported in a cardiac myocardial resonance imaging (CMR) study; 6-month treatment of empagliflozin reduced LVMi in T2DM patients with CAD compared with placebo, and the degree of reduction was greater in those with higher baseline LVMi [30]. As described above, SGLT2 inhibitors could reduce the risk of HF in patients with T2DM, whereas DPP4 inhibitors did not. The reduction of LV hypertrophy, which is not clear in studies with the DPP4 inhibitors, might play some roles in the HF prevention by SGLT2 inhibitors. Most of the echocardiographic studies with SGLT2 or DPP-4 inhibitors were of small scale and had limitations. Recently, it has been suggested that SGLT2 inhibitors may reduce the risk of both HHF and CV death in patients with HFrEF irrespective of the presence or absence of T2DM. It may make a hypothesis that the preventive effect of SGLT2 inhibitors on might not be related with the echocardiographic changes associated with T2DM. Large-scale RCT studies are required to clarify how SGLT2 inhibitors modify the structural and functional changes in the heart of T2DM patients, and whether or how these changes could be related to reduction of HF incidence.
Changes in left ventricular (LV) mass and diastolic function after treatment with an SGLT-2 inhibitor. Changes in LV mass index and lateral e′ velocity from baseline (Pre-EMPA) to the follow-up study (Post-EMPA) after 3 months treatment with empagliflozin, an SGLT-2 inhibitor, in 10 patients with T2DM and established cardiovascular disease [27]
Summary
The incidence of HF is remarkably increased in patients with T2DM irrespective of CAD and contributes to shortened life expectancy. LV hypertrophy, diastolic dysfunction and subclinical systolic dysfunction are frequently observed in T2DM patients even without other risk factors such as hypertension, and these structural and functional changes could be related to HF development. Among antihyperglycemic drugs, SGLT2 inhibitors are reported to decrease the incidence of HF hospitalization and that of CV death. It is suggested that SGLT2 might reduce LV mass, and which may be one of the possible causes of HF reduction. More reliable, large-scale studies with echocardiography or other imaging modalities are required to investigate the mechanisms of the beneficial effects of SGLT2 inhibitors.
References
Elley CR, Kenealy T, Robinson E, et al. Glycated haemoglobin and cardiovascular outcomes in people with type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–301.
Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162(938–948):e2.
Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88.
Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circulation. 2019;140:e294–324.
Seferović PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–72.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Vaur L, Gueret P, Lievre M, et al. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care. 2003;26:855–60.
Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the strong heart study. J Am Coll Cardiol. 2001;37:1943–9.
Aguilar D, Deswal A, Ramasubbu K, et al. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–7.
Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the atherosclerosis risk in the community study. Circ Heart Fail. 2015;8:448–54.
De Marco M, de Simone G, Roman MJ, et al. Cardiac geometry and function in diabetic or prediabetic adolescents and young adults: the strong heart study. Diabetes Care. 2011;34:2300–5.
Levelt E, Mahmod M, Piechnik SK, et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. 2016;65:44–52.
Moir S, Hanekom L, Fang Z-Y, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart. 2006;92:1414–9.
Ng ACT, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–401.
Wang Y, Yang H, Huynh Q, et al. Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. JACC Cardiovasc Imaging. 2018;11:1390–400.
Boonman-De Winter LJMM, Rutten FH, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55:2154–62.
Ernande L, Audureau E, Jellis CL, et al. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J Am Coll Cardiol. 2017;70:1704–16.
Leung M, Wong VW, Hudson M, et al. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging. 2016;9:1–9.
Nogueira KC, Furtado M, Fukui RT, et al. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor—a pilot study. Diabetol Metab Syndr. 2014;6:1–7.
Fujiwara T, Yoshida M, Nakamura T, et al. Dipeptidyl peptidase-4 inhibitors are associated with improved left ventricular diastolic function after acute myocardial infarction in diabetic patients. Heart Vessels. 2015;30:696–701.
Oe H, Nakamura K, Kihara H, et al. Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial. Cardiovasc Diabetol. 2015;14:2–5.
Leung M, Leung DY, Wong VW. Effects of dipeptidyl peptidase-4 inhibitors on cardiac and endothelial function in type 2 diabetes mellitus: a pilot study. Diabetes Vasc Dis Res. 2016;13:236–43.
Yamada H, Tanaka A, Kusunose K, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;16:1–12.
McMurray JJV, Ponikowski P, Bolli GB, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018;6:8–17.
Verma S, Garg A, Yan AT, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–3.
Matsutani D, Sakamoto M, Kayama Y, et al. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17:1–12.
Soga F, Tanaka H, Tatsumi K, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:1–8.
Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes and coronary artery disease: the EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019. https://doi.org/10.1161/CIRCULATIONAHA.119.042375.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author received honoraria from AstraZeneca, Ono pharmaceutical, Boehringer-Ingelheim, Eli Lilly and Company, Astellas Pharma, Mitsubishi Tanabe Pharma and Daiichi-Sankyo.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iwakura, K. Heart failure in patients with type 2 diabetes mellitus: assessment with echocardiography and effects of antihyperglycemic treatments. J Echocardiogr 17, 177–186 (2019). https://doi.org/10.1007/s12574-019-00446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-019-00446-9