Abstract
Purpose of Review
Type 2 diabetes (T2D) is associated with an increased risk of heart failure (HF), with diabetic cardiomyopathy (DbCM) referring to abnormal heart structure in the absence of other driving cardiac factors such as hypertension, coronary artery disease (CAD), and valvular heart disease. Stage B DbCM is commonly asymptomatic and represents a form of stage B HF; DbCM thus represents a transitional phenotype prior to onset of symptomatic HF. The pathogenesis of DbCM is not fully elucidated but involves hyperglycemia, insulin resistance, increased free fatty acids (FFA), lipotoxicity, oxidative stress, advanced glycation end product (AGE) formation, activation of the renin–angiotensin–aldosterone system (RAAS) with an increase in angiotensin II, and dyshomeostasis of calcium, which all contribute to left ventricular hypertrophy (LVH) and cardiac systolic and diastolic dysfunction. Although DbCM is an established pathogenic process, it is underrecognized clinically due to its commonly asymptomatic nature. Raising awareness to identify high-risk individuals with stage B HF due to DbCM, who may subsequently progress to overt HF (stage C/D HF), as well as identifying new pharmacological agents and approaches to prevent functional decline, may help reduce this global health problem. The aim of this review is to focus on stage B DbCM; provide data on diagnostic approaches, current therapies, and potential therapies under investigation; and highlight the need to raise awareness and interdisciplinary dialogue among clinicians and researchers.
Recent Findings
There are no currently approved therapeutic strategies to treat or prevent progression of stage B DbCM, but multiple attempts are being made to target different pathogenic mechanisms involved in the development of DbCM. Recent cardiovascular (CV) outcome trials (CVOTs) have identified newer therapeutic agents with CV benefit, such as sodium-glucose cotransporter-2 (SGLT-2) inhibitors that reduce hospitalization for HF and glucagon-like peptide-1 (GLP-1) receptor agonists that reduce major adverse CV events (MACE), though without consistent effect on HF outcomes. Recent clinical practice guidelines recommend screening patients at high risk for HF. Further definition and interdisciplinary discussion of high-yield populations to screen, appropriate subsequent evaluation and intervention are needed to advance this area.
Summary
DbCM is a complex entity that results from multiple pathogenic mechanisms triggered by impairment of glucose and lipid metabolism over many years. DbCM is commonly asymptomatic and represents a form of stage B HF. It is an underrecognized process that may progress to functional decline and overt HF. Although newer medications approved for the treatment of T2D may play an important role in reducing the risk of HF complications, less focus has been placed on earlier recognition and treatment of DbCM while asymptomatic. Additional efforts should be made to further study and target this stage in order to decrease the overall burden of HF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of HF in T2D is estimated between 9 and 22%, or four times that of the general population, and higher in adults with T2D over 60 years old [1]. The presence of T2D itself confers increased risk of mortality, with increased risk for CV mortality, HF-related hospitalization, and longer hospital stay [2]. Hence, understanding how to identify and prevent progression of HF in T2D is of pressing importance.
DbCM is a specific form of heart disease characterized by the presence of abnormal heart structure due to underlying metabolic derangements associated with diabetes mellitus (DM), not due to known risk factors such as hypertension and CAD. While DbCM has been described in both type 1 and type 2 DM, early descriptions of DbCM did not specify type of diabetes. Underlying mechanisms of DbCM in type 1 and type 2 DM likely overlap, with duration and increased glycemia contributing to increased risk of DbCM in both [3]. Herein, type of diabetes is specified if done so in the studies cited, otherwise referred to as DM.
Described in 1972 when found in postmortem analyses of 4 patients with DM, cardiomegaly, and HF without a clear cause, DbCM was initially attributed to diabetic microangiopathy and abnormal metabolism seen in diabetes [4]. This was later confirmed in the Framingham study with data suggesting that diabetes was another cause of cardiomyopathy conferring a 4- to fivefold increased risk of HF after adjusting for other risk factors [5]. The presence of DM confers a high risk for the development of cardiomyopathy [6] and additionally induces important changes in myocardial structure and function even prior to overt symptoms related to cardiac disease [7, 8].
Clinical practice guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) [9], as well as consensus recommendations from the recent Universal Definition and Classification of HF [10••], identify 4 stages of HF, spanning at risk (stage A), asymptomatic structural heart disease or with abnormal cardiac biomarkers (stage B or pre-HF), symptomatic (stage C), and refractory (stage D). Unlike New York Heart Association (NYHA) class, which is based on symptoms and is bidirectional, reflecting improvements or decline of functional status, the HF staging classification categorizes HF based on underlying pathophysiology, which is typically progressive.
It is estimated that the number of patients with left ventricular systolic dysfunction (LVSD) in stage B HF is 4 times higher than in the clinically symptomatic stages C and D HF combined [11, 12]. However, these high-risk individuals are usually clinically underrecognized.
While achieving glucose control has been the cornerstone of preventing DM complications, no consensus has been reached regarding the best strategies to prevent, identify, or treat DbCM [8]. Thus, raising awareness and identifying the high-risk individuals with stage B DbCM, who may subsequently progress to overt HF, as well as identifying novel therapeutic approaches to prevent and/or treat stage B DbCM, are critical in order to reduce the global health burden of HF.
In this review, we will summarize the pathogenic mechanisms involved in the development of DbCM (focusing on stage B DbCM as a pivotal stage for identifying individuals at risk) and provide data on diagnostic approaches, current therapies, and potential therapeutic strategies, with the goal of raising interdisciplinary awareness and dialogue on the underrecognized entity of stage B DbCM.
Definition of DbCM
HF is a multifactorial clinical syndrome resulting from anatomic or functional alterations of ventricular filling or blood ejection and is not synonymous with the terms cardiomyopathy or left ventricular (LV) dysfunction, as these describe cardiac structural or functional changes. In most patients with HF, systolic and diastolic abnormalities may concur, regardless of the ejection fraction (EF) [6]. In patients with DM, different factors have been associated with HF and include age, duration of DM, poor glycemic control, urinary albumin-to-creatinine ratio (UACR), peripheral vascular disease, ischemic heart disease, and obesity [13,14,15,16,17,18].
Pathophysiologic Connection Between Diabetes and the Heart
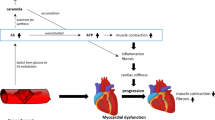
DbCM is a complex entity that results in cardiac remodeling and impairment of excitation and contraction coupling. The altered glucose and lipid metabolism seen in T2D promotes increased oxidative stress, inflammation, cellular and endothelial damage, mitochondrial dysfunction, fibrosis, and systolic and diastolic dysfunction [8, 19] (Fig. 1).
Pathophysiologic links between diabetes and the heart. Type 2 diabetes (T2D) is a complex metabolic disorder. Hyperglycemia plays a crucial role in the pathophysiology of complications related to T2D. Multiple pathways illustrated here (polyol pathway, hexosamine pathway, PKC pathway, AGE pathway) contribute to an increased risk of diabetic cardiomyopathy. Abbreviations: AGEs advanced glycation end products, Ang II angiotensin II, ER endoplasmic reticulum, FFA free fatty acids, GLUT glucose transporters, LVH left ventricular hypertrophy, O-GlcNAc O-linked N-acetylglucosamine, PDK pyruvate dehydrogenase kinase, PFK phosphofructokinase, PKC protein kinase C, RAAS renin–angiotensin–aldosterone system, ROS reactive oxygen species. Figure created with BioRender.com
Hyperinsulinemia, Insulin Resistance, Lipid Accumulation
Insulin is an anabolic hormone that exerts metabolic and mitogenic properties. The metabolic effects of insulin occur after a short and low hormonal exposure, while the mitogenic effects occur after a prolonged and high hormonal exposure. The two main signaling pathways, metabolic and mitogenic, through which insulin acts are the phosphatidylinositol 3-kinase (PI3K)/AKT (also known protein kinase B or PKB) and mitogen-activated protein kinase (MAPK)/Ras pathways, respectively [20]. Insulin resistance mediates hyperglycemia, hyperlipidemia, and lipotoxicity. Hyperinsulinemia and insulin resistance promote LVH through the activation of the MAPK/Ras pathway [21] and promote the increase of free fatty acids (FFA) to maintain energy production, which leads to a shift in energy production, decreased glucose utilization, and intracellular accumulation of FFA, favoring lipotoxicity. This subsequently results in loss of metabolic substrate flexibility in the diabetic heart and increased production of reactive oxygen species (ROS) that further contributes to stress and cardiomyocyte apoptosis, making the heart susceptible to ischemia–reperfusion injury [21, 22] (Fig. 1).
Hyperglycemia
Hyperglycemia induces oxidative stress through the activation of multiple pathways of non-oxidative glucose metabolism such as the AGE pathway, the polyol pathway, the protein kinase C (PKC) pathway, and the hexosamine pathway, resulting in mitochondrial damage, impairment in cardiac protein contractility, disruption in calcium handling, and changes in gene expression seen in the diabetic heart [23, 24]. Abnormal cell metabolism and oxidative stress lead to endoplasmic reticulum (ER) stress, cardiomyocyte death, endothelial dysfunction and damage, inflammation, and profibrotic responses [8]. The increase in collagen deposition and expression of profibrotic molecules such as transforming growth factor beta 1 (TGFβ-1) cause abnormal extracellular matrix (ECM) deposition. AGEs have directly been implicated in aberrant ECM changes [21]. Furthermore, hyperglycemia activates RAAS and increases angiotensin II (Ang II), inducing proliferation of cardiac fibroblasts and contributing to LVH while also exacerbating insulin resistance, hypertension, and hyperlipidemia [22] (Fig. 1).
Inflammation
Diabetes can be characterized as a chronic state of inflammation, and a maladaptive proinflammatory response contributes to the impairments seen in DbCM. Different alterations of the innate immune system as well as activation of multiple proinflammatory cytokines (tumor necrosis factor alpha via activation of nuclear factor kappa B), interleukins (IL) 6 and 8, monocyte chemotactic protein 1 (MCP-1), and adhesion molecules (such as intercellular adhesion molecule 1 and vascular cell adhesion molecule 1) are present in the diabetic heart and contribute to abnormal cardiac remodeling and function [3].
Identifying Stage B Cardiomyopathy
As noted, DbCM is a form of stage B HF, also known as pre-HF. The challenge in recognizing stage B HF is the fact that these patients are asymptomatic or have minimal symptoms. High clinical suspicion on the basis of the clinical risk factors (Table 1) is needed; adjunctive testing, as outlined below, may then be applied to support clinical judgment regarding presence of DbCM.
Biomarkers
As noted in the Universal Definition and Classification of HF, elevated concentrations of B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) are criteria to help identify the presence of stage B HF. Serum concentrations of these biomarkers correlate with the presence and severity of HF, with increasing evidence supporting their use for screening and early intervention to prevent HF [6, 25]. Furthermore, therapeutic strategies such as accelerated up-titration of RAAS blockers and beta-blockers for primary prevention of cardiac events for patients with T2D have been shown as a safe and effective intervention in patients pre-selected by NT-proBNP levels [26]. It is important to consider that both BNP and NT-proBNP may be increased in a variety of conditions, including kidney disease, atrial arrhythmias, and advanced age, and may be decreased in patients with obesity or HF with preserved EF and in Black patients, hence limiting universal cutoffs. Nonetheless, efforts are ongoing toward standardizing diagnostic criteria for use of BNP or NT-proBNP in identifying patients at risk for HF during stage A/B HF to support prevention of HF (ACC/AHA Class of Recommendation IIa) [27, 28].
Echocardiographic Criteria
Multiple echocardiographic abnormalities have been noted in stage B HF. These include higher LV mass, increased wall thickness, left atrial enlargement (LAE), LV diastolic dysfunction, and impaired global longitudinal strain (GLS).
Specifically, the echocardiographic definition of stage B HF includes the presence of at least one of the following [29,30,31]:
-
LVH, defined as left ventricular mass index (LVMi) > 115 g/m2 in men and > 95 g/m2 in women
-
LAE, defined as left atrial volume index (LAVi) ≥ 34 ml/m2
-
Abnormal ratio of mitral inflow peak early diastolic velocity (E) to tissue Doppler mitral annular early diastolic velocity (e′), defined as E/e′ ≥ 13
-
Impaired GLS, defined as GLS < 18%
According to Yang et al. [30], LAE is the strongest predictor of stage B HF, followed by impaired GLS and abnormal E/e′. Furthermore, diastolic dysfunction (DD), which is present in at least 50% of asymptomatic patients with T2D, is associated with LAE; impaired GLS is also commonly found in asymptomatic patients with stage B DbCM and is a sensitive marker of systolic dysfunction; and LVH represents an early change in myocardial structure [32••].
Cardiopulmonary Exercise Test (CPET)
Exercise intolerance is a common manifestation of HF, yet many patients with stage B HF may not declare typical exertional symptoms of HF due to sedentary lifestyle or limited exertion on a day-to-day basis. However, when evaluated with exercise testing, impaired functional capacity may be identified. Cardiopulmonary exercise testing (CPET) enables measurement of peak oxygen uptake (VO2) and other variables to assess exercise capacity, and when integrated with cardiac imaging and invasive hemodynamic monitoring, CPET provides a comprehensive characterization of multisystem reserve capacity, thus providing an important role in delineating previously unappreciated symptoms [33].
Early Detection of DbCM
The 2017 update from the AHA, ACC, and Heart Failure Society of America (HFSA) on the management of HF incorporates recommendations for evaluating patients at risk of developing HF, based on the multicenter, randomized STOP-HF (The St. Vincent’s Screening to Prevent Heart Failure) study [25], in which BNP-based screening of patients at risk for HF (defined by presence of hypertension, DM, or known vascular disease and without LVSD or HF) resulted in reduction of asymptomatic LV dysfunction and fewer hospitalizations. Another single-center, small, randomized trial, PONTIAC (“NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease”) [26], found that accelerated up-titration of RAAS and beta-blockers reduced CV events in patients with T2D and elevated NT-proBNP and without cardiac disease at baseline when compared to usual care. Of note, in STOP-HF and PONTIAC, the concentrations of BNP (≥50 pg/ml) or NT-proBNP (>125 pg/ml) used to identify eligible patients were considerably lower than those used for diagnosis of symptomatic HF.
Berg et al. [34] developed a clinical risk prediction score for hospitalization for HF (HHF) using data from the placebo arm of the SAVOR-TIMI 53 CVOT, externally validated by data from the placebo arm of the DECLARE-TIMI 58 study and identified 5 key independent clinical risk predictor variables of HHF: prior HF, history of atrial fibrillation, CAD, estimated glomerular filtration rate (eGFR) and UACR. Looking broadly at the interconnection between T2D and CV disease (CVD), the stage of elevated UACR and depressed eGFR corresponds to the microvascular complication stage of T2D, often associated with longstanding or inadequately controlled hyperglycemia. Thus, from a diabetes management perspective, this clinical stage, in which other microvascular complications are noted or suspected, may also prompt consideration of screening for DbCM.
Despite the recommendation that patients at risk of developing HF should be screened for HF, further study and interdisciplinary dialogue are needed to more precisely define who to screen for DbCM, when to screen for DbCM, and the extent of additional evaluation needed prior to making a diagnosis of DbCM, as well as how to prevent further progression once diagnosed.
As mentioned before, DbCM refers to an entity that excludes other underlying causes such as CAD or valvular heart disease. However, there is no reason to believe that a patient with DbCM could not get CAD. Part of the reason for identifying DbCM would be to recognize the possible subsequent complications, in the absence of other clinical disease or complications, allowing opportunity for prevention. Moreover, identifying a “clean” phenotype that lacks obvious CAD, prior MI, etc., could contribute to the better understanding of this entity as well as allow the development of therapies for this specific phenotype.
Current Glucose-Lowering Medications and Risk of HF
In this section, we review evidence surrounding current glucose-lowering medications and their effects on risk of HF or use in established HF.
The general management for patients with T2D includes achieving and maintaining glycemic control, with early studies establishing the relationship between degree of glycemia and incidence of HF. Recent studies have focused on the CV safety of glucose-lowering medications, as mandated by the US Food and Drug Administration (FDA); have assessed MACE, i.e., CV death, MI, or stroke; and many have included HF complications as a secondary endpoint. Notably, HF benefits seen in recent CVOTs did not correlate with glycemic control achieved, suggesting non-glycemic mechanisms may be at play.
Metformin
Metformin is the preferred initial pharmacotherapy for the treatment of hyperglycemia in T2D [35] that has demonstrated safety and efficacy and may reduce the risk of CV events and death [36]. Although a reduction in HF among patients with T2D has not been a prominent feature of studies examining effects of metformin, some data have suggested lower rates of incident HF among those treated with metformin. For example, in a retrospective cohort study [37], Tseng et al. reported a dose-dependent association between metformin use and a 40% reduction in HHF. More recently, Richardson and colleagues [38] examined rates of new-onset HF among metformin versus sulfonylurea users, reporting a 15% lower rate of HHF. However, neither of these studies specifically examined patients with DbCM.
An ongoing trial, Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular Outcomes VA-IMPACT (ClinicalTrials.gov NCT02915198) is evaluating metformin in patients with prediabetes and established CVD, may provide further information on the effect on HF.
Sulfonylureas
There are limited data regarding the use of sulfonylureas and the development of HF in patients with T2D [1]. However, a recent RCT [39] comparing linagliptin vs. glimepiride in 6042 patients with T2D and elevated CV risk resulted in noninferior risk of a composite CV outcome and no difference in HHF (HR 1.21, 95% CI 0.92–1.59) or investigator-reported HF events (HR 1.06, 95% CI 0.85–1.32).
Insulin
Insulin alone or in combination with other pharmacologic therapies is used to achieve glycemic targets in patients with T2D. Different studies have included insulin and have not demonstrated increased rates of HF [1]. The ORIGIN trial, a CVOT that randomized 12,537 participants with dysglycemia to receive insulin glargine vs. standard of care, found no difference in CV outcomes, including HF hospitalization [40].
Insights from CVOTs with DPP-4i, GLP-1 RA, and SGLT2i
In 2008, the US FDA mandated that all medications for T2D demonstrate CV safety in adequately powered CVOTs. Since then, various medications, including dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon like peptide 1 receptor analogues (GLP1-RA), and sodium-glucose cotransporter-2 inhibitors (SGLT2i), have undergone large, rigorous CVOTs [41] and have provided further insights of their efficacy in populations at high risk of CV events. Most of the DPP4 inhibitors (i.e., sitagliptin, linagliptin, and alogliptin) have shown CV neutrality for all MACE endpoints including HF hospitalization [42,43,44]. However, a 27% increase in HF hospitalization with saxagliptin vs. placebo (HR 1.27, 95% CI 1.07–1.51) was seen [45]. In sum, DPP-4 inhibitor trials have not shown evidence of benefits in HF, and specific DPP4 inhibitors may increase the risk of HF hospitalization in high-risk patients; thus, their use is recommended with caution in patients at risk for HF.
Recent evidence from large RCTs has demonstrated the benefits of SGLT2i in reducing MACE and HHF [46]. The first of the SGLT2i CVOTs, the EMPA-REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes Patients) [47], showed that HHF was reduced by 35% with empagliflozin treatment. Notably, the reduction in adverse HF events has been consistently demonstrated in other SGLT2i trials [48, 49], including in dedicated HF outcome trials [50, 51]. These studies have positioned SGLT2i as important therapeutic agents for treatment of T2D [1, 35, 52].
Several GLP1-RA have demonstrated reduction in MACE in patients with T2D with established atherosclerotic CVD (ASCVD) or at high risk of ASCVD but have not demonstrated consistent reductions in HF adverse outcomes. HHF was lower with albiglutide vs. placebo in the Harmony Outcomes trial [53] (HR 0.71, 95% CI 0.53–0.94) and non-significantly lower with liraglutide vs. placebo (HR 0.85, 95% CI 0.70–1.04) in the LEADER trial [54]. Meta-analyses of the GLP1-RA CVOTs [55] indicate an overall HR of 0.91 (0.83–0.99) for effects of GLP1-RA on HHF. However, dedicated studies evaluating the potential of GLP1-RA therapy in patients with HF have not shown improvements in outcomes or LVEF [56, 57], though this remains an active area of study.
Despite the advances of the recent CVOTs, significant gaps remain in our understanding, largely because HF itself has not traditionally been characterized in the large CVOTs. Detailed characterization in future studies [58], including functional class and clinical stage, EF, and HF therapy regimen, will better identify treatments for the various stages of HF treatment and prevention.
Additional Therapeutic Strategies
A meta-analysis [59] of 6 angiotensin-converting enzyme (ACE) inhibitor studies that stratified data by diagnosis of DM and included 2398 patients with DM showed similar reductions in mortality in patients with LV systolic dysfunction, both with DM (RR 0.84; 95% CI, 0.70–1.00) and without DM (RR 0.85; 95% CI, 0.78–0.92). Importantly, various studies of angiotensin receptor blockers (ARBs) had similar benefits in reducing CV morbidity and mortality outcomes which were not modified by having DM at baseline [1, 2, 60,61,62]. In addition to the benefits shown in the ACE inhibitor and ARB trials, a subgroup analysis [63] of the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) showed that in patients with HF with reduced ejection fraction, the angiotensin receptor-neprilysin inhibitor (ARNi) sacubitril-valsartan was beneficial compared to enalapril irrespective of glycemic status. Additional information may come from the PARADISE-MI study (ClinicalTrials.gov NCT02924727), which is evaluating the efficacy and safety of sacubitril/valsartan compared to ramipril on morbidity and mortality in high-risk patients following an MI. Mineralocorticoid receptor antagonists (MRAs) have also shown benefits in patients with HF in patients with and without DM. A subset analysis [64] of 1483 patients with DM with LVSD from the EPHESUS trial (the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) showed that treatment with eplerenone reduced the primary composite endpoint of CV mortality or CV hospitalization by 17%.
Finally, 3 FDA-approved beta-blockers (carvedilol, metoprolol succinate, and bisoprolol) have been shown to reduce morbidity and mortality in patients with DM and HF [1].
Thus, the four key pillars of treatment of HF with reduced ejection fraction include SGLT2i, ARNi, MRA, and evidence-based BB. All appear to be similarly beneficial for those with and without diabetes. Given the higher baseline risk of HF among patients with diabetes, the anticipated absolute risk reduction of these therapies is greater.
Potential Therapeutic Strategies for Stage B DbCM
No strategies to treat and/or prevent stage B DbCM have been specifically approved. Yet multiple attempts to target cardiac remodeling, oxidative stress, inflammation, and metabolic disturbances have been made to restore the different alterations in the diabetic heart [8].
Phosphodiesterase Inhibitors
Cyclic guanosine monophosphate (cGMP) acts as a secondary messenger, transducing nitric oxide (NO) and natriuretic peptide coupled signaling and stimulating phosphorylation by protein kinase G [65]. Cyclic GMP phosphodiesterase type 5 (PDE5) protein is upregulated in myocardial hypertrophy, and chronic inhibition of PDE5 in animal models has shown improvements in LVH and ischemia/reperfusion injuries and prevented cardiac remodeling. A small RCT [66] using sildenafil in 59 men with non-ischemic DbCM for 3 months and assessed by magnetic resonance imaging improved LV torsion and strain, LV mass/volume ratio, LV contraction, and circulating markers such as MCP-1 and TGF-β. The role of phosphodiesterase type 9A (PDE9A) in the development of cardiac hypertrophy has been also explored as it is also upregulated in cardiac hypertrophy and HF. PDE9A regulates natriuretic peptide, reverses heart disease independent of NO-synthase (NOS) activity, and it may become a potential therapeutic agent [65].
Anti-inflammatory Agents
Inflammation has been described as part of the complex pathogenesis of HF. Different data suggest that targeting inflammation without affecting the lipid profile may reduce CVD. In the CANTOS study [67], canakinumab, a therapeutic monoclonal antibody targeting IL1β in over 10,000 patients with previous MI and high-sensitivity C-reactive protein (hsCRP), significantly reduced levels of high-sensitivity CRP and IL-6 as well as the rate of recurrent CV events when compared to placebo, all independent of lipid-level lowering mechanisms. The subsequent analysis of the CANTOS trial [68] showed that canakinumab significantly reduced HHF in patients with prior MI and elevated hsCRP, suggesting that targeting inflammation and especially IL-1β may provide further benefits in patients with HF. However, further studies to understand the potential role of canakinumab in DbCM are required.
Colchicine is an anti-inflammatory drug with broad cellular effects that significantly lowered the risk of ischemic CV events in patients after a recent MI [69], as well as in patients with chronic CAD [70]. Thus, further studies to evaluate the role of colchicine in DbCM could also be of interest.
Agents Targeting Oxidative Stress
Zinc has been studied for its putative roles in reducing inflammation and oxidative stress. Polymorphisms in the gene that encodes metallothionein, which binds to zinc under physiological conditions, affects metal ion homeostasis, and regulates cellular redox status and signaling, are involved in DM-related CV complications, suggesting a potentially beneficial role of zinc in patients with DM [8, 71, 72].
Aldose reductase inhibitors (ARI) prevent glucose from being metabolized in the polyol pathway and have been studied for the treatment of diabetic neuropathy [73,74,75,76], yet the effects on cardiac function and performance were not clear in these studies. A small study with the ARI zopolrestat [75] which evaluated its beneficial effects on asymptomatic cardiac abnormalities in patients with type 1 or type 2 DM and neuropathy showed a clinically significant increase in LVEF (P < 0.02), cardiac output (P < 0.03), LV stroke volume (P < 0.004), and exercise LVEF (P < 0.001) as compared to placebo. Additional information could come from the ARISE-HF study (ClinicalTrials.gov NCT04083339) which is evaluating the safety and efficacy of the ARI AT-001 in patients with T2DM and Stage B DbCM. Interestingly this study is evaluating whether this ARI can improve or prevent the decline of functional capacity in patients with DbCM and without other causes of cardiac disease including CAD, stroke, valvular disease, arrhythmia, and other forms of cardiomyopathy.
Conclusions
Both T2D and HF represent global health problems and are closely intertwined. DbCM is a complex entity that has been recognized for almost 50 years and is the result of multiple pathogenic mechanisms triggered by alterations in glucose and lipid metabolism. Stage B DbCM represents a unique window of opportunity for prevention of HF and HF complications. While advances have been made in treating patients with established HF, significant progress needs to be made regarding early identification of patients with DbCM at risk of development or progression of HF, with continued incorporation of evidence-based approaches to slow the physiologic and clinical progression of DbCM.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–324. https://doi.org/10.1161/CIR.0000000000000691.
MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–85. https://doi.org/10.1093/eurheartj/ehn153.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38. https://doi.org/10.1161/CIRCRESAHA.117.311586.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. https://doi.org/10.1016/0002-9149(72)90595-4.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. https://doi.org/10.1016/0002-9149(74)90089-7.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52. https://doi.org/10.1161/CIR.0b013e31829e8807.
Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, et al. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: a systematic review and meta-analysis. Diab Vasc Dis Res. 2018;15(6):477–93. https://doi.org/10.1177/1479164118787415.
Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607. https://doi.org/10.1038/s41569-020-0339-2.
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-479. https://doi.org/10.1161/CIRCULATIONAHA.109.192065.
•• Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and writing committee of the Universal Definition of Heart Failure. J Card Fail. 2021. The Universal Definition and classification of HF identifies 4 stages of HF. The staging classification categorizes HF based on underlying pathophysiology, typically progressive, which differs from NYHA as the latter is based on symptoms and only reflects improvements or decline of functional status.
Frigerio M, Oliva F, Turazza FM, Bonow RO. Prevention and management of chronic heart failure in management of asymptomatic patients. Am J Cardiol. 2003;91(9A):4F-9F. https://doi.org/10.1016/s0002-9149(02)03335-0.
Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113(24):2851–60. https://doi.org/10.1161/CIRCULATIONAHA.105.600437.
Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43(12):2236–41. https://doi.org/10.1016/j.jacc.2003.10.074.
Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. https://doi.org/10.2337/diacare.27.3.699.
Carr AA, Kowey PR, Devereux RB, Brenner BM, Dahlof B, Ibsen H, et al. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol. 2005;96(11):1530–6. https://doi.org/10.1016/j.amjcard.2005.07.061.
Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115(11):1371–5. https://doi.org/10.1161/CIRCULATIONAHA.106.661405.
Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103(22):2668–73. https://doi.org/10.1161/01.cir.103.22.2668.
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–84. https://doi.org/10.2337/diacare.27.8.1879.
Teodoro JS, Nunes S, Rolo AP, Reis F, Palmeira CM. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2018;9:1857. https://doi.org/10.3389/fphys.2018.01857.
Kahn CR. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–51. https://doi.org/10.1146/annurev.me.36.020185.002241.
Nunes S, Soares, Pereira F, Reis F. The role of inflammation in diabetic cardiomyopathy. Int J Interferon Cytokine Mediator Res. 2012;4:59–73.
Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic cardiomyopathy: current and future therapies. Beyond glycemic control. Front Physiol. 2018;9:1514. https://doi.org/10.3389/fphys.2018.01514.
Brahma MK, Pepin ME, Wende AR. My sweetheart is broken: role of glucose in diabetic cardiomyopathy. Diabetes Metab J. 2017;41(1):1–9. https://doi.org/10.4093/dmj.2017.41.1.1.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. https://doi.org/10.2337/diabetes.54.6.1615.
Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66–74. https://doi.org/10.1001/jama.2013.7588.
Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365–72. https://doi.org/10.1016/j.jacc.2013.05.069.
Ibrahim NE, Burnett JC Jr, Butler J, Camacho A, Felker GM, Fiuzat M, et al. Natriuretic peptides as inclusion criteria in clinical trials: a JACC: heart failure position paper. JACC Heart Fail. 2020;8(5):347–58. https://doi.org/10.1016/j.jchf.2019.12.010.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–51. https://doi.org/10.1016/j.cardfail.2017.04.014.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. https://doi.org/10.1093/ehjci/jev014.
Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non-ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18(11):1331–9. https://doi.org/10.1002/ejhf.643.
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–91. https://doi.org/10.1016/j.echo.2012.10.008.
•• Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. https://doi.org/10.1016/j.jacc.2017.11.019. Diastolic dysfunction, associated with LAE, impaired GLS, a sensitive marker of systolic dysfunction, and LVH, represent early changes in myocardial structure and are found in patients with asymptomatic DbCM.
Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607–16. https://doi.org/10.1016/j.jchf.2016.03.022.
Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation. 2019;140(19):1569–77. https://doi.org/10.1161/CIRCULATIONAHA.119.042685.
American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24. https://doi.org/10.2337/dc21-S009.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. https://doi.org/10.1056/NEJMoa0806470.
Tseng CH. Metformin use Is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: a retrospective cohort analysis. J Am Heart Assoc. 2019;8(21):e011640. https://doi.org/10.1161/jaha.118.011640.
Richardson Jr TL, Hackstadt AJ, Hung AM, Greevy RA, Grijalva CG, Griffin MR, et al. Hospitalization for heart failure among patients with diabetes mellitus and reduced kidney function treated with metformin versus sulfonylureas: a retrospective cohort study. J Am Heart Assoc. 2021:e019211. https://doi.org/10.1161/jaha.120.019211.
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 Ddabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155–66. https://doi.org/10.1001/jama.2019.13772.
Investigators OT, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–28. https://doi.org/10.1056/NEJMoa1203858.
Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8–16. https://doi.org/10.1016/j.diabres.2019.02.014.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. https://doi.org/10.1056/NEJMoa1501352.
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. https://doi.org/10.1001/jama.2018.18269.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. https://doi.org/10.1056/NEJMoa1305889.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. https://doi.org/10.1056/NEJMoa1307684.
Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr, Kalyani RR, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76(9):1117–45. https://doi.org/10.1016/j.jacc.2020.05.037.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29. https://doi.org/10.1016/S0140-6736(20)31824-9.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. https://doi.org/10.1016/S0140-6736(18)32590-X.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190.
American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–50. https://doi.org/10.2337/dc21-S010.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. https://doi.org/10.1016/S0140-6736(18)32261-X.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85. https://doi.org/10.1016/S2213-8587(19)30249-9.
Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19(1):69–77. https://doi.org/10.1002/ejhf.657.
Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–8. https://doi.org/10.1001/jama.2016.10260.
Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351–66. https://doi.org/10.1016/j.jacc.2018.04.070.
Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41(9):1529–38. https://doi.org/10.1016/s0735-1097(03)00262-6.
Cohn JN, Tognoni G, Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–75. https://doi.org/10.1056/NEJMoa010713.
Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–8. https://doi.org/10.1016/S0140-6736(09)61913-9.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–906. https://doi.org/10.1056/NEJMoa032292.
Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9(1). https://doi.org/10.1161/CIRCHEARTFAILURE.115.002560.
O’Keefe JH, Abuissa H, Pitt B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: results from EPHESUS. Diabetes Obes Metab. 2008;10(6):492–7. https://doi.org/10.1111/j.1463-1326.2007.00730.x.
Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519(7544):472–6. https://doi.org/10.1038/nature14332.
Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, et al. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation. 2012;125(19):2323–33. https://doi.org/10.1161/CIRCULATIONAHA.111.063412.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–99. https://doi.org/10.1161/CIRCULATIONAHA.118.038010.
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505. https://doi.org/10.1056/NEJMoa1912388.
Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–47. https://doi.org/10.1056/NEJMoa2021372.
Giacconi R, Bonfigli AR, Testa R, Sirolla C, Cipriano C, Marra M, et al. +647 A/C and +1245 MT1A polymorphisms in the susceptibility of diabetes mellitus and cardiovascular complications. Mol Genet Metab. 2008;94(1):98–104. https://doi.org/10.1016/j.ymgme.2007.12.006.
Yang L, Li H, Yu T, Zhao H, Cherian MG, Cai L, et al. Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications. Am J Physiol Endocrinol Metab. 2008;294(5):E987–92. https://doi.org/10.1152/ajpendo.90234.2008.
Greene DA, Arezzo JC, Brown MB. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study Group. Neurology. 1999;53(3):580–91. https://doi.org/10.1212/wnl.53.3.580.
Ikeda T, Iwata K, Tanaka Y. Long-term effect of epalrestat on cardiac autonomic neuropathy in subjects with non-insulin dependent diabetes mellitus. Diabetes Res Clin Pract. 1999;43(3):193–8. https://doi.org/10.1016/s0168-8227(99)00015-7.
Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, et al. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27(2):448–54. https://doi.org/10.2337/diacare.27.2.448.
Roy TM, Broadstone VL, Peterson HR, Snider HL, Cyrus J, Fell R, et al. The effect of an aldose reductase inhibitor on cardiovascular performance in patients with diabetes mellitus. Diabetes Res Clin Pract. 1990;10(1):91–7. https://doi.org/10.1016/0168-8227(90)90086-9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Stanton, A.M., Vaduganathan, M., Chang, LS. et al. Asymptomatic Diabetic Cardiomyopathy: an Underrecognized Entity in Type 2 Diabetes. Curr Diab Rep 21, 41 (2021). https://doi.org/10.1007/s11892-021-01407-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-021-01407-2