Abstract
The gustatory cells in taste buds have been identified as paraneuronal; they possess characteristics of both neuronal and epithelial cells. Like neurons, they form synapses, store and release transmitters, and are capable of generating an action potential. Like epithelial cells, taste cells have a limited life span and are regularly replaced throughout life. However, little is known about the molecular mechanisms that regulate taste cell genesis and differentiation. In the present study, to begin to understand these mechanisms, we investigated the role of Mash1-positive cells in regulating adult taste bud cell differentiation through the loss of Mash1-positive cells using the Cre-loxP system. We found that the cells expressing type III cell markers—aromatic L-amino acid decarboxylase (AADC), carbonic anhydrase 4 (CA4), glutamate decarboxylase 67 (GAD67), neural cell adhesion molecule (NCAM), and synaptosomal-associated protein 25 (SNAP25)—were significantly reduced in the circumvallate taste buds after the administration of tamoxifen. However, gustducin and phospholipase C beta2 (PLC beta2)—markers of type II taste bud cells—were not significantly changed in the circumvallate taste buds after the administration of tamoxifen. These results suggest that Mash1-positive cells could be differentiated to type III cells, not type II cells in the taste buds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian taste buds are mainly observed in the gustatory papillae (fungiform, foliate, and circumvallate papillae) of the tongue (Finger and Simon 2000). Taste buds are multicellular end organs that comprise several distinct cell types. Taste bud cells are differentiated from surrounding epithelial cells, undergo continuous renewal, and are regularly replaced throughout the lifespan (Beidler and Smallman 1965; Farbman 1980; Delay et al. 1986; Stone et al. 2002).
Mash1 (Ascl1) is a mammalian achaete-scute homolog of the proneural gene, which encodes basic helix-loop-helix (bHLH) transcription factors (Johnson et al. 1990; Guillemot and Joyner 1993). Mash1 is expressed in precursors of the both central and peripheral neurons (Lo et al. 1991; Guillemot et al. 1993). A null mutation in the Mash1 gene eliminates most olfactory neurons, sympathetic and parasympathetic neurons, and enteric neurons of the foregut, suggesting that Mash1 plays a role in determining the cell fate of specific neural lineages (Guillemot et al. 1993; Sommer et al. 1996; Blaugrund et al. 1996).
Previous studies indicated that Mash1 is also expressed in the taste papillae of mouse embryos and in a subset of adult taste bud cells (Seta et al. 1999; Kusakabe et al. 2002; Miura et al. 2003, 2005; Nakayama et al. 2008). Recently, we observed that Mash1 was expressed in some basal cells and in the majority of differentiated type III taste cells, but never in type II taste cells of adult taste buds (Seta et al. 2006). Furthermore, we demonstrated that Mash1 is required for the expression of aromatic L-amino acid decarboxylase (AADC) and glutamate decarboxylase 67 (GAD67) in type III cells in the taste buds using Mash1 knockout (KO) mice (Seta et al. 2011; Kito-Shingaki et al. 2014). However, as Mash1 KO mice die prior to taste bud formation in the taste papillae, it is not possible to determine how Mash1 affects the differentiation of adult taste bud cells. A conditional gene recombination system can be used to explore the Mash1 function in adult taste buds. In Ascl1CreERT2 mice, the entire coding region of the endogenous achaete-scute complex homolog 1 (Mash1) gene is replaced by a CreERT2 fusion protein. Cre activity is induced after administering tamoxifen. CAG-floxed neo-DTA mice contain the CAG promoter, which is followed by the neomycin resistance cassette that is flanked by the loxP sequence and the diphtheria toxin A (DTA) gene. Ascl1CreERT2::CAG-floxed neo-DTA mice are generated by breeding Ascl1 CreERT2 knock-in mice with CAG-floxed neo-DTA mice. Tamoxifen-inducible Cre-mediated recombination results in the removal of the loxP-flanked DNA sequence, and the DTA gene is driven by the CAG promoter in Mash1-expressing cells.
In the present study, we investigated the role of Mash1-positive cells in the differentiation of adult taste bud cells, utilizing several transgenic mouse strains (Ascl1CreERT2, CAG-floxed neo-DTA, and GAD67-GFP) to explore the mechanisms that regulate taste bud cell differentiation.
Materials and methods
Animals
All of the animals used in this study were maintained and handled according to the protocols approved by Kyushu Dental University Animal Care. Adult animals used in this study were Ascl1 CreERT2 knock-in mice (The Jackson Laboratory, stock no: 012882), CAG-floxed neo-diphtheria toxin A (DTA) mice (Matsumura et al. 2004), and glutamate decarboxylase 67 (GAD67)-GFP knock-in mice (Tamamaki et al. 2003). Ascl1CreERT2::CAG-floxed neo-DTA::GAD67-GFP(MGD) mice were generated by breeding Ascl1 CreERT2 knock-in mice, CAG-floxed neo-DTA mice with GAD67-GFP knock-in mice, and were referred to as MGD mice. The genotyping of mice used the following primers:
Ascl1 CreERT2 mutant sense, 5′-AACTTTCCTCCGGGGCTCGTTTC-3′;
Ascl1 CreERT2 mutant antisense, 5′-CGCCTGGCGATCCCTGAACATG-3′; GAD67-GFP sense, 5′-GGCACAGCTCTCCCTTCTGTTTGC-3′;
GAD67-GFP mutant antisense, 5′-CTGCTTGTCGGCCATGATATAGACG-3′; CAG-floxed neo-DTA sense, 5′-GCCTTCTATCGCCTTCTTGACGAGTTCTTC-3′;
CAG-floxed neo-DTA antisense, 5′-CTACATAACCAGGTTTAGTCCCG-3′.
Tissue preparation
MGD mice were injected intraperitoneally with tamoxifen (100 mg/kg; Sigma, T5648) in sunflower seed oil (Sigma, S5007), which has the effect of translocating Cre-ERT2 to the nucleus, where it excises the reporter gene.
At the age of 6–8 weeks, MGD and Ascl1 CreERT2::GAD67-GFP (MG) (the control) mice were administered tamoxifen once every 24 h for 3, 5, and 10 days, according to Kim et al. (2011). Ascl1 CreERT2::GAD67-GFP (MG) (the control) mice were administered tamoxifen once every 24 h for 10 days. Mice were anesthetized by intraperitoneally administering pentobarbital (50 mg/kg) and perfusing through the left ventricle with 4% paraformaldehyde (PFA) in phosphate buffer, pH 7.4. The tongues of the perfused mice were fixed overnight in the same fixative and embedded in OCT compound (Sakura, Torrance, CA, USA). Cryostat sections (6–8 µm) were mounted on MAS-coated Superfrost slides (Matsunami, Japan) and stored in airtight boxes at − 80 °C.
Immunohistochemistry
For immunohistochemical staining, the sections were blocked for 2 h in 5% goat serum in phosphate-buffered saline (PBS) and incubated with the primary antibodies rabbit anti-aromatic L-amino acid decarboxylase (AADC) (1:200; Gene Tex), goat anti-carbonic anhydrase IV (CA4) (1:200; R&D Systems), rabbit anti-gustducin (1:1000; Santa Cruz), rabbit anti-phospholipase C beta2 (PLC beta2) (1:1000; Santa Cruz), rabbit anti-neural cell adhesion molecule (NCAM), and rabbit anti-synaptosomal-associated protein 25 (SNAP25) overnight at 4 °C in a humidified chamber. After rinsing with PBS, sections were incubated with the secondary antibodies Alexa Fluor 488 conjugated donkey anti-rabbit IgG (1:1000) or Alexa Fluor 546 conjugated donkey anti-goat IgG (1:1000) overnight at 4 °C. Slides were rinsed with PBS and coverslipped with Vectashield (Vector Laboratories, USA). Negative controls were performed by omitting each of the primary antibodies.
All images were obtained using a fluorescence microscope (BZ-9000; KEYENCE, Osaka, Japan). Digital images were contrasted and color adjusted, and plates were created using Adobe Photoshop CS5 for Macintosh.
Statistical analyses
Statistical significance was assessed using one-way ANOVA and analysis of variance followed by the Tukey–Kramer test for each group. Significance was defined as p < 0.05.
Results
After administering tamoxifen to MGD mice for 3, 5, and 10 days, there was no significant difference between the control and experimental groups in the size and shape of the circumvallate papilla. Moreover, there was no significant difference in the distribution or number of taste buds in the circumvallate papillae between the tamoxifen-treated MGD mice and the control mice (Table 1).
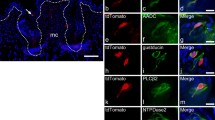
To investigate the influence of the loss of Mash1-positive cells in the differentiation of taste bud cells, we performed immunohistochemistry using taste cell markers. PLC beta2 is a marker of type II and a subset of type III taste cells (Clapp et al. 2001). Immunoreactivity of PLC beta2 was present in a large subset of mouse circumvallate taste buds (Fig. 1a–h). Gustducin is a marker of a subset of type II taste cells (Clapp et al. 2001; Yee et al. 2001). Immunoreactivity of gustducin was also present in a large subset of mouse circumvallate taste buds (Fig. 1i–p). The expression of these type II cell markers was not significantly different from that in the control (Fig. 2). Next we investigated the influence of the loss of Mash1 on the expression of type III cell markers in taste buds. GAD67 is a subset of type III cell markers (Kito-Shingaki et al. 2014). The expression of GAD67 in taste bud cells was missing in Mash1 mutant mice. In MGD mice, after the administration of tamoxifen for 10 days, GAD67-expressing taste cells were significantly reduced compared with those of control mice (Fig. 3a–h). AADC, CA4, NCAM, and SNAP25 are also type III cell markers (Yee et al. 2003, Seta et al. 2011); these type III cell markers were found at a rate of about 5–7 immunoreactive cells per taste bud in the control mice (Figs. 3e, m, u, 4e, m). The observed reduction in the number of immunopositive cells for type III cell markers depended on the length of administration of tamoxifen (Fig. 5). AADC and CA4 levels were significantly reduced to approximately 75% of those in the control mice after the administration of tamoxifen for 5 days and 10 days, respectively (Fig. 3i–x). On the other hand, NCAM and SNAP25 were significantly reduced compared to those of the control after the administration of tamoxifen for 10 days (Fig. 4a–p; Table 2).
Expression of type II cell markers (PLCbeta2 in a–h, gustducin in i–p) after the administration of tamoxifen in mouse circumvallate papilla. Dashed line indicates the outline of a taste bud. The expression of these type II cell markers was not significantly different from that of the control. Scale bars = 10 µm
Expression of type III cell markers (GAD67 in a–h, AADC in i–p, and CA4 in q–x) after the administration of tamoxifen in mouse circumvallate papilla. Dashed line indicates the outline of a taste bud. A reduction was observed in the number of immunopositive cells for type III cell markers depending on the length of administration of tamoxifen. Scale bars = 10 µm
Expression of type III cell markers (NCAM in a–h and SNAP25 in i–p) after the administration of tamoxifen in mouse circumvallate papilla. Dashed line indicates the outline of a taste bud. A reduction was also observed in the number of immunopositive cells for type III cell markers depending on the length of administration of tamoxifen. Scale bars = 10 µm
Discussion
This study shows the effect of the loss of Mash1-positive cells in adult taste bud cells. We found that there was no significant difference in the number and size of taste buds in the adult circumvallate papilla between the control and experimental groups. After administering tamoxifen for 10 days, the expression of type III cell markers was significantly reduced compared with that of the control mice, but the expression of type II cell markers was not significantly different from that of the control.
Recent studies have demonstrated that Mash1 is not only expressed in the precursors of the nervous system but also in neuroendocrine cells and sensory organs. Studies evaluating the loss of function of Mash1 revealed that Mash1 appears to act during a late stage of neuronal differentiation, neuroendocrine cells and olfactory neurons differentiation (Guillemot et al. 1993). Furthermore, gain of function of Mash1 experiments showed that Mash1 may play a role in regulating subtype-specific aspects of neural differentiation (Lo et al. 1998). Recently, we demonstrated that AADC-IR- and GAD67-expressing type III cells disappeared although no significant differences were observed in gene expression in the developing papillae or taste bud formation in the soft palate of Mash1 mutant mice (Seta et al. 2011; Kito-Shingaki et al. 2014). In the present study, we observed that the tamoxifen-induced elimination of Mash1-expressing cells did not affect taste bud morphology and formation in adult mice. These results indicate that Mash1 does not play a role in taste bud cell differentiation from the surrounding epithelial cells.
Based on the ultrastructural features of taste bud cells, mammalian taste buds possess four distinct morphologically identifiable types of cells (Murray 1986).Among these taste bud cell types, only type III cells make synaptic contact with nerve terminals; these cells are considered sour taste receptor cells. Mash1 was expressed in the subset of basal cells and also in that of mature taste cells (Seta et al. 1999). In this study, we observed that type II cell markers in the experimental group were unchanged compared with those of the control mice. This result suggests that the tamoxifen-induced elimination of Mash1-expressing basal cells does not affect type II cell differentiation, and there are two types of basal cells: one is a Mash1-expressing basal cell and the other is a Mash1-negative basal cell. We previously demonstrated that AADC- and GAD67-expressing cells were missing in Mash1 KO mice. The type III cell markers NCAM and SNAP25 were, however, expressed in the soft palate epithelial tissue of Mash1 KO mice according to RT-PCR, and NCAM-immunopositive cells were observed in the soft palate taste buds of Mash1 KO mice (Seta et al. 2011). In this study, the expression of all type III cell markers was significantly reduced compared with that of the control at 10 days after the administration of tamoxifen. There were, however, differences in the reduction rates of the type III cell markers. AADC, CA4, and GAD67 expression was reduced to a greater extent than NACM and SNAP25 expression. Our results suggest that there are two subsets of type III cells in taste buds: (1) Mash1 appears to be necessary for the differentiation of a subset of type III cells—AADC-positive and GAD67-positive cells in the taste buds, and (2) Mash1 does not appear to be necessary for the differentiation of a subset of type III cells—NACM and SNAP25-positive cells. Taken together, our investigations revealed that Mash1 may play an essential role in the differentiation of a subset of type III cells (AADC-, CA4-, and GAD67-expressing cells). Unfortunately, MGD mice do not completely lose Mash1-expressing cells with the administration of tamoxifen. Thus, the precise function of Mash1 in taste bud cell differentiation remains to be determined.
In conclusion, the present study indicates that Mash1 is required to regulate the expression of type III cell markers in adult taste buds. Further studies that target transcription factors will provide a better understanding of taste bud cell differentiation.
References
Beidler LM, Smallman RI (1965) Renewal of cells within taste buds. J Cell Biol 27:263–272
Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD (1996) Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development 122:309–320
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC (2001) Immunocytochemical evidence for co-expression of type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2:6
Delay RJ, Kinnamon JC, Roper SD (1986) Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J Comp Neurol 253:242–252
Farbman AI (1980) Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet 13:349–357
Finger TE, Simon SA (2000) Cell biology of taste epithelium. In: Finger TE, Silver WL, Restrepo D (eds) The neurobiology of taste and smell. Wiley-Liss, New York, pp 287–314
Guillemot F, Joyner AL (1993) Dynamic expression of the murine achaete-scute homologue Mash-1 in the developing nervous system. Mech Dev 42:171–185
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476
Johnson JE, Birren SJ, Anderson DJ (1990) Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature 346:858–861
Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE (2011) Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE 6(3):e18472
Kito-Shingaki A, Seta Y, Toyono T, Kataoka S, Kakinoki Y, Yanagawa Y, Toyoshima K (2014) Expression of GAD67 and Dlx5 in the taste buds of mice genetically lacking Mash1. Chem Senses 39:403–414
Kusakabe Y, Miura H, Hashimoto R, Sugiyama C, Ninomiya Y, Hino A (2002) The neural differentiation gene Mash-1 has a distinct pattern of expression from the taste reception-related genes gustducin and T1R2 in the taste buds. Chem Senses 27:445–451
Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ (1991) Mammalian achaete-scute homolog 1 is transiently expressed by specially-restricted subsets of early neuroepithelial and neural crest cells. Genes Dev 5:1524–1537
Lo L, Tiveron MC, Anderson DJ (1998) Mash1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development 125:609–620
Matsumura H, Hasuwa H, Inoue N, Ikawa M, Okabe M (2004) Lineage-specific cell disruption in living mice by Cre-mediated expression of diphtheria toxin A chain. Biochem Biophys Res Commun 321:275–279
Miura H, Kusakabe Y, Kato H, Miura-Ohnuma J, Tagami M, Ninomiya Y, Hino A (2003) Co-expression pattern of Shh with Prox1 and that of Nkx2.2 with Mash1 in mouse taste bud. Gene Expr Patterns 3:427–430
Miura H, Kato H, Kusakabe Y, Ninomiya Y, Hino A (2005) Temporal changes in NCAM immunoreactivity during taste cell differentiation and cell lineage relationships in taste buds. Chem Senses 30:367–375
Murray RG (1986) The mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res 95: 175-88
Nakayama A, Miura H, Shindo Y, Kusakabe Y, Tomonari H, Harada S (2008) Expression of the basal cell markers of taste buds in the anterior tongue and soft palate of the mouse embryo. J Comp Neurol 509:211–224
Seta Y, Toyono T, Takeda S, Toyoshima K (1999) Expression of Mash1 in basal cells of rat circumvallate taste buds is dependent upon gustatory innervation. FEBS Lett 444:43–46
Seta Y, Stoick-Cooper CL, Toyono T, Kataoka S, Toyoshima K, Barlow LA (2006) The bHLH transcription factors, Hes6 and Mash1, are expressed in distinct subsets of cells within adult mouse taste buds. Arch Histol Cytol 69:189–198
Seta Y, Oda M, Kataoka S, Toyono T, Toyoshima K (2011) Mash1 is required for the differentiation of AADC-positive type III cells in mouse taste buds. Dev Dyn 240:775–784
Sommer L, Ma Q, Anderson DJ (1996) Neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci 8:221–241
Stone LM, TanS-S Tam PPL, Finger TE (2002) Analysis of cell lineage relationships in taste buds. J Neurosci 22:4522–4529
Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T (2003) Green fluorescent protein expression and colocalization with calretinin, paravalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467:60–79
Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC (2001) “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 440:97–108
Yee CL, Jones KR, Finger TE (2003) Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol 459:15–24
Acknowledgements
The work was supported by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research no. 15K11018 to Y.S.). The authors would like to thank Enago (www.enago.jp) for performing a review of the English language used in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takagi, H., Seta, Y., Kataoka, S. et al. Mash1-expressing cells could differentiate to type III cells in adult mouse taste buds. Anat Sci Int 93, 422–429 (2018). https://doi.org/10.1007/s12565-018-0431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-018-0431-4