Abstract
Mammalian taste bud cells are composed of several distinct cell types and differentiated from surrounding tongue epithelial cells. However, the detailed mechanisms underlying their differentiation have yet to be elucidated. In the present study, we examined an Ascl1-expressing cell lineage using circumvallate papillae (CVP) of newborn mice and taste organoids (three-dimensional self-organized tissue cultures), which allow studying the differentiation of taste bud cells in fine detail ex vivo. Using lineage-tracing analysis, we observed that Ascl1 lineage cells expressed type II and III taste cell markers both CVP of newborn mice and taste organoids. However, the coexpression rate in type II cells was lower than that in type III cells. Furthermore, we found that the generation of the cells which express type II and III cell markers was suppressed in taste organoids lacking Ascl1-expressing cells. These findings suggest that Ascl1-expressing precursor cells can differentiate into both type III and a subset of type II taste cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taste buds are peripheral sensory end organs used for the reception of chemical substances in food and are a cell population composed of many taste cells concentrated in the gustatory papillae on the tongue surface. A single taste bud contains about 50–100 taste cells, which are classified as types I–IV based on their morphological and immunohistochemical characteristics (Murray 1973; Finger and Simon 2000). Type I cells expressing nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) are the most abundant cell type within each taste bud and are generally considered glial-like, whereas type II and III cells function as taste receptors (Lindeman 1996; Yang et al. 2004; Barlow and Klein 2015). Type II cells express various immunohistochemical markers, including phospholipase C β2 (PLCβ2) and gustducin (Kim et al. 2003; Clapp et al. 2004). Type III cells are characterized by the expression of various markers, such as aromatic L-amino acid decarboxylase (AADC), carbonic anhydrase IV (Car4), and glutamate decarboxylase 67 (GAD67) (DeFazio et al. 2006; Huang et al. 2006, 2008; Seta et al. 2007; Chandrashekar et al. 2009). Because they form synapses with gustatory nerve fibers, type III cells are considered more neural-like than types I and II (DeFazio et al. 2006; Chaudhari and Roper 2010). These various taste cell types, each of which has specific characteristics, are differentiated from basal cells (type IV cells) within taste buds and are regularly replaced throughout life (Beidler and Smallman 1965; Farbman 1980; Delay et al. 1986; Stone et al. 1995). Recently, leucine-rich repeat-containing G-protein coupled receptor (Lgr) 5-positive cells were identified as adult taste stem cells in the posterior tongue that give rise to type I–III taste cells (Yee et al. 2013; Ren et al. 2014). Several signaling pathways and molecules are known to be involved in the development or homeostasis of taste buds, e.g., Wnt signaling, sonic hedgehog (Shh) signaling, insulin signaling, and bone morphogenetic proteins (Zhou et al. 2006; Nguyen and Barlow 2010; Gaillard et al. 2015; Castillo-Azofeifa et al. 2017; Takai et al. 2019). Nevertheless, the detailed mechanisms underlying the developmental process by which mature taste cells are elongated from taste stem/progenitor cells via precursors in the basal side of the taste bud remain unclear. Currently, the taste organoid culture system has been established as an experimental tool to better understand the mechanisms of the cell differentiations and functions of taste buds in vivo cellular environment (Ren et al. 2014, 2017). Taste organoids are three-dimensional self-organized tissue cultures that provide many exploratory possibilities for revealing key features of in vivo real taste organs.
Achaete-scute complex-like 1 (Ascl1), one of the basic helix–loop–helix transcription factors, is specifically expressed in subsets of neuronal precursors in both the developing central and peripheral nervous systems. The expression of Ascl1 (also known as Mash1) is essential for the differentiation of neurons in the nervous system, neuroendocrine cells, and sensory organs (Lo et al. 1991; Guillemot et al. 1993; Guillemot and Joyner 1993; Tomita et al. 1996). In our previous study, we found that within the mature taste buds of adult mice, Ascl1 is detected in some basal cells and a subset of type III cells although not in type II cells (Seta et al. 2006). Using Ascl1 knockout mice, we also found that Ascl1 is required for the expression of GAD67 and AADC in the epithelium of the circumvallate papillae (CVP) and taste buds of the soft palate during the embryonic stages (Seta et al. 2011; Kito-Shingaki et al. 2014). However, in their immature state, both type II and III cells have been shown to express Ascl1 (Miura et al. 2006). Furthermore, fate-mapping analysis has revealed that most Ascl1-expressing precursor cells can differentiate into type III cells and possibly PLCβ2 (often used as a marker for type II cells)-expressing cells in adult mouse taste buds (Hsu et al. 2021). Thus, it remains unclear exactly how Ascl1-expressing cells are involved in the taste cell differentiation.
In the present study, we investigated the role of Ascl1 in the differentiation of taste bud cells using the native taste tissues of newborn mice and taste organoids that recapitulate in vivo processes of the taste cell differentiation under ex vivo conditions. Using lineage-tracing analysis, we observed that Ascl1 lineage cells expressed type II and III taste cell markers in taste buds during their initial development and in taste organoids. Furthermore, we found that the generation of type II and III cells was suppressed in taste organoids lacking Ascl1-expressing cells. Collectively, these findings suggest that Ascl1-expressing precursor cells may differentiate into both type II and type III taste cells.
Materials and methods
Animals
All animal experiments were approved by Kyushu Dental University Animal Care (approval no: 20–03). Ascl1CreERT2 knockin (stock No: 012882) and CAG-floxed red fluorescent protein variant (tdTomato; stock No: 007905) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). CAG-floxed neo-diphtheria toxin A (DTA) mice were described previously (Matsumura et al. 2004) and purchased from the RIKEN BioResource Center (Ibaraki, Japan, BRC No: RBRC01347). Ascl1CreERT2 knockin (hereafter A) mice were bred with CAG-floxed tdTomato mice to generate Ascl1CreERT2: CAG-floxed tdTomato (hereafter AT) mice. A mice were bred with CAG-floxed DTA mice to generate Ascl1CreERT2: CAG-floxed DTA (hereafter AD) mice. Genotyping was performed via polymerase chain reaction (PCR) using genomic DNA extracted from the tails of mice and the following primers: Ascl1CreERT2 mutant sense, 5′-AACTTTCCTCCGGGGCTCGTTTC-3′; Ascl1CreERT2 mutant antisense, 5′-CGCCTGGCGATCCCTGAACATG-3′; CAG-floxed tdTomato sense, 5′-AAGGGAGCTGCAGTGGAGTA-3′; CAG-floxed tdTomato antisense, 5′-CCGAAAATCTGTGGGAAGTC-3′; CAG-floxed DTA sense, 5′-GCCTTCTATCGCCTTCTTGACGAGTTCTTC-3′; and CAG-floxed DTA antisense, 5′-CTACATAACCAGGTTTAGTCCCG-3′. For newborn mice tissue collection, 8 weeks to 6 months old male and female mice were mated (AT mice, n = 14; AD mice, n = 12), and their pups of both sexes were used (AT mice, n = 27; AD mice, n = 12; littermates, n = 12). For organoid experiment, 6 weeks to 6 months old mice from both sexes were used (AT mice, n = 9; AD mice, n = 18; A mice, n = 18).
Tissue preparation
Timed-pregnant females were utilized for newborn mice tissue collection. Embryonic day (E) 0.5 was designated as noon of the day on which a vaginal plug was detected. To induce Cre recombination in embryos at E18.5, tamoxifen (Sigma-Aldrich, St. Louis, MO, cat. no. T5648) was dissolved in sunflower seed oil (Sigma-Aldrich, cat. no. S5007) at a concentration of 6.7 mg/mL, and a single dose of tamoxifen solution was given through oral gavage to the pregnant dams (dose: 100 mg/kg body weight). Administering tamoxifen allowed CreERT2 to translocate into the nucleus and excise LoxP-flanked DNA regions. AT and AD pups and littermate controls were collected on postnatal day (P) 7. For immunohistochemical analyses, thin frozen Sects. (6–8-μm thick) of newborn mouse tissues were prepared as previously described (Hsu et al. 2021).
Immunohistochemistry
For microtomy, cultured taste organoids were solidified in iPGell (GenoStaff, Tokyo, Japan, cat. no. PG20-1), fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) overnight at 4 °C, and soaked in 10%, 20%, and 30% sucrose in series, respectively, overnight at 4 °C. Solidified gel with organoids was embedded in O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) and sliced at a thickness of 6–8 µm on a cryostat. The sections of the CVP tissues and taste organoids were rehydrated in PBS and incubated with primary antibodies against the following proteins overnight at 4 °C: AADC (1:200; Gene Tex, Irvine, CA, cat. no. GTX30448), Car4 (1:200; R&D Systems, Minneapolis, MN, cat. no. AF2414), gustducin (1:1,000; Santa Cruz Biotechnology, Dallas, TX, cat. no. sc-395), PLCβ2 (1:1,000; Santa Cruz Biotechnology, cat. no.sc-206), and NTPDase2 (1:400; R&D Systems, cat. no. AF5797). The sections were then washed three times with PBS before they were incubated with secondary antibodies against the primary antibody overnight at 4 °C. Secondary antibodies were used for AADC, gustducin, and PLCβ2 [1:1,000; Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG); Thermo Fisher Scientific, Waltham, MA], Car4 (1:1,000; Alexa Fluor 488 donkey anti-goat IgG; Thermo Fisher Scientific), and NTPDase2 (1:500; Alexa Fluor 488 donkey anti-sheep IgG; Abcam, Cambridge, UK). Subsequently, slides were washed with PBS and cover slipped using Fluoro-KEEPER with DAPI (Nacalai Tesque, Kyoto, Japan, cat. no. 12745–74). Whole-mount immunostaining of taste organoids was conducted as described previously (Ren et al. 2014). Briefly, culture medium containing organoids was removed from each well into 2.0-mL siliconized tubes. Organoids were washed with PBS and immersed for 15 min in 4% PFA in PBS. The fixed organoids were incubated with primary antibodies at 4 °C overnight, after which they were incubated for 2 h with secondary antibodies against the primary antibody and washed with PBS. Anti-gustducin (1:500; Santa Cruz Biotechnology) and anti-Car4 (1:20; R&D Systems) were used as primary antibodies. Secondary antibodies were used for gustducin (1:500; Alexa Fluor 488 goat anti-rabbit IgG; Thermo Fisher Scientific) and Car4 (1:500; Alexa Fluor 488 donkey anti-goat IgG; Thermo Fisher Scientific). The immunofluorescence of labeled taste cells was visualized using a fluorescence microscope (BZ-X810 or BZ-9000; KEYENCE, Osaka, Japan). The brightness and contrast of immunostained digital images were optimized using Photoshop CC 2018 for Windows (Adobe, San Jose, CA).
Taste organoid culture
Taste organoids were generated as described previously (Ren et al. 2017). In short, CVP tissues were removed from the tongues isolated from A, AT, and AD adult mice and minced using fine microscissors, after which they were treated with trypsin. Cells dissociated from the CVP tissues were seeded directly onto ultralow-attachment 24-well plates (Corning, NY, cat. no. 3473) with 5% chilled Matrigel (Corning, cat. no. 356231) in 0.5 mL of culture medium per well. The complete culture medium was composed of 20% DMEM/F12 (Thermo Fisher Scientific, cat. no. 11320–033), 50% Wnt3a-conditioned medium generated from a Wnt3a-producing cell line (gifted from Dr. Hans Clevers, Hubrecht Institute, Netherlands, and Dr. Toshiro Sato, Keio University, Japan; selected using 125 µg/mL Zeocin), 20% R-spondin-conditioned medium generated from an R-spondin2 cell line (gifted from Dr. Jeffrey Whitsett, University of Cincinnati, Cincinnati, OH; selected using 600 µg/mL Zeocin), and 10% Noggin-conditioned medium generated from a Noggin-producing cell line (gifted from Dr. Peihua Jiang, Monell Chemical Senses Center, Philadelphia, PA; selected using 600 µg/mL Zeocin) and supplemented with 1% N2 (Thermo Fisher Scientific, cat. no. 17502–048), 2% B27 (Thermo Fisher Scientific, cat. no. 17504–044), epidermal growth factor (50 ng/mL; PeproTech, Cranbury, NJ, cat. no. 315–09), Y27632 (10 µM; Sigma-Aldrich, cat. no. Y0503), and penicillin–streptomycin (1 × ; Nacalai Tesque, cat. no. 26252–94). The culture medium was changed for the first time after 7 days of culture and every 2 or 3 days thereafter. For Cre activation, 0.5 µL of 4-hydroxytamoxifen (Sigma-Aldrich, cat. no. SML1666) was added to 0.5 mL of culture medium per well during the first 7 days of culture.
Counting taste cells and organoid colonies
The numbers of labeled taste cells and tdTomato-positive cells were counted manually in taste buds and taste organoids, including a tdTomato-positive reaction in each section. Subsequently, the percentages of type II and type III taste cells coexpressing tdTomato and taste cell marker proteins were determined based on the number of cells counted. Only cells with clearly visible whole cell bodies and 4′, 6-diamidino-2-phenylindole (DAPI)-stained nuclei were counted in each section to prevent counting the same cell more than once. To determine whether an organoid was positive for Car4 or gustducin, we identified at least one cell with morphology similar to that of a native taste cell, e.g., a spindle-shaped cell and containing a large spherical nucleus near the middle of the cell.
Quantitative real-time PCR
CVP tissues removed from the tongues of P7 AD mice and littermate controls were digested using Dispase I (FUJIFILM Wako Chemical, Osaka, Japan, cat. no. 386–02,271) in DMEM (FUJIFILM Wako Chemical, cat. no. 043–30,085) at 4 °C overnight. The epithelia of the CVP were manually peeled off from the underlying connective tissue using fine forceps under a microscope. RNA was extracted using Sepasol-RNA I Super G (Nacalai Tesque, cat. no. 09379–55) and treated with TURBO DNase (Thermo Fisher Scientific, cat. no. AM2238) to remove genomic DNA contaminants. RNA samples were converted to synthesized cDNA using Transcriptor Universal cDNA Master (Roche Diagnostics, Mannheim, Germany, cat. no. 05893151001) and were analyzed via quantitative PCR using an Eco Real-Time PCR System (Illumina Inc., San Diego, CA) and PrimeTime Gene Expression Master Mix (Integrated DNA Technologies, San Diego, CA, cat. no. 1055772). Primers and TaqMan probes were obtained from Applied Biosystems (Foster, CA; β-actin, Mm00607939_s1; Ascl1, Mm04207567_g1; Ddc (encoding for AADC), Mm00516688_m1; Plcb2, Mm01338057_m1; and Entpd2 (encoding for NTPDase2), Mm00515450_m1). Standard curves were used to quantify the samples. The non-reverse transcribed control was conducted for each sample. β-actin was used as a control to normalize the individual gene expression levels.
Statistical analyses
Data are presented as mean + standard error of the mean. Microsoft Excel was used for statistical analyses. Welch’s t-test was used to compare the mRNA expression in the CVP of AD and control pups.
Results
Ascl1-CreERT2 labels type II and III cells in newly developed taste buds
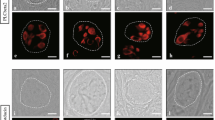
To examine whether the differentiation of Ascl1-expressing cells into taste cells can be traced in taste buds during their initial development, we used Ascl1CreERT2: CAG-floxed tdTomato (AT) mice at P7. Following a single dose of tamoxifen administered to pregnant dams, Ascl1 lineage cells were robustly labeled with tdTomato expression within the CVP epithelium of AT mice at P7. The tdTomato-expressing cells were observed mainly in the upper cleft region and dorsal surface of the CVP (arrow in Fig. 1a). Approximately 5, and no more than 10, tdTomato-expressing cells were typically detected per circumvallate papilla in AT mice at P7.
tdTomato expression in the circumvallate papillae (CVP) of Ascl1CreERT2: CAG-floxed tdTomato (AT) mice at P7. a The tdTomato-positive cell was identified in the CVP epithelium of P7 AT pups after dams received a dose of tamoxifen. The arrow indicates the tdTomato-positive cell. The white dotted line delimits the basement membrane. mc mesenchymal core. In taste buds of P7 AT pups, tdTomato-positive cells expressed type III cell markers (carbonic anhydrase IV (Car4), b–d; aromatic L-amino acid decarboxylase (AADC), e–g) and type II cell markers (gustducin, h–j; phospholipase C β2 (PLCβ2), k–m). tdTomato-positive cells expressing type I cell marker were not observed (nucleoside triphosphate diphosphohydrolase-2 (NTPDase2), n–p). Nuclei are labeled with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 50 µm in a; 10 µm in d, g, j, m, and p
Using antibodies against taste cell markers, we performed immunohistochemistry on the sections of the CVP from tamoxifen-treated AT mice at P7 to investigate the cell fate of Ascl1-expressing precursors. Car4- and AADC-positive taste cells were labeled with tdTomato in taste buds that were newly developed, similar to the taste buds of adults (Hsu et al. 2021), and a subset of gustducin- and PLCβ2-positive taste cells was also labeled with tdTomato (Fig. 1b–m). Cells expressing tdTomato had a high rate of coexpression with type III cell markers (Car4, 77.8%, Fig. 2a; AADC, 81.5%, Fig. 2b). However, the coexpression rate in type II cells was lower than that in type III cells (gustducin, 36.0%, Fig. 2c; PLCβ2, 38.5%; Fig. 2d). Although NTPDase2-positive taste cells, with a typical elongated shape, tightly wrap adjacent taste cells, which makes counting individual cells and determining the coexpression rate in type I cells difficult as Miura et al. (2014) noted, NTPDase2-positive cells appeared to be not labeled with tdTomato (Fig. 1n–p).
Coexpression ratios for tdTomato and taste cell markers in the circumvallate papillae taste buds of P7 Ascl1CreERT2: CAG-floxed tdTomato pups after tamoxifen induction. The left two panels show type III cell marker results (carbonic anhydrase IV (Car4), a; aromatic L-amino acid decarboxylase (AADC), b). The right two panels show type II cell marker results (gustducin, c; phospholipase C β2 (PLCβ2), d). The number of tdTomato-positive cells, cell-type marker-positive cells, and coexpression cells are presented in the Venn diagram. The coexpression rate of each type of cell marker is presented in parentheses in the overlapping region of the Venn diagram. Coexpression rates were calculated as the number of coexpressing cells in the total number of tdTomato-positive cells. For the cell counting per taste cell marker, 13–20 CVP sections containing tdTomato-expressing cells prepared from 9–12 pups were analyzed
Ascl1 lineage cells can be traced in cultured taste organoids
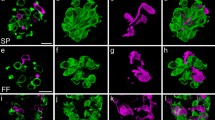
Because the growth process of taste organoids is considered similar to the generation process of taste cells in vivo, we applied an organoid culture system in this study. To trace the lineages of the Ascl1-expressing cells of the taste bud under ex vivo conditions, we cultured cells dissociated from the CVP of AT mice and produced taste organoids. During the first 7 days of culture, the organoid culture medium contained hydroxytamoxifen, i.e., the functional metabolite of tamoxifen, which activated Cre. Under this condition, we observed numerous Ascl1-CreERT2-driven tdTomato-positive cells within the mature taste organoids (Fig. 3a). To assess which taste bud cells were localized tdTomato, we performed immunostaining of hydroxytamoxifen-treated taste organoids derived from AT mice. Some of tdTomato-expressing cells were coexpressed with Car4 (Figs. 3e–g and 4a), AADC (Figs. 3h–j and 4b). A subset of them were coexpressed with PLCβ2 (Figs. 3k–m and 4d), and a small subset were coexpressed with gustducin (Figs. 3b–d and 4c). Additionally, tdTomato expression was not detected in NTPDase2-positive cells, which were considered type I cells (Fig. 3n–p). Therefore, Ascl1-expressing precursor cells predominantly give rise to type III cells, and a subset may give rise to type II cells in cultured organoids.
tdTomato expression in taste organoids derived from Ascl1CreERT2: CAG-floxed tdTomato (AT) mice. a Representative image of the whole taste organoid derived from AT mice after tamoxifen treatment. The organoid was cultured for 20 days. Immunohistochemistry of whole-mount (gustducin, b–d) and sections (carbonic anhydrase IV (Car4), e–g; aromatic L-amino acid decarboxylase (AADC), h–j; phospholipase C β2 (PLCβ2), k–m; nucleoside triphosphate diphosphohydrolase-2 (NTPDase2), n–p) of taste organoids derived from AT mice. Arrows indicate colocalization of signals of tdTomato and each taste cell marker. Taste cell marker-positive cells that did not colocalize with tdTomato are indicated by arrowheads. Nuclei are labeled with DAPI. Scale bars, 100 µm in a; 20 µm in d; 10 µm in g, j, m, and p
Coexpression ratios for tdTomato and taste cell markers in taste organoids at 20 days derived from Ascl1.CreERT2: CAG-floxed tdTomato mice. The left two panels show type III cell marker results (carbonic anhydrase IV (Car4), a; aromatic L-amino acid decarboxylase (AADC), b). The right two panels show type II cell marker results (gustducin, c; phospholipase C β2 (PLCβ2), d). The tdTomato-positive cell, cell-type marker-positive cell, and coexpression cell numbers are presented in the Venn diagram. Each type of cell marker’s coexpression rate is presented in parentheses in the overlapping Venn diagram region. Coexpression rates were calculated as the coexpressing cell number in the total tdTomato-positive cell number. The number of cells were counted using the sections prepared from two independent experiments (n = 3 animals per experiment)
Gene expression in mouse CVP epithelium lacking Ascl1-expressing cells
Because we suspected that Ascl1 is expressed while differentiating from taste precursor cells to type III cells and a subset of type II cells, we investigated whether the loss of Ascl1-expressing cells affects newly developed taste buds. We used Ascl1CreERT2: CAG-floxed neo-diphtheria toxin A (AD) mice because Ascl1 knockout mice typically show mortality within 24 h of birth; hence, it is not possible to analyze their developed taste buds located in the tongue papillae. In AD mice, tamoxifen administration induces specific cell death in Ascl1-expressing cells, and this strategy was used previously to investigate the Ascl1-expressing cell lineage in adult mice (Takagi et al. 2018). The mRNA expression of some taste cell markers was analyzed quantitatively via PCR using the CVP epithelium of AD mice and that of littermates as a control at P7 following the administration of tamoxifen to pregnant dams at E18.5. Although the mRNA expression level of Ascl1 in the CVP epithelium of AD mice at P7 significantly decreased compared with that in the CVP epithelium of control mice, the expression levels of Ddc and Plcb2 were not significantly different between the two groups (Fig. 5a–c). The mRNA expression level of Entpd2 remained unchanged between the two groups (Fig. 5d).
Quantitative PCR analyses to evaluate taste cell marker gene expression in the circumvallate papillae (CVP) of Ascl1CreERT2: CAG-floxed neo-diphtheria toxin A (AD) mice and their littermates (controls). Their CVP epithelium was collected after dams received a tamoxifen dose. Ascl1 (a), Ddc (encoding for AADC, type III cell marker, b), Plcb2 (type II cell marker, c), and Entpd2 (encoding for NTPDase2, type I cell marker, d) mRNA expression levels were normalized using β-actin and evaluated statistically using Welch’s t-test. Each dot shows an individual mouse. n = 6 for each group. Data are presented as mean + standard error of the mean
Loss of Ascl1-expressing cells affects taste cell generation in cultured taste organoids
Next, using AD mice and Ascl1CreERT2 (A) mice as controls, we evaluated the changes in the generation of mature taste cells when Ascl1-expressing cells were absent from taste organoids. Taste organoids derived from both AD and A mice were cultured and treated with hydroxytamoxifen, which caused the death of Ascl1-expressing cells isolated from AD mice under ex vivo conditions. We performed immunohistochemistry using antibodies against taste cell markers to determine if taste cells were present in the taste organoids of each group, and we counted the number of the organoid colonies that contained immunoreactive cells in three independent experiments per group. The number and percentage of the organoid colonies that contained Car4-positive taste cells in the AD group was smaller than that in the control group (Fig. 6a, c). Furthermore, the number and percentage of the organoid colonies containing gustducin-positive taste cells was decreased in the AD group compared with that in the control group (Fig. 6b, d). Therefore, Ascl-expressing cells are important for differentiation of both type II and III taste cells in organoids.
Number and percentages of the organoid colonies containing taste cells in the absence of Ascl1-expressing cells. The taste organoids derived from both Ascl1CreERT2: CAG-floxed neo-diphtheria toxin A (AD) mice and Ascl1CreERT2 (A) mice as controls were cultured with organoid culture medium added hydroxytamoxifen. The organoid colonies containing carbonic anhydrase IV (Car4, type III cell marker)-positive cells (a, c) and gustducin (type II cell marker)-positive cells (b, d) were counted and determine the percentages after whole-mount immunohistochemistry. The organoid colonies were counted in three independent experiments per group
Discussion
In the present study, we found that Ascl1 lineage cells express both type II and III cell markers in both the native taste tissues of Ascl1CreERT2: CAG-floxed tdTomato newborn mice and taste organoids derived from the CVP region of the transgenic mice. Additionally, using Ascl1CreERT2: CAG-floxed neo-DTA mice, we found that the generation of type II and III cells was suppressed in cultured taste organoids when Ascl1-expressing cells were absent.
Ascl1 is best known for its role in the regulation of neurogenesis, but it also plays an important role in other tissues, e.g., the olfactory epithelium (Lo et al. 1991; Guillemot et al. 1993; Guillemot and Joyner 1993; Tomita et al. 1996). Previous studies have shown that Ascl1 is expressed during cell differentiation from basal cells to elongated cells within taste buds (Miura et al. 2006; Nakayama et al. 2008), and we revealed previously that type III cells expressing AADC and GAD67 are not detected in Ascl1 knockout mouse embryos (Seta et al. 2011; Kito-Shingaki et al. 2014). In another investigation, the inducible cell death of Ascl1-expressing cells in adult mice resulted in a significant decrease in the number of type III taste cells (Takagi et al. 2018). Using Ascl1-CreERT2 2, 5, and 10 days after tamoxifen induction, we also showed that Ascl1-CreERT2-labeled cells express AADC, Car4, and PLCβ2 in adult taste buds (Hsu et al. 2021). These studies suggest that Ascl1-expressing postmitotic taste precursor cells predominantly differentiate into type III cells within mature taste buds. According to a previous report, both type II and III cells are derived from Ascl1-expressing basal cells (Miura et al. 2006). Moreover, we previously observed that a small population of Ascl1-CreERT2-driven tdTomato-positive cells was immunoreactive for gustducin in adult taste buds, with a tdTomato and gustducin coexpression rate of < 10% (Hsu et al. 2021). This study’s results showed that the ratio in AT newborn mice nascent taste buds was 36% (Fig. 2c), indicating different gustducin and Ascl1-CreERT2-driven tdTomato coexpression rates in newborns and adults. Miura et al. (2005) demonstrated that the coexpressing ratio of the gustducin and neural cell adhesion molecule, a type III taste cell marker, gradually decreases during development using in situ hybridization and immunohistochemistry. Taken together with this study’s results, the molecular mechanisms regulating the taste cell differentiation may vary between initially developed and adult taste buds. Because taste cells are constantly and rapidly replaced via turnover, taste buds contain a mixture of taste cells with different lifespans at the maintenance stage in an adult tongue. Type II taste cells always have a shorter lifespan (approximately 8 days) than that of type III cells and are replaced by newly differentiated cells within a faster cycle (Perea-Martinez et al. 2013). In the present study, we tested taste buds that were newly developed in the newborn CVP, finding that Ascl1-expressing cells labeled in late embryos differentiate into type II and III taste cells in the first postnatal week. Mouse taste bud primordia appear as a thickening of the epithelium on the dorsal surface of the tongue at E12.0 (Mistretta and Bosma 1972). After E15.5, the taste bud primordia continue to develop without the addition of cells from the surroundings (Golden et al. 2021; Barlow 2021). The complete differentiation of nascent taste buds occurs postnatally, with the expression of specific taste cell markers not appearing until the first week (Barlow 2015). Additionally, the supply of new taste cells, generated from the differentiation of progenitor cells adjacent to taste buds into taste buds, begins during P2 to P9 (Golden et al. 2021). At the first postnatal week in developmental stage, when we collected the tissues in this study, it is assumed that the replacement of taste bud cells has not yet occurred, so taste buds of P7 mice are composed of early-born taste cells expressing specific taste cell markers allowing for a clearer analysis of the cell lineage within initially developed taste buds.
Organoid cultures derived from mouse taste tissues are practical for investigating taste systems as they provide more comprehensible insights into the effects of genetic or other manipulations (Qin et al. 2018). In the present study, taste organoids were produced from AT mice with tamoxifen to trace Ascl1-expressing cells ex vivo. Sections were then prepared and immunostained to examine the taste cell marker localization. As expected, the ratio of gustducin-positive cells in tdTomato-expressing cells was lower than that of type III cell markers, consistent with the in vivo results. Moreover, a relatively high PLCβ2-positive cell percentage was observed in tdTomato-expressing cells (Fig. 4). This may be attributed to the unique organoid culture environment, such as additional growth factors or decoupling of signals from other tissues, although this has not been tested.
Taste cell marker expression was examined with quantitative real-time PCR to evaluate the P7 CVP epithelium of AD mice. As expected, Ascl1 mRNA was detected at a lower level than control, while Ddc and Plcb2 mRNA was not detected enough to make a statistically significant difference. In our experiment, a single tamoxifen dose was administered to E18. Consequently, it was presumed that cells whose cell fate had already been determined toward AADC- or PLCβ2-expressing cells before E18 were not killed by diphtheria toxin and remained at CVP epithelium. If we could remove Ascl1-expressing cells with tamoxifen in newborn mice for a longer term, a remarkable difference might be detected. Further investigations are required to address this.
Various molecules and multiple signaling pathways, for example, Shh signaling, Wnt/β-catenin signaling, Notch signaling, and R-spondin proteins, have been reported to regulate the development and regeneration of gustatory cells in a complex manner (Gaillard et al. 2015; Castillo-Azofeifa et al. 2017; Seta et al. 2003; Barlow 2015; Ren et al. 2017; Lin et al. 2021). Lgr5-expressing cells located at the bottom of the CVP trench area and base of the CVP taste buds have been identified as taste stem cells, and in an ex vivo culture system, sorted single Lgr5-positive cells were found to give rise to all three types of mature taste cells (Ren et al. 2014). With the use of organoids, it is possible to recreate the cell differentiation process of some organs, including taste buds, in detail. Indeed, Ren et al. (2017) noted that the growth and differentiation of cultured organoids mimic the regeneration of taste bud cells in vivo. Specifically, they found that when Notch signaling was inhibited pharmacologically, the number of type I cells was reduced, whereas the maturation of type II and III cells was accelerated in cultured taste organoids, indicating that blocking Notch signaling altered the cell fate from type I cells to type II or III cells. In the nervous system, Notch signaling induces the expression of transcription factors, such as Hes1 and Hes5 that act in a downregulatory manner on the function and expression of Ascl1, and plays crucial roles in maintaining stem cell characteristics and promoting glial cell differentiation. Conversely, when Notch signaling is absent, Ascl1 expression prevails, leading the cell toward a neuronal fate (Schuurmans and Guillemot 2002). Since we used a diphtheria-toxin cell-killing genetic model in Ascl1-expressing cells to specifically delete cells in late embryos and in organoids, it was not possible to survey whether the cell fate differs when Ascl1 is not genetically expressed in taste precursor cells. Future studies using transgenic mice that can carry out taste precursor cell-specific Ascl1 deficiency will be able to address this. In the present study, the conditional cell death of Ascl1-expressing cells under a Cre-loxP recombination system resulted in suppression of type II and III taste cell generation in cultured taste organoids. Thus, Ascl1-expressing precursor cells appear to differentiate predominantly into type III taste cells and a subset of type II taste cells. However, it is not yet possible to determine the cell populations within type II cells with which Ascl1 is involved. The expression of the critical regulators of type II taste precursor cell specification, including Pou2f3 (Skn-1a) and Eya1 (Matsumoto et al. 2011; Ohmoto et al. 2021), or the expression of the taste receptors could be used to determine these cell populations within type II taste cells in future studies. It will also be important to examine the interaction of Ascl1 with various transcription factors and signaling pathways that are known to regulate the differentiation of taste cells.
In summary, we found that Ascl1-CreERT2-driven tdTomato-positive cells were immunoreactive for type II and III taste cell markers. Furthermore, the generation of type II and III taste cells was suppressed in the absence of Ascl1-expressing cells. Overall, these findings suggest that Ascl1-expressing precursor cells may differentiate into a subset of type II cells in addition to type III cells in newborn mice and in cultured taste organoids.
Data availability
All the relevant data is provided in this manuscript and associated figures.
References
Barlow LA (2021) The sense of taste: development, regeneration, and dysfunction. Wires Mech Dis 14:e1547
Barlow LA (2015) Progress and renewal in gustation: new insights into taste bud development. Development 142:3620–3629
Barlow LA, Klein OD (2015) Developing and regenerating a sense of taste. In: Current Topics in Developmental Biology. Elsevier, pp 401–419
Beidler LM, Smallman RL (1965) Renewal of cells within taste buds. J Cell Biol 27:263–272
Castillo-Azofeifa D, Losacco JT, Salcedo E, Golden EJ, Finger TE, Barlow LA (2017) Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development 144:3054–3065
Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zucker CS (2009) The taste of carbonation. Science 326:443–445
Chaudhari N, Roper SD (2010) The cell biology of taste. J Cell Biol 190:285–296
Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC (2004) Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol 468:311–321
DeFazio RA, Dvoryanchikov G, Maruyama Y, KimJW PE, Roper SD, Chaudhari N (2006) Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26:3971–3980
Delay RJ, Kinnamon JC, Roper SD (1986) Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J Comp Neurol 253:242–252
Farbman AI (1980) Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet 13:349–357
Finger TE, Simon SA (2000) Cell biology of taste epithelium. In: Finger TE, Silver WL, Restrepo D (eds) The Neurobiology of Taste and Smell. Wiley-Liss, New York, pp 287–314
Gaillard D, Xu M, Liu F, Millar SE, Barlow LA (2015) β-catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLOS Genet 11:e1005208
Golden EJ, Larson ED, Shechtman LA, Trahan GD, Gaillard D, Fellin TJ, Scott JK, Jones KL, Barlow LA (2021) Onset of taste bud cell renewal starts at birth and coincides with a shift in SHH function. eLife 10:e64013
Guillemot F, Joyner AL (1993) Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev 42:171–185
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476
Hsu CC, Seta Y, Matsuyama K, Kataoka S, Nakatomi M, Toyono T, Gunjigake KK, Kuroishi KN, Kawamoto T (2021) Mash1-expressing cells may be relevant to type III cells and a subset of PLCβ2-positive cell differentiation in adult mouse taste buds. Cell Tissue Res 383:667–675
Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJP, Zuker CS (2006) The cells and logic for mammalian sour taste detection. Nature 442:934–938
Huang YA, Maruyama Y, Stimac R, Roper SD (2008) Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste: sour taste mechanisms in mouse taste buds. J Physiol 586:2903–2912
Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A (2003) Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312:500–506
Kito-Shingaki A, Seta Y, Toyono T, Kataoka S, Kakinoki Y, Yanagawa Y, Toyoshima K (2014) Expression of GAD67 and Dlx5 in the taste buds of mice genetically lacking Mash1. Chem Senses 39:403–414
Lindeman B (1996) Taste reception. Physiol Rev 76:719–766
Lin X, Lu C, Ohmoto M, Choma K, Margolskee RF, Matsumoto I, Jiang P (2021) R-spondin substitutes for neuronal input for taste cell regeneration in adult mice. Proc Natl Acad Sci 118:e2001833118
Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ (1991) Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev 5:1524–1537
Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K (2011) Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci 14:685–687
Matsumura H, Hasuwa H, Inoue N, Ikawa M, Okabe M (2004) Lineage-specific cell disruption in living mice by Cre-mediated expression of diphtheria toxin A chain. Biochem Biophys Res Commun 321:275–279
Mistretta CM, Bosma JF (1972) Topographical and histological study of the developing rat tongue, palate and taste buds. Third Symp Oral Sensat Percept Mouth Infant Springf I1:163–187
Miura H, Kato H, Kusakabe Y, Ninomiya Y, Hino A (2005) Temporal changes in NCAM immunoreactivity during taste cell differentiation and cell lineage relationships in taste buds. Chem Senses 30:367–375
Miura H, Kusakabe Y, Harada S (2006) Cell lineage and differentiation in taste buds. Arch Histol Cytol 69:209–225
Miura H, Scott JK, Harada S, Barlow LA (2014) Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev Dyn 243:1286–1297
Murray R (1973) The ultrastructure of taste buds. In: Friedmann I (ed) The Ultrastructure of Sensory Organs. North Holland, Amsterdam, pp 1–81
Nakayama A, Miura H, Shindo Y, Kusakabe Y, Tomonari H, Harada S (2008) Expression of the basal cell markers of taste buds in the anterior tongue and soft palate of the mouse embryo. J Comp Neurol 509:211–224
Nguyen HM, Barlow LA (2010) Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci 11:129
Ohmoto M, Kitamoto S, Hirota J (2021) Expression of Eya1 in mouse taste buds. Cell Tissue Res 383:979–986
Perea-Martinez I, Nagai T, Chaudhari N (2013) Functional cell types in taste buds have distinct longevities. PLoS ONE 8:e53399
Qin Y, Sukumaran SK, Jyotaki M et al (2018) Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLOS Genet 14:e1007058
Ren W, Aihara E, Lei W, Gheewala N, Uchiyama H, Margolskee RF, Iwatsuki K, Jiang P (2017) Transcriptome analyses of taste organoids reveal multiple pathways involved in taste cell generation. Sci Rep 7:4004
Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P (2014) Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci 111:16401–16406
Schuurmans C, Guillemot F (2002) Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol 12:26–34
Seta Y, Kataoka S, Toyono T, Toyoshima K (2007) Immunohistochemical localization of aromatic L-amino acid decarboxylase in mouse taste buds and developing taste papillae. Histochem Cell Biol 127:415–422
Seta Y, Oda M, Kataoka S, Toyono T, Toyoshima K (2011) Mash1 is required for the differentiation of AADC-positive type III cells in mouse taste buds. Dev Dyn 240:775–784
Seta Y, Seta C, Barlow LA (2003) Notch-associated gene expression in embryonic and adult taste papillae and taste buds suggests a role in taste cell lineage decisions. J Comp Neurol 464:49–61
Seta Y, Stoick-Cooper CL, Toyono T, Kataoka S, Toyoshima K, Barlow LA (2006) The bHLH transcription factors, Hes6 and Mash1, are expressed in distinct subsets of cells within adult mouse taste buds. Arch Histol Cytol 69:189–198
Stone LM, Finger TE, Tam PP, Tan SS (1995) Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci 92:1916–1920
Takagi H, Seta Y, Kataoka S, Nakatomi M, Toyono T, Kawamoto T (2018) Mash1-expressing cells could differentiate to type III cells in adult mouse taste buds. Anat Sci Int 93:422–429
Takai S, Watanabe Y, Sanematsu K, Yoshida R, Margolskee RF, Jiang P, Atsuta I, Koyano K, Ninomiya Y, Shigemura N (2019) Effects of insulin signaling on mouse taste cell proliferation. PLoS ONE 14:e0225190
Tomita K, Nakanishi S, Guillemot F, Kageyama R (1996) Mash1 promotes neuronal differentiation in the retina. Genes Cells 1:765–774
Yang R, Stoick CL, Kinnamon JC (2004) Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol 471:59–71
Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P (2013) Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells Dayt Ohio 31:992–1000
Zhou Y, Liu H-X, Mistretta CM (2006) Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol 297:198–213
Acknowledgements
We are grateful to Dr. Jeffrey Whitsett (University of Cincinnati) for the R-spondin2 stable cell line, Dr. Hans Clevers (Hubrecht Institute) and Dr. Toshiro Sato (Keio University) for the Wnt3a stable cell line, and Dr. Peihua Jiang (Monell Chemical Senses Center) for the Noggin stable cell line. We thank Ms. M. Ishikawa for secretarial assistance.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI grant JP20K18461.
Author information
Authors and Affiliations
Contributions
Y.S., conceptualization and study design; K.M., S.T., N.S., T.T., and Y.S., methodology; K.M., S.K., and T.T., investigation; K.M., T.T., and Y.S., project administration; K.M., M.N., and S.K., visualization; K.M., writing—original draft; Y.S., T.K., and M.N., writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were approved by Kyushu Dental University Animal Care (approval No: 20–03).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuyama, K., Takai, S., Shigemura, N. et al. Ascl1-expressing cell differentiation in initially developed taste buds and taste organoids. Cell Tissue Res 392, 631–641 (2023). https://doi.org/10.1007/s00441-023-03756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03756-8