Abstract
Our objective was to show morphological changes of the fornix in autopsies of patients with temporal lobe epilepsy, which may potentially serve for post-mortem diagnosis. Epileptic and non-epileptic autopsy brains were obtained from the council of forensic medicine between the years 2005 and 2007. In both non-epileptic and epileptic autopsies the mean cross-sectional areas and fiber densities of the right and left fornices were calculated and analyzed. The numbers of myelinated and unmyelinated fibers, and the total number of fibers forming each fornix were counted. The total number of fibers in the right fornix was always greater than in the left fornix, in both epileptic and non-epileptic autopsies. The mean total number of fornix fibers was significantly reduced in epileptics compared with non-epileptics, in both the right (p = 0.043) and left (p = 0.043) sides. The electron-microscopic sections showed that myelinated axons outnumbered unmyelinated axons in both epileptic and non-epileptic autopsies. However, the reduction in the number of unmyelinated fibers was only statistically significant for the right fornix in right epileptic autopsies (p = 0.021). Although the reduction in the number of myelinated fibers was not statistically significant, electron-microscopic evaluations showed myelin degeneration of the myelinated fibers in the right fornix of the right temporal lobe in epileptic autopsies. In conclusion, our results suggest that unmyelinated fiber loss is functionally important, and may have functional consequences of diagnostic value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesial temporal lobe epilepsy is associated with clinically well defined epileptic syndrome—EEG abnormalities in the temporal lobe, memory dysfunction, and hippocampal atrophy (French et al. 1993). Mesial temporal lobe sclerosis (MTS) is the major pathological abnormality in approximately 60% of patients with intractable temporal lobe epilepsy (Babb and Brown 1987).
The common histological findings in MTS are neuronal cell loss and gliosis in the CA1, CA3, and CA4 regions, and in the dentate gyrus of the hippocampus (Bronen 1992; Garcia et al. 1994; Jack et al. 1990; Mamourian et al. 1998; Margerison and Corsellis 1966). Although the cornu ammonis and dentate gyrus are the major sites of the disease in MTS, morphological abnormalities have also been demonstrated in other temporal lobe structures, for example the amygdala, entorhinal cortex, subiculum, and parahippocampal gyrus (Corsellis 1957). The fornix, a well defined tract which consists of efferent and afferent fibers of the hippocampal formation, is affected in numerous neurologic conditions including temporal lobe epilepsy, Alzheimer’s disease, and memory disorders (Bilir et al. 1998). Numerous MRI studies measuring the volume and width of the fornix on patients with MTS have suggested that the fornix is reduced in size (Baldwin et al. 1994; Bilir et al. 1998; Kim et al. 1995; Kodama et al. 2003; Kuzniecky et al. 1999; Ng et al. 1997; Oikawa et al. 2001) and asymmetry (Mamourian et al. 1998) and shows signs of axonal degeneration (Concha et al. 2005).
In this study we add to this evidence by providing electron-microscopic data on the average number of fibers forming the column of the fornix. In addition, we have measured cross-sectional area, fiber density, and ratio of myelinated to unmyelinated fibers for non-epileptic and epileptic human autopsy brains.
Methods
Eight autopsies were used in this study. Four of the autopsies (two male, two female) were from right temporal lobe epilepsy patients (age range 24–48 years) and four were non-epileptic autopsies (three male, one female; age range 27–61 years). Only right temporal lobe epilepsy autopsies were selected to avoid confusion in the interpretation of the data obtained. The study was approved by the ethics committee of the forensic medicine council. All procedures were carried out by the same researcher who was unaware of the clinical data.
Autopsy procedure
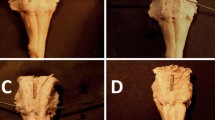
Autopsies with intracranial pathologies other than those associated with right temporal lobe epilepsy were not included in this study. The calvaria were opened by routine autopsy dissections. The brain was removed en bloc from the skull. Cerebellum and diencephalon were removed and then the inferior surface of the column of the fornix and the anterior commissure were exposed (Fig. 1). Each fornix was taken from the same region, immediately superior to the anterior commissure. Special care was taken to fix the brain tissue as soon as possible to avoid extensive degeneration.
Light-microscopic procedure
The temporal lobe, including the hippocampus, was removed and fixed in 10% formalin solution for ten days. The blocks were then dehydrated in an ascending alcohol series and embedded in paraffin. Coronal sections (5 μm thick) from the mid-hippocampus, including all sectors of the hippocampus, were cut with a sliding microtome. The sections were stained with cresyl violet to evaluate cell loss and gliosis.
Electron-microscopic procedure
Each fornix specimen was cut into 1-mm slices and fixed in 2.5% phosphate-buffered (0.1 M and pH 7.2) glutaraldehyde for 2 h. The specimens were then postfixed for an hour in 1% OsO4. As the size of the whole fornix was too large for preparation, fornix slices were divided into two equal parts and processed separately. They were then dehydrated in a graded alcohol series and embedded in epoxy resin. Semi-thin sections (1 μm thick) of each part were stained with toluidine blue for light-microscopic evaluation and thin sections (60 nm thick) were contrasted with uranyl acetate and lead citrate and examined with a Jeol (Tokyo, Japan) 1200 SX TEM.
The semi-thin sections of each fornix sector were photographed at ×40 final magnification for measurement of the total cross-sectional area of each fornix. The boundary of each fornix sector was outlined with Adobe Photoshop 6.0. The cross-sectional area of each fornix sector section then was calculated by use of Clemex Vision PE software (demonstration version). The total cross-sectional area of each fornix was then calculated.
Sections for fiber counts were obtained from constant regions of the right and left fornices in both right temporal lobe epilepsy autopsies and non-epileptic autopsies. Data on the number of myelinated and unmyelinated nerve fibers were obtained by examination of electron-microscopic images. The mean total number of myelinated and unmyelinated fibers forming the fornices were computed from the cross-sectional areas and the densities of the myelinated and unmyelinated fibers for the right and left fornices. Thin sections were obtained from two parts of each fornix. Every fifth section and a total of five sections from each part were examined. Five photographs were taken at a magnification of ×10,000 from each grid. The first photograph was randomly taken then the other four photographs were taken by sliding the grid in four different directions. The numbers of myelinated and unmyelinated fibers were counted at each site within a photographic area measuring 52 μm2. The nerve fibers touching the lower horizontal and right vertical borders of the photographs were excluded from the counts. Finally, from the nerve fiber densities and cross-sectional areas, nerve-fiber counts of the fornices of epileptic and non-epileptic cases were calculated.
Statistical analysis was by use of the Mann–Whitney U test.
Results
Light-microscopic sections of the hippocampus from four right temporal lobe epilepsy autopsies showed clear evidence of cell loss in the dentate gyrus of the right hippocampus (Fig. 2a) whereas non-epileptic autopsies showed a regular hippocampal appearance in all sectors of the hippocampus, on both sides (Fig. 2b).
a Light-microscopic section of the hippocampus, from the right temporal lobe of an epilepsy patient, stained with cresyl violet. The area in the ellipse shows cell loss in the dentate gyrus of the hippocampus. ×200. b Light-microscopic section of the sectors of the hippocampus in a normal autopsy. CA1 cornu ammonis 1, CA2 cornu ammonis 2, CA3, cornu ammonis 3, CA4 cornu ammonis 4, Dg Dentate gyrus. ×40. c Electron-microscopic appearance of the myelinated and unmyelinated fibers of the fornix in a normal autopsy. Myelinated fiber (thick arrow), unmyelinated fiber (thin arrow). Bar, 500 nm. d Electron-microscopic appearance of the myelinated and unmyelinated fibers of the fornix in a right temporal lobe epileptic autopsy. Myelin degeneration (thick arrow). Bar, 500 nm
The total number of fornix fibers was always greater on the right side and the mean total number of fibers was higher on the right than the left side, in both epileptic (p = 0.029) and non-epileptic (p = 0.021) autopsies (Table 1). The total mean number of fornix fibers was significantly lower in epileptic cases, compared with non-epileptics, for both right (p = 0.043) and left sides (p = 0.043).
The electron-microscopic sections showed that myelinated axons outnumbered unmyelinated axons in both epileptic and non-epileptic autopsies (Fig. 2c, d). A decrease in the number of myelinated and unmyelinated fibers was observed in the epileptic autopsies for both fornices. However, the reduction was statistically significant for the unmyelinated fibers of the right fornix only (p = 0.021). Although the reduction in the number of myelinated fibers was not statistically significant, electron microscopic evaluations showed myelin degeneration of myelinated fibers in the right fornix in right temporal lobe epilepsy autopsies (Fig. 2d).
The mean forniceal cross-sectional areas were similar in epileptic and non-epileptic autopsies, for both the left and right sides. Mean densities of myelinated and unmyelinated fibers were calculated and are shown in Table 1 for both non-epileptic and epileptic cases.
Discussion
This study gives a far more accurate estimate of fiber numbers than earlier studies with the light microscope and also enables distinction of myelinated fiber loss from unmyelinated fiber loss in autopsies of temporal lobe epilepsy patients.
In this study, the fornix specimens were taken from a constant region, immediately superior to the anterior commissure, to avoid variations of regional fiber numbers. Chance et al. (1999) calculated the number of fibers forming the fornix superior to the anterior commissure in normal men as 1,020,200 on the left and 1,138,300 on the right; in healthy women it was 1,255,800 on the left and 1,242,200 on the right. Daitz (1953) reported 912,000 fibers in the human post-commissural fornix. In this study, the corresponding figures were 1,277,780 ± 102,588 on the right and 817,010 ± 98,994 on the left. Average fiber counts in non-epileptic autopsies on the right side were slightly higher than those in previous studies. This might be because of the use of electron microscopy in this study to calculate the density, because electron microscopy may be sensitive to small axons, for example unmyelinated fibers.
In this study there was a relative reduction in the cross-sectional area on the right side in epileptic autopsies, in correspondence with previous clinical studies (Kuzniecky et al. 1999). We suggest that relative reduction of the cross-sectional area in unilateral epileptic autopsies may be because of the significant reduction of unmyelinated fibers. Although we found a reduction in the number of myelinated axons also, the loss was not statistically significant. In addition, there was a decrease in the average density of unmyelinated fibers in both the right and left fornices in the autopsies of right temporal lobe epilepsy cases. The fornix contains not only the efferents of the hippocampus but also the afferents. Therefore, a reduction in the unmyelinated fibers may result not only in efferent loss but also in deafferentation of the hippocampal fibers.
The neuronal loss observed in the dentate gyrus of the hippocampus in this study may be the result of Wallerian or transneuronal degeneration causing a decrease in the number of fornix fibers in the epileptic autopsies (Baldwin et al. 1994; Kodama et al. 2003; Ng et al. 1997).
Histological studies have demonstrated bilateral and/or unilateral hippocampal cell loss in patients with MTS (Babb 1991; Corsellis 1970). Recently, MRI studies reported bilateral changes in the fornix of patients with unilateral temporal lobe epilepsy (Concha et al. 2005; Ng et al. 1997). In accordance with previous MRI and histological studies, the results of this study revealed unilateral neuronal loss and gliosis. A bilateral decrease in the number of fornix fibers was observed in epileptic autopsies; this may be because of the degeneration of commissural fibers interconnecting the two hippocampi (Kuzniecky et al. 1999). Our findings suggest that temporal lobe epilepsy is associated with bilateral limbic system pathology even in patients with unilateral temporal lobe epilepsy.
This electron-microscopic study has shown that the fornix is composed mainly of myelinated fibers and fewer unmyelinated fibers (Table 1). Mamourian et al. (1998), reported fornix myelin loss in MTS patients. This study also showed degeneration in the myelin sheath of the myelinated fibers in temporal lobe autopsies. However, there was a striking reduction in the number of unmyelinated fibers whereas less change was observed for myelinated fibers.
MRI studies have shown that most myelinated fibers in the fornix terminate in hypothalamic nuclei (Miller et al. 1994; Saeki et al. 2001); the termination of the unmyelinated fibers is unknown.
Precise knowledge of morphological changes in cerebral structures is important for diagnosis of neurological disorders and for understanding the underlying pathology. This electron-microscopic study revealed unmyelinated fiber loss in autopsies from temporal lobe epilepsy cases; this may be functionally more important than loss of myelinated fibers and may serve for post-mortem diagnosis or have functional implications that may be of diagnostic value.
References
Babb TL, Brown WJ (1987) Pathological findings in epilepsy. In: Engel JJ (ed) Surgical treatment of the epilepsies. Raven, New York, pp 511–540
Babb TL (1991) Bilateral pathological damage in temporal lobe epilepsy. Can J Neurol Sci 18:645–648
Baldwin GN, Tsuruda JS, Maravilla KR, Hamill GS, Hayes CE (1994) The fornix in patients with seizures caused by unilateral hippocampal sclerosis: detection of unilateral volume loss on MR images. AJR Am J Roentgenol 162:1185–1189
Bilir E, Craven W, Hugg J et al (1998) Volumetric MRI of the limbic system: anatomic determinants. Neuroradiology 40:138–144
Bronen RA (1992) Epilepsy: the role of MR imaging. AJR Am J Roentgenol 159:1165–1174
Chance SA, Highley JR, Esiri MM, Crow TJ (1999) Fiber content of the fornix in schizophrenia: lack of evidence for a primary limbic encephalopathy. Am J Psychiatry 156:1720–1724
Concha L, Beaulieu C, Gross DW (2005) Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 57:188–196
Corsellis JA (1957) The incidence of Ammon’s horns sclerosis. Brain 80:193–208
Corsellis JA (1970) The neuropathology of temporal lobe epilepsy. Mod Trends Neurol 5:254–270
Daitz HM (1953) Note on the fibre content of the fornix system in man. Brain 76:509–512
French JA, Williamson PD, Thadani VM et al (1993) Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol 34:774–780
Garcia PA, Laxer KD, Barbaro NM, Dillon WP (1994) Prognostic value of qualitative magnetic resonance imaging hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia 35:520–524
Jack CR Jr, Sharbrough FW, Twomey CK et al (1990) Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 175:423–429
Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N (1995) Clinial significance of asymmetry of the fornix and mamillary body on MR in hippocampal sclerosis. AJNR Am J Neuroradiol 16:509–515
Kodama F, Ogawa T, Sugihara S et al (2003) Transneuronal degeneration in patents with temporal lobe epilepsy: evaluation by MR imaging. Eur Radiol 13:2180–2185
Kuzniecky R, Bilir E, Gilliam F, Faught E, Martin R, Hugg J (1999) Quantitative MRI in temporal lobe epilepsy: evidence for fornix atrophy. Neurology 53:496–501
Mamourian AC, Cho CH, Saykin AJ, Poppito NL (1998) Association between size of the lateral ventricle and asymmetry of the fornix in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 19:9–13
Margerison JH, Corsellis JAN (1966) Epilepsy and temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89:499–530
Miller MJ, Mark LP, Yetkin FZ et al (1994) Imaging white matter tracts and nuclei of the hypothalamus: an MR-anatomic comparative study. AJNR Am J Neuroradiol 15:117–121
Ng SES, Lau TN, Hui FKH et al (1997) MRI of the fornix and mamillary body in temporal lobe epilepsy. Neuroradiology 39:551–555
Oikawa H, Sasaki M, Tamakawa Y, Kamei A (2001) The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology 43:205–210
Saeki N, Sunami K, Kubota M et al (2001) Heavily T2-weighted MR imaging of white matter tracts in the hypothalamus: normal and pathologic demonstrations. AJNR Am J Neuroradiol 22:1468–1475
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozdogmus, O., Cavdar, S., Ersoy, Y. et al. A preliminary study, using electron and light-microscopic methods, of axon numbers in the fornix in autopsies of patients with temporal lobe epilepsy. Anat Sci Int 84, 2–6 (2009). https://doi.org/10.1007/s12565-008-0001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-008-0001-2