Abstract
The aim of this study was to assess the MR imaging findings of transneuronal degeneration of limbic system in the patients with temporal lobe epilepsy, and to detect the influence of surgery on the anatomy of the limbic system. Axial and coronal T1- and T2-weighted MR images were retrospectively analyzed in 34 patients with temporal lobe epilepsy, focusing on transneuronal degeneration. In 17 of the 34 patients, MR images were also analyzed after selective amygdalo-hippocampectomy. Atrophy of the fornix, mamillary body, mamillothalamic tract (MTT), and thalamus ipsilateral to the epileptic focus was demonstrated on MR images in 14.7, 17.6, 8.8, and 11.8% of the 34 patients, respectively. Focal hyperintensity of the thalamus was found on T2-weighted images in 8.8% of the 34 patients. In 17 patients who were evaluated before and after surgery, transneuronal degeneration was seen more frequently after surgery: fornix (11.8 vs 29.4%), mamillary body (11.8 vs 52.9%), MTT (5.9 vs 11.8%), and thalamus (11.8 vs 11.8%). Transneuronal degeneration of the limbic system is clearly demonstrated by MR imaging in patients with temporal lobe epilepsy, and surgical intervention induces transneuronal degeneration more frequently.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well established as transneuronal degeneration that neuronal damage caused by a focal brain lesion can influence the function and morphology of remote intact regions following the interruption of neural circuits [1]. Because the synaptic connections form closed neuronal system in specified neurons of the central nervous system, this sequential chain reaction may occur. Beginning at the injured or dead neurons, axon-to-somadendritic degeneration is referred to as anterograde transneuronal degeneration, and somadendritic-to-axonal degeneration is referred to as retrograde transneuronal degeneration [2]. Many experimental [3, 4] and pathological studies have shown the existence of transneuronal degeneration at regions remote from the primary lesion; anterograde transneuronal degeneration in the substantia nigra after massive basal ganglia infarction [5]. Functional changes at sites remote from the primary lesion have also been observed by single photon emission computed tomography (SPECT) and positron emission tomography (PET) [6, 7], whereas anatomical changes at remote sites have been demonstrated in patients by CT and MR imaging [8, 9, 10, 11].
The most common cause of the temporal lobe epilepsy is hippocampal sclerosis. In hippocampal sclerosis, cell loss and astrogliosis occur in the mesial temporal cortex, hippocampal formation, parahippocampal gyrus, amygdala, and entorhinal cortex. The hippocampus is connected to the fornix, mamillary body, and anterior thalamic nucleus, forming part of the limbic system called the circuit of Papez. It has been previously suggested that neuronal damage in the hippocampus may cause atrophy of the ipsilateral limbic system as a result of transneuronal degeneration. Cakirer et al. reported that this finding was found on MR images in 21.9% of the patients with medically intractable epilepsy [12]; however, there have been only limited studies concerning transneuronal degeneration of the limbic system in patients with temporal lobe epilepsy [13, 14, 15, 16].
In the present study, we evaluated the frequency and the MR imaging features of transneuronal degeneration of the limbic system in patients with temporal lobe epilepsy. We also detected the influence of surgery on the anatomy of the limbic system.
Subjects and methods
Thirty-four patients with temporal lobe epilepsy, who underwent MR examination consecutively from 1991 through 1999, were reviewed retrospectively. There were 19 men and 15 women, ranging in age from 3 to 54 years (mean age 28.6 years). All patients were diagnosed as temporal lobe epilepsy on the basis of electroencephalograms, MR imaging, PET, and/or intracranial electroencephalographic recordings. They were also confirmed to have an epileptic focus in the unilateral temporal lobe. The epileptic foci were located in the right temporal lobes in 14 patients and in the left temporal lobes in 20 patients. There was no case with bilateral involvement of the temporal lobe. Time intervals between onset of epilepsy and MR examination ranged from 1 to 45 years (median 19.6 years).
We obtained MR images with a 1.5-T system (Magnetom Vision, Siemens, Erlangen, Germany) using a regular head coil. T1-weighted spin-echo images and T2-weighted fast spin-echo images were obtained in the axial and coronal planes, which were parallel and vertical to the long axis of the hippocampus, respectively. T1-weighted images were obtained with TR/TE/no. of excitations=500 ms/14 ms/2, and T2-weighted images were obtained with TR/TE/no. of excitations=3500 ms/90 ms/1. The slice thickness was 5 mm with no intersection gap and the matrix was 256×256.
Magnetic resonance images were retrospectively analyzed by two experienced neuroradiologists (F.K., T.O.), who were blinded to the clinical information. They evaluated the size and the signal intensity on T2-weighted images of the following structures composing the limbic system: hippocampus; fornix; mamillary body; mamillothalamic tract (MTT); and thalamus. Only abnormalities that were identified in both the axial and coronal planes were defined as abnormal findings. When there was a difference of opinion concerning MR imaging findings between the two observers, the final judgment was reached by consensus.
We statistically compared each finding between the groups with hippocampal sclerosis and without hippocampal sclerosis on MR imaging. Limbic structures were visually classified into two grades according to their size and scored as follows: 0=no change; and 1=decrease in size. They were statistically analyzed using the sign test. A difference of p<0.05 was considered statistically significant. Seventeen of the 34 patients underwent selective amygdalo-hippocampectomy for intractable epilepsy and follow-up MR images were also obtained. Time intervals between surgery and follow-up MR examination ranged from 9 days to 12.7 months (median 6.5 months). In these patients, postoperative MR images were assessed in the same manner and an asymmetrical decrease in the size of the limbic structures was compared between before and after surgery.
Results
Among 17 patients who underwent surgery, 4 patients were not diagnosed because the tissue samples obtained were too small for pathological evaluation. Eight patients were found to have neuronal loss and gliosis in the hippocampus and were diagnosed as having hippocampal sclerosis. Two patients had tumorous lesions in the temporal lobe (one was a cavernous hemangioma and the other was a dysembryoplastic neuroepithelial tumor). The remaining 3 patients were suspected to have hippocampal sclerosis.
Abnormal findings of the limbic system outside the hippocampus on MR images were revealed in 11 (32.4%) of the 34 patients. Table 1 reveals the MR imaging data on each structure composing the limbic system. In 15 (44.1%) of the 34 patients, there was a small hippocampus ipsilateral to the epileptic focus. The signal intensity of the hippocampus was increased on T2-weighted images in 7 patients (20.6%). In only 1 (2.9%) of the 34 patients was it found in contralateral side of the epileptic focus. Atrophy of the fornix ipsilateral to the epileptic focus was found in 5 (14.7%) of the 34 patients. Only one (2.9%) of all the patients, who had a cavernous hemangioma, showed atrophy of the fornix on the contralateral side. Atrophy of the mamillary body ipsilateral to the epileptic focus was found in 6 (17.6%) of all the patients. Only one patient (2.9%), the same patient with a cavernous hemangioma, had contralateral atrophy of the mamillary body. Atrophy of the MTT ipsilateral to the epileptic focus was found in 3 (8.8%). Atrophy of the thalamus was also found in 3 (8.8%). Atrophy of the MTT and thalamus was not seen on the contralateral side. A focal increase of the signal intensity in the thalamus on T2-weighted images was found in 3 (8.8%) of the 34 patients, but no such increase was identified in the fornix, mamillary body, or MTT.
When evaluation was limited to the 15 patients with hippocampal sclerosis on MR images, the frequency of atrophy in the limbic system increased as follows: atrophy of the fornix in 4 (26.7%); of the mamillary body in 4 (26.7%); of the MTT in 2 (13.3%); and of the thalamus in 3 (20%; Table 2). The frequency of the atrophy of fornix and thalamus was significantly higher in the patients with hippocampal sclerosis than those without hippocampal sclerosis on MR imaging.
Length of history was 18.5±12.1 years (range 3–38 years) in the group with abnormal findings of the limbic system on MR images and 24.6±14.4 years (range 3–45 years) in the group with normal findings of those structures. No correlation was recognized between the interval from the onset of epilepsy and atrophy of any structure in the limbic system.
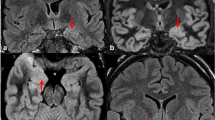
When comparing the prevalence of the findings before and after surgery in the 17 patients who underwent surgery, the frequency of limbic system atrophy tended to be higher after surgery than before surgery (Table 3). Limbic system atrophy ipsilateral to the epileptic focus was found in the following structures after the surgery: 5 (29.4%) in the fornix (Fig. 1); 9 (52.9%) in the mamillary body; 2 (11.8%) in the MTT (Fig. 2); and 2 (11.8%) in the thalamus. The frequency of mamillary body atrophy was significantly higher after surgery than before surgery (p=0.015), but there was no significant difference for the other structures.
Atrophy of the ipsilateral fornix, mamillary body, and thalamus. a Axial and b coronal T2-weighted MR images obtained 2 months after selective amygdalo-hippocampectomy reveal that the ipsilateral fornix is smaller than that on the other side (arrow). c Axial T1-weighted MR image reveals asymmetry of the mamillary body. The left mamillary body is smaller than the right side (arrow). d Axial T2-weighted MR image reveals the atrophy of the left thalamus. A subtle hyperintense area is also identified in the left thalamus (arrows)
Right hippocampal sclerosis and atrophy of the ipsilateral mamillothalamic tract (MTT). a Coronal T2-weighted MR image reveals asymmetry of the hippocampus. The right MTT is visualized as smaller area than that on the left side (arrow). b On an axial T2-weighted MR image, the right MTT cannot be identified (arrow)
Discussion
Long-term abnormal electrical activity in the patients with temporal lobe epilepsy causes damage to extratemporal structures as well as the hippocampus [14]. This phenomenon is well known as diaschisis, in which a focal brain lesion influences both the function and morphology of the intact regions remote from, but connected to, the primary injury [1]. The classical types of neuronal degenerations are Wallerian degeneration and transneuronal degeneration. Wallerian degeneration is afferent axonal degeneration that occurs after injury to the axon or cell body. This type of degeneration does not extend to other neurons through synaptic connections, in general; however, in some specified groups of neurons, degeneration can be transferred to both afferent and efferent neurons via synapses and this is called transneuronal degeneration. It occurs more commonly in a closed neuronal system, such as the limbic system [2].
Extratemporal changes in patients with temporal lobe epilepsy are also recognized as a kind of transneuronal degeneration. Seizure propagation can influence cerebral blood flow and metabolism in the extratemporal regions. Hypoglucose metabolism and hypoperfusion in the extratemporal areas can be observed on PET and SPECT in patients with temporal lobe epilepsy [17, 18]. Such phenomena are observed mainly in the thalamus ipsilateral to the epileptic focus and are thought to be caused by the interruption of afferent axonal supply. Anatomical changes also occur. For example, Margerison and Corsellis reported that autopsy revealed widespread neuronal loss and gliosis in the thalamus, amygdala, and cerebellum of patients with temporal lobe epilepsy [19]. Although anatomical changes of the limbic system distant from the primary lesion have recently been demonstrated using MR imaging [13, 14, 16, 20], only a few reports have been published.
Transneuronal degeneration in the limbic system of patients with temporal lobe epilepsy is thought to be related to the circuit of Papez, which is a neuronal circuit in the limbic system that is related to memory and emotion. This circuit is a closed chain of neuronal connections, which is composed of the hippocampus, mamillary body, MTT, and anterior thalamic nuclei (Fig. 3). Efferent neurons from the hippocampus connect to the mamillary body via the fornix and then neuronal activity is transferred from the mamillary body to the anterior thalamic nuclei via the MTT. Next, anterior thalamic nuclei project to the cingulate gyrus via thalamocingulate fibers and this circuit is completed by connections from the cingulate gyrus to the hippoccampus via the cingulum and the parahippocampal gyrus.
Neuroanatomic diagram of the circuit of Papez. The circuit of Papez starts in the hippocampus (1), which is connected to the mamillary body by first efferent neurons via the fornix (2). In the mamillary body (3), the first neurons are connected to the second neurons through synapses. The circuit continues via the mamillothalamic tract (4) to reach the anterior thalamic nucleus (5), and then is projected to the cingulate gyrus (6) via the thalamocingulate fibers. Finally, the circuit is completed by the transfer of impulses from the cingulum to the hippocampus via the parahippocampal gyrus (7)
Previous reports have shown asymmetrical fornix and mamillary body in 20–50% of temporal lobe epilepsy patients on MR images [13, 14, 16]. In those reports, all the patients were intractable temporal lobe epilepsy patients with histologically proven hippocampal sclerosis. In comparison with such reports, the frequency of fornix and mamillary body asymmetry was lower (14.7 and 17.6%, respectively) in our study. This difference may be mainly related to patient selection. Because we retrospectively reviewed temporal lobe epilepsy patients who were consecutively examined by MR imaging, this study included various grade of epilepsy from intractable cases with hippocampal sclerosis undergoing surgery to mild cases with no MR abnormalities. When evaluation was limited to the patients with hippocampal sclerosis confirmed on MR imaging, the frequency of fornix and mamillary body asymmetry increased to 26.7%. This datum is similar to the result reported by Kim et al., who reported that asymmetrically small fornix was shown in 39% of cases [16].
Our data compared between before and after surgery proved that the more severe neuronal damages are, the more limbic system atrophy occurs. The frequency of limbic system atrophy was higher (11.8–52.9%) after surgery than before surgery (5.9–11.8%). Kim et al. reported the same result compared between presurgical and postsurgical groups, which described that the frequency of asymmetrical small fornix and mamillary bodies increased more than twice after surgery [16]; thus, the neuronal damage caused by surgery probably interrupted efferent axonal activity, resulting in atrophic change of the entire limbic system. Although a significant difference was found only for the mamillary body in our study, further investigations are warranted to achieve a careful assessment of other structures in a larger number of cases. On the other hand, there was no correlation between the time interval from the onset of epilepsy and atrophy of each structure in the limbic system. Some of the previous reports reveal that the time interval might correlate with the abnormal MR findings, but Oikawa et al. reported that the extent of the abnormality of the limbic system did not correlate with time interval [20]. This is because the length of the history does not always indicate the clinical severity. To evaluate the clinical severity precisely, we need another appropriate indicator including not only the length of the history, but also severity of the epilepsy itself.
To our knowledge, most previous studies evaluated the mamillary body and the fornix, and evaluation of transneuronal degeneration of the MTT and thalamus has only been performed in a few previous studies [15]. Kataoka et al. [3] demonstrated one case of abnormal appearance of the MTT and mamillary body on MR images in patient with simple and complex partial seizures. In this study, we found atrophy of the MTT and thalamus in 8.8% of the patients. Its frequency was lower than that of atrophy of the fornix and mamillary body, suggesting that structures directly connected to the hippocampus might be more influenced by abnormal electrical activity than those indirectly connected.
There are still some limitations to our retrospective analysis using conventional MR images to depict asymmetry of the limbic system. Firstly, due to its unique morphology and small size, there are some difficulties in detecting asymmetry of the limbic structures. These structures are fairly small compared with the slice thickness of 5 mm used in our study. If a slice tilted, the findings can easily be modified by the partial-volume effect. Secondly, the fornix and mamillary body are difficult to evaluate for abnormal high signal intensity on T2-weighted images, because of the surrounding cerebrospinal fluid. These findings are likely to be influenced by the partial-volume effect. Thirdly, asymmetry of the limbic structures is not rare in normal volunteers [21]. For example, Kim et al. reported a unilateral small fornix in 6% of the normal subjects [16].
In order to avoid such errors and to improve the accuracy of detecting transneuronal degeneration in the limbic system, the following points should be considered: a small field of view (e.g., 17–18 cm2) and a thinner slice thickness (e.g., 3 mm) may contribute less artifacts including partial-volume effects. Volumetry using magnetization prepared rapid acquisition gradient-echo sequences may also be valuable for assessing atrophy [22]. Although it is time-consuming and requires appropriate post-processing systems, volumetry has the advantage of absolute measurements in bilateral mesial temporal sclerosis [23]. Fluid-attenuated inversion recovery images may also be the most useful for evaluation of transneuronal degeneration in the limbic system [23]. In fact, the frequency of atrophy and/or a high signal intensity of the limbic structures is reported to be increased by using such techniques [13, 16, 24], so a large-scale prospective study using these methods may be warranted in the future.
Yune et al. reported extratemporal hypoperfusion on interictal SPECT in the patients with temporal lobe epilepsy [17]. Ipsilateral hypoperfusion of the thalamus was found even in the case without mesial temporal sclerosis on MRI, and they concluded that the interictal brain SPECT should be useful for the localization of a seizure focus. They suggested that hypoperfusion and hypometabolism on SPECT and PET could be observed before the changes of anatomical structures on MRI. Therefore, development of MRI technique [25] might be able to reveal subtle anatomical changes using appropriate sequences, thus, extratemporal transneuronal degeneration should be useful for lateralization of the epileptic focus in patients with temporal lobe epilepsy clinically.
Although there were some problems with the MR imaging methods used in our retrospective study, the incidence of transneuronal degeneration in the limbic system observed in patients with temporal lobe epilepsy was far from low. The circuit of Papez seems to play an important role in the development of transneuronal degeneration and the remote effect may be a common finding equal to hippocampal sclerosis.
Conclusion
In conclusion, MR imaging enables us to evaluate transneuronal degeneration in the limbic system. We demonstrated extrahippocampal limbic system abnormalities on conventional MR images in patients with temporal lobe epilepsy. The MR images revealed transneuronal degenerations ipsilateral to the epileptic focus.
References
Feeney DM, Baron JC (1986) Diaschisis. Stroke 17:817–830
Torch WC, Hirano A, Solomon S (1977) Anterograde transneuronal degeneration in the limbic system: clinical–anatomic correlation. Neurology 27:1157–1163
Kataoka K, Hayakawa T, Yamada K, Mushiroi T, Kuroda R, Mogami H (1989) Neuronal network disturbance after focal ischemia in rats. Stroke 20:1226–1235
Matthews MA (1973) Death of the central neuron: an electron microscopic study of thalamic retrograde degeneration following cortical ablation. J Neurocytol 2:265–288
Forno LS (1983) Reaction of the substantia nigra to massive basal ganglia infarction. Acta Neuropathol (Berlin) 62:96–102
Sakashita Y, Matsuda H, Kakuda K, Takamori M (1993) Hypoperfusion and vasoreactivity in the thalamus and cerebellum after stroke. Stroke 24:84–87
Baron JC, Bousser MG, Comar D, Castaigne P (1980) "Crossed cerebellar diaschisis" in human supratentorial brain infarction. Trans Am Neurol Assoc 105:459–461
Ogawa T, Yoshida Y, Okudera T, Noguchi K, Kado H, Uemura K (1997) Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology 204:255–262
Ogawa T, Okudera T, Inugami A, Noguchi K, Kado H, Yoshida Y, Uemura K (1997) Degeneration of the ipsilateral substantia nigra after striatal infarction: evaluation with MR imaging. Radiology 204:847–851
Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, Takahashi M (1994) Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 192:539–543
Nakane M, Teraoka A, Asato R, Tamura A (1992) Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke 23:328–332
Cakirer S, Basak M, Mutlu A, Galip GM (2002) MR imaging in epilepsy that is refractory to medical therapy. Eur Radiol 12:549–558
Baldwin GN, Tsuruda JS, Maravilla KR, Hamill GS, Hayes CE (1994) The fornix in patients with seizures caused by unilateral hippocampal sclerosis: detection of unilateral volume loss on MR images. Am J Roentgenol 162:1185–1189
Chan S, Erickson JK, Yoon SS (1997) Limbic system abnormalities associated with mesial temporal sclerosis: a model of chronic cerebral changes due to seizures. Radiographics 17:1095–1110
Yamada K, Shrier DA, Rubio A, Yoshiura T, Iwanaga S, Shibata DK, Patel U, Numaguchi Y (1998) MR imaging of the mamillothalamic tract. Radiology 207:593–598
Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N (1995) Clinical significance of asymmetry of the fornix and mamillary body on MR in hippocampal sclerosis. Am J Neuroradiol 16:509–515
Yune MJ, Lee JD, Ryu YH, Kim DI, Lee BI, Kim SJ (1998) Ipsilateral thalamic hypoperfusion on interictal SPECT in temporal lobe epilepsy. J Nucl Med 39:281–285
Rausch R, Henry TR, Ary CM, Engel J Jr, Mazziotta J (1994) Asymmetric interictal glucose hypometabolism and cognitive performance in epileptic patients. Arch Neurol 51:139–144
Magerison JH, Corsellis JAN (1966) Epilepsy and the temporal lobes: a clinical, electroencephalographic, and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89:499–530
Oikawa H, Sasaki M, Tamakawa Y, Kamei A (2001) The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology 43:205–210
Supprian T, Hofmann E (1997) The fornix of the human brain: evidence of left/right asymmetry on axial MRI scans. Surg Radiol Anat 19:105–109
Sitoh YY, Tien RD (1998) Neuroimaging in epilepsy. J Magn Reson Imaging 8:277–288
Meiners LC (2002) Role of MR imaging in epilepsy. Eur Radiol 12:499–501
Meiners LC, Gils A, Jansen GH, Kort G de, Witkamp TD, Ramos LMP, Valk J, Debets RMC, van Huffelen AC, van Veelen CWM, Mali WPTM (1994) Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. Am J Neuroradiol 15:1547–1555
El-Koussy M, Mathis J, Loveblad KO, Stepper F, Kiefer C, Schroth G (2002) Focal status epilepticus: follow-up by perfusion and diffusion MRI. Eur Radiol 12:568–574
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodama, F., Ogawa, T., Sugihara, S. et al. Transneuronal degeneration in patients with temporal lobe epilepsy: evaluation by MR imaging. Eur Radiol 13, 2180–2185 (2003). https://doi.org/10.1007/s00330-003-1875-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-1875-y