Abstract
The aim of this study was to investigate the effects of melatonin (MT) feed supplementation on the antioxidant capacity, immune defense, and intestinal flora in Procambarus clarkii (P. clarkii). Six groups of P. clarkii were fed test feeds containing different levels of MT: 0 mg/kg (control), 22.5, 41.2, 82.7, 165.1, and 329.2 mg/kg for a duration of 2 months. The specific growth rate, hepatosomatic index, and condition factor were recorded highest in the test group of shrimp fed an MT concentration of 165.1 mg/kg. Compared to the control group, the rate of apoptosis was lower in hepatopancreas cells of P. clarkii supplemented with high concentrations of MT. Analyses of antioxidant capacity and immune-response-related enzymes in the hepatopancreas indicated that dietary supplementation of MT significantly augmented both the antioxidant system and immune responses. Dietary MT supplementation significantly increased the expression levels of antioxidant-immunity-related genes and decreased the expression levels of genes linked to apoptosis. Dietary MT was associated with an elevation in the abundance of the Firmicutes and a reduction in the abundance of the Proteobacteria in the intestines; besides, resulting in an increase in the abundance of beneficial bacteria, such as Lactobacilli. The broken-line model indicated that the suitable MT concentration was 154.09–157.09 mg/kg. MT supplementation enhanced the growth performance of P. clarkii, exerting a positive influence on the intestinal microbiota, and bolstered both immune response and disease resistance. Thus, this study offered novel perspectives regarding the application of dietary MT supplementation within the aquaculture field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melatonin (MT) is a derivative of tryptophan, a steroidal hormone, chemically referred to as N-acetyl-5-methoxytryptamine (Herrero et al. 2007). Initially MT discovered as a secretion product of the pineal gland, it is now understood to also be synthesized in other animal organs (Agapito et al. 1995; Meyer-Rochow 2001; Reiter et al. 2013, 2014). The lipophilic and hydrophilic properties of MT enable it to readily traverse different physiological barriers and distribute across organs and bodily fluids (Cruz et al. 2014; Hardeland et al. 2010). MT serves multiple physiological functions and impart a broad range of biological effects (Ahmadi et al. 2024). One of its primary functions is to convey information about the day-night cycle, thereby synchronizing physiological parameters with environmental factors and contributing to the formation of the biological clock (Reiter 1991).
MT plays a regulatory role in the reproductive, neurological, immunological, and digestive systems of animals (Reiter et al. 2009; Sébastien et al. 2021; Zhernakova and Rybnikova 2008). MT has close relationship in numerous physiological functions in aquatic animals, such as enhancing antioxidant capacity, immune responses, digestive enzyme activity, and the regulation of blood lymphocyte parameters (Maciel et al. 2009; Sainath et al. 2013; Yang et al. 2023). Furthermore, MT possesses potent free radical scavenging properties. Both MT itself and its metabolites mitigate oxidative stress by promoting the upregulation of antioxidant enzymes and attenuating the activation of pro-oxidant enzymes (Cai et al. 2021; Reiter et al. 2016; Zhang and Zhang 2014). For example, following exposure to glyphosate and feeding 80 mg/kg MT, the Chinese mitten crab (Eriocheir sinensis) experienced a significantly increased survival rate, antibacterial capabilities, and increased lipase, amylase, and trypsin activities (Song et al. 2020b). Moreover, the addition of MT to diet increases weight gain, specific growth rate, and the digestive enzyme activity of crayfish (Cherax destructor). MT also boosts the antioxidative potential of the hepatopancreas and improves immunological metrics within blood lymphocytes (Yang et al. 2023). However, there are currently no studies of the impacts of adding MT to feed on the growth performance and antioxidant capacity and immune defense of P. clarkii.
The intestine serves as a pivotal organ not only for digestion and nutrient absorption but also as a vital element of the immune system (Wittig and Zeitz 2003). Shrimp intestines contain a diverse array of microbes that comprise the gut microbiota (Duan et al. 2018). Such microbes not only contribute to food digestion but also influence the immune function of organisms by producing immune factors and promoting the formation of mucosal barriers (Liu et al. 2022; Wu et al. 2016; Yu et al. 2012; Zhao et al. 2022). When the gut microbiota in an animal is healthy, it can synthesize and release beneficial metabolites. Such metabolites have a positive impact on the well-being of the host and aid in balancing the gut’s microbial ecosystem, reducing the risk of disease (Cai and Kang 2023; Koh et al. 2016). However, an imbalanced or unhealthy gut microbiota may have adverse effects on the host’s immune system (Wang et al. 2023b; Zhan et al. 2022b). Feed additives play a substantial role in aquaculture by influencing the composition and functionality of the gut microbiota, thereby impacting the development and general health of aquatic organisms (Meng et al. 2023; Wang et al. 2023a; Zhan et al. 2022a). A recent study revealed that the supplementation of glycerol monolaurate in the diet of Chinese mitten crabs enhanced their blood lymphocyte immune enzyme activity and antimicrobial peptide expression, and improved the gut microbiota composition, thereby enhancing the growth and antioxidative capacity of crabs (Fu et al. 2022). Another study indicated that the inclusion of 1.0 g/kg to 2.0 g/kg of fennel extract to the diet of Yellow River carp was beneficial in modulating the gut microbiota, thereby enhancing their growth and antioxidative capacity (Li et al. 2023a). Currently, there is a lack of research investigating the mechanisms leading to the effects of MT supplementation on the intestinal microbiota of P. clarkii.
P. clarkii is a significant economic crustacean, originally found in the southeastern USA and northern Mexico (Ou et al. 2013). It was introduced to China from Japan during the 1920s, and is notable for its delectable taste, high protein content, relatively low fatty levels, favorable fatty acid composition, and low cholesterol content. Thus, freshwater crayfish are considered an ideal food source (Chen et al. 2018; 2022b). Due to its high growth rate, high reproductive capacity, and strong environmental adaptability, P. clarkii is widely distributed throughout freshwater wetlands in China, including rice paddies and river channels (Yi et al. 2018). However, in recent years, artificial cultivation of crayfish has been plagued by health challenges (Zhu et al. 2023). A weakened immune system, inadequate antioxidant and stress responses, increased susceptibility to disease, increased mortality, and reduced tolerance to transport are problems that must be addressed in artificial farming (Zhang et al. 2023). Therefore, in recent years, suitable dietary immune enhancers such as probiotics (include yeasts, microalgae, and bacteria), functional sugars (such as fucoidan), etc., have been widely employed in aquaculture to augment the innate immune responses of shrimp and crayfish (Amenyogbe 2023; Michalak et al. 2023; Xu et al. 2014). In this study, we chose MT as a feed supplement to evaluate its effects on the growth, intestinal microbiota, antioxidant capacity, and immune defense of P. clarkii. Our results have the potential to facilitate the formulation of suitable cultivation strategies for P. clarkii and offer innovative perspectives for future investigations into the feed nutrition of crayfish.
Materials and Methods
The present study was approved by the Committee on the Ethics of Animal Experiments of East China Normal University (Shanghai, China). The protocols for crayfish were implemented under the Guidance of the Care and Use of Laboratory Animals in China (20,201,001).
Experimental Diet
MT powder (purity ≥ 99.0%, China National Pharmaceutical Group Corporation, Shanghai, China) was dissolved in 1 mL of ethanol (Yang et al. 2023). The solution was then sprayed onto basal feed and the prepared pellets were dried at 37 °C for 24 h. The formula of the basic feed and setting of different melatonin concentration groups are shown in our previous study (Li et al. 2023d).
Experimental Animals and Experimental Design
P. clarkii were gained from an aquaculture base (Jiading District, Shanghai, China). The specific breeding process is consistent with our previous studies (Li et al. 2023d).
Sample Collection
The feeding trial extended over a period of 8 weeks. In order to assess the growth performance of P. clarkii, the number of shrimps in each tank was counted at the beginning and end of the trial. Upon the conclusion of the 8-week feeding experiment, a 24-h starvation period was implemented before sample collection. From each tank, three P. clarkii were chosen at random and pooled to form a solitary sample for each group. The survival rate (SR), body length growth rate (BLGR), and weight gain rate (WGR) of the cultured P. clarkii can be seen in previous studies (Li et al. 2023d). In addition, we also measured specific growth rate (SGR), hepatosomatic index (HSI), feed conversion ratio (FCR), and condition factor (CF). The formula is as follows:
Hepatopancreatic tissues of shrimps were removed for histological analysis, and the remaining were immediately frozen in liquid nitrogen at – 80 °C. Intestinal flora was analyzed for the 0 and 165.1 mg/kg MT group (n = 6). Crude protein, crude fat, ash, and moisture content of the basal diet and whole shrimp body were determined by methods as described in the literature (AOAC 1995).
Determination of Hepatopancreas Cell Apoptosis
Hepatopancreatic tissues from each group (0, 82.7, and 329.2 mg/kg) were fixed in 4% paraformaldehyde. The processing process is consistent with previous studies (Li et al. 2023d). Apoptosis was detected following the method of Li et al (2022). The formula is in the Supplementary Material.
Analysis of Antioxidant Indicators and Immune Enzymes
Pre-cooled physiological saline was mixed with hepatopancreas samples in a 9:1 ratio and homogenized using an electric homogenizer. The homogenate was centrifuged at 4 °C and 2500 rpm for 10 min, and the supernatant was collected for analyses of antioxidant and immune indicators. Acid phosphatase (ACP, A060-2–2), alkaline phosphatase (AKP, A059-2–2), lysozyme (LZM, A050-1–1), phenol oxidase (PO, H247-1–2), nitric oxide synthetase (NOS, A014-2–2), nitric oxide (NO, A013-2–1), acetyl-CoA ascorbic acid (ASA, A009-1–1), and total antioxidant capacity (T-AOC, A015-3–1) were analyzed using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Identification of Genes Involved in Immune Response and Antioxidant Capacity in the Hepatopancreas
Upon completion of the experiment, total RNA was extracted from the hepatopancreas of the shrimps from each group. Total RNA was reverse transcribed into first-strand cDNA following assessment of the extracted RNA’s concentration and purity. The primer sequences of the target genes were formed by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China) (Table 1). Relative gene expression was calculated by the 2–ΔΔCT method (Livak and Schmittgen 2001). All procedures were consistent with our previous studies (Li et al. 2023d).
Intestinal Microbiota DNA Extraction and Sequencing
DNA was extracted from the intestinal contents of shrimp using the Fast DNA Stool Mini Kit (QIAamp, Germany) according to the instructions of manufacturer. Sequencing was performed applying the V3–V4 region of the 16S rRNA gene, amplified with barcode primers V341F (5′-CCTACGGGNGGCWGCAG-3′) and V805R (5′-GACTACHVGGGTATCTAATCC-3′). Refer to Supplementary Materials for more information.
Statistical Analysis
All data were expressed as the mean ± standard error of the mean (mean ± SEM). SPSS 23.0 software (IBM, Armonk, NY, USA) was used to analyze the data. The Shapiro–Wilk test was used to test the normality of the data and the Leven’s test was used to test the homogeneity of the variance before data analysis. Data were analyzed using one-way ANOVA followed by Duncan’s multiple comparisons. Statistical significance was defined when P < 0.05. All graphs were generated by GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA, USA).
Result
Growth Performance
As the concentration of MT increased, the specific growth rate (SGR) and hepatosomatic index (HSI) exhibited an initial increase followed by a subsequent decrease in quadratic models (p < 0.001). The feed conversion ratio (FCR) showed an initial decrease followed by an increase in quadratic models (p < 0.001). In addition, the results of condition factor (CF) were significant both linearly and quadratically (p < 0.05). When the MT concentration reached 165.1 mg/kg, the SGR, HSI, and CF reached their highest values, while the FCR reached its lowest value. Moreover, the SGR and HSI values were significantly higher in the 165.1 mg/kg group than other groups (p < 0.05), while the FCR values were considerably lower than those in other groups (p < 0.05) (Table 2).
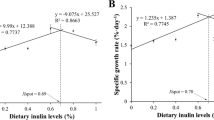
Relationship between dietary MT level and SGR broken-line model (y = 0.0047x + 1.2881, R2 = 0.9381) indicated that the most suitable dietary MT level for P. clarkii in our study was 157.09 mg/kg (Fig. 1A). The broken-line model (y = − 0.2862x + 184.43, R2 = 0.9322) derived from the correlation between MT level and FCR demonstrated that 154.09 mg/kg represented the optimal level for the growth of P. clarkii (Fig. 1B). Additionally, the broken-line model (y = 0.0078x + 6.5599, R2 = 0.9124) derived from the correlation between MT level and HSI suggested that the most favorable growth for P. clarkii was observed at 154.58 mg/kg (Fig. 1C).
Nutrient Composition of Whole Body
The impact of MT concentration on the proximate composition of whole shrimp is shown in Table 3. The levels of MT in feed did not exert a notable impact on the moisture or ash contents between the different groups (p > 0.05). However, the incorporation of 82.7, 165.1, and 329.2 mg/kg of MT in the feed resulted in a noteworthy elevation of the crude protein content in shrimp bodies when contrasted with the control group. The group with 165.1 mg/kg of MT showed a 4.4% increase in crude protein content than the control group. Conversely, the inclusion of 41.2, 82.7, 165.1, and 329.2 mg/kg of MT in feed significantly reduced the crude fat content in shrimp bodies. The group with 165.1 mg/kg of MT showed a 6.1% decrease in the crude fat content compared to the control group.
Immune Response-Related Substance Content and Enzyme Activities
As shown in Table 4, with increasing MT concentrations, the activities of PO, LZM, AKP, and ACP demonstrated an initial rise succeeded by a decrease, showing a quadratic model (p < 0.05). When the MT concentration was 165.1 mg/kg, the activities of PO, LZM, and ACP reached their highest levels, exhibiting a notable increase than other groups (p < 0.05). When the MT concentration was 82.7 mg/kg, the AKP activity peaked, exhibiting a substantial increase compared to the activity levels observed in the other groups (p < 0.05). The NO content exhibited an initial increase followed by a decrease as the MT concentration increased (Table 5). When the MT concentration was 165.1 mg/kg, NO content reached its maximum, which was considerably higher than that in the other groups (p < 0.05). When the MT concentration was 82.7 mg/kg, NOS activity was highest. In comparison with the control group, the NOS activity in P. clarkii fed 82.7 mg/kg MT increased approximately 1.13 times (p < 0.05).
Apoptosis Rate and Antioxidant Capacity-Related Enzyme Activities
We measured apoptosis rates in the groups supplemented with different concentrations of MT (Fig. 2). Fluorescence intensity analysis revealed that the apoptosis rate of hepatopancreas cells decreased with increases in MT concentration. The quantity of positive cells identified in the groups administered 82.7 mg/kg and 329.2 mg/kg were notably lower compared to the control group (p < 0.05). Besides, as shown in Table 5, the MT concentration in the feed was 165.1 mg/kg, and the ASA content and T-AOC capacity in P. clarkii reached their maximum, significantly higher than the other groups (p < 0.05).
Effects of Melatonin on Antioxidant, Immune, and Apoptosis-Related Genes
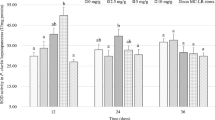
As demonstrated in Fig. 3, the mRNA expressions of the antioxidant-related enzymes catalase (CAT) and superoxide dismutase (SOD) depicted an initial rise, succeeded by a decline with increasing dietary MT concentration in quadratic models (p < 0.05). When the MT concentration was 82.7 mg/kg, the mRNA level of CAT reached its highest point, which was significantly higher compared to the control group (p < 0.05). When the MT concentration in the diet was 165.1 mg/kg, the mRNA level of SOD reached its peak, exhibiting a significantly higher than the control group (p < 0.05).
Effect of dietary MT level on the genes’ expression involved in antioxidation, immune, and apoptosis in the hepatopancreas of P. clarkii. Values are means (n = 4) with standard errors represented by the vertical bars. Different lowercase letters indicate significant differences between the groups (P < 0.05). Abbreviations: CAT, catalase; SOD, superoxide dismutase; LZM, lysozyme; proPo, prophenoloxidase; Cytc, cytochrome C; Adj. R2, adjusted R squared
The mRNA levels of the immune-related enzymes LZM and proPo also followed a trend of initially increasing and then decreasing as the MT concentration in the diet increased. When the MT concentration was 165.1 mg/kg, the mRNA level of proPo reached its highest, which was considerably higher than the control group (p < 0.05). The mRNA level of LZM reached its highest point when the MT concentration was 41.2 mg/kg. Conversely, the mRNA expression levels of the apoptosis-related enzymes Caspase3 and Cytc initially decreased and then increased with increasing MT concentrations in the diet. When the MT concentration added to the diet was 82.7 mg/kg, the mRNA level of Caspase3 reached its lowest point. When the MT concentration was 165.1 mg/kg, the mRNA level of Cytc reached its lowest point, which was considerably lower than that of the other groups (p < 0.05).
Sequencing of 16S rRNA, and Annotation and Evaluation of Species
The intestinal flora of P. clarkii fed dietary supplementation with MT were divided into phylum, genus, family, order, and class. Across all 12 samples from both groups (0 and 165.1 mg/kg), the majority of phylotypes belonged to three core phyla: Proteobacteria in group C (0.654) and group DM (0.585); Firmicutes in group C (0.202) and group DM (0.272); Bacteroidetes in group C (0.139) and group DM (0.139) (Fig. 4A). When P. clarkii was fed dietary supplementation with MT, the distribution patterns of the three predominant phyla within each group were comparable across the various MT concentration groups, yet distinct differences were observed in both abundance and variation trends, group C different species were 557 and group DM different species were 474 (Fig. 4B). PCA plots showed that the groups fed MT for 2 months at concentrations of 0 and 165.1 mg/kg exhibited significant clustering, suggesting a favorable grouping effect (Fig. 4C).
Analysis of Species and Genera
Boxplots of C and DM examined in terms of genera showed significant in Thermomonas, Clostridium sensu stricto, Cloacibacterium, and Lactococcus (Fig. 5A). After 2 months of feeding with 165.1 mg/kg of MT, the relative abundance of Citrobacter, Candidatus Bacilloplasma, Aeromonas, Lactobacillus, Prevotella, Bacteroides, Thermomonas, Gemmobacter, Dysgonomonas, and Anaerorhabdus furcosa was high in the gut (Fig. 5B). The abundance of Lactobacillus faecis, Enterococcus durans, Lactococcus lactis, Butyricicoccus pullicaecorum, Aeromonas media, Acinetobacter johnsonii, Bdellovibrio sp., and Bacteroides sartorii in the gut of P. clarkii fed 165.1 mg/kg MT for 2 months increased and the relative abundance of beneficial bacteria also increased (Fig. 6). In comparison with the control group, the relative abundance of Lactobacillus intestinalis, Bosea sp., alpha proteobacterium, Vibrio cholerae, and Bacteroides plebeius in the intestinal tract decreased with MT supplementation.
P. clarkii was fed dietary melatonin supplementation at concentrations of 0 (C) and 165.1 (DM) mg/kg. A The top 10 boxplots representing the abundance of different genera. Species with zero relative abundance were not present in any of the groups. B Heat map of differences between the two groups at the genus level. Sample information is on the horizontal axis and species labeling information is on the vertical axis. Higher relative abundance is in red; lower relative abundance is in blue
P. clarkii was fed dietary melatonin supplementation at concentrations of 0 (C) and 165.1 (DM) mg/kg. Phylogenetic tree between populations and heat map of species abundance in both groups. The evolutionary tree is shown on the left. Different colors of the branches represent different phyla. The tip of each branch is an OTU
Discussion
MT secreted by the pineal gland is an indole-class hormone with diverse physiological functions (Pandi-Perumal et al. 2006). Previous studies have indicated that supplementing animal feed with MT can result in improvement in their growth performance (Akbarian et al. 2014). Feed supplementation is crucial for the long-term sustainability of the aquaculture sector as well as for the health and development of animals. However, studies on the impact of adding MT to shrimp feed remain relatively scarce. Defining the nutritional sources and quantities in the diet components of the study subject during biological dietary research is advantageous to ensuring the controllability of environmental factors (Glenny et al. 2020). Therefore, to avoid interference from MT, the basal diet in this study was modified in accordance with Yu et al. (2021). By evaluating fundamental growth indexes, antioxidant capacity, immune defense, and intestinal microbiota-related indicators, we elucidated the impact of MT supplementation on the growth of P. clarkii, and established the optimal dosage of MT.
This investigation demonstrated that the growth performance of P. clarkii was improved after the addition of MT to the diet. The diet supplemented with 165 mg/kg MT group substantially enhanced the WGR, SGR, HIS, and CF of P. clarkii, which is consistent with our previous findings on survival (SR), weight gain rate (WGR), and body length growth rate (BLGR) (Li et al. 2023d). Nevertheless, significant growth promotion impact was not observed at higher doses than this. Similar improvements in growth were reported in Pacific white shrimp (Litopenaeus vannamei) (Ye et al. 2024) and crayfish (Cherax destructor) (Yang et al. 2023) upon achieving an appropriate level of dietary MT content. But there are also studies that show that adding melatonin to feed does not improve the growth index of gilthead sea bream (Sparus aurata L.) (Amri et al. 2020). We speculate that this is due to the difference in the dose of MT supplementation in the feed and the species studied. The broken-line regression analyses of SGR, FCR, and HSI in our study indicated that the ideal dietary MT concentrations for juvenile P. clarkii were 157.09, 154.09, and 154.58 mg/kg, respectively. In conclusion, the optimal dietary MT levels for enhancing growth performance ranged from 154.09 to 157.09 mg/kg.
Research has revealed that proteins are essential constituents in the construction and maintenance of cells, and they can also be converted into energy (Gao et al. 2010). In this study, we observed an increase in the crude protein content in shrimp bodies following supplementation with MT, thus, indicating that the addition of MT to feed can benefit the growth of P. clarkii. Furthermore, we observed a reduction in the crude fat content of the whole shrimp. Studies have shown that dietary MT can promote lipid transport, up-regulate lipid oxidation, and down-regulate lipid synthesis, thereby reducing lipid accumulation in the hepatopancreas (Ye et al. 2024). We speculate that MT may enhance lipid metabolism in P. clarkii. Similar results were found when feeding Litopenaeus vannamei with methanotrophic (Methylococcus capsulatus) bacteria powder (Chen et al. 2022a).
The hemolymph of crustaceans contains the prophenoloxidase (proPO) activation system, which is pivotal to the immune system and functions to defend against pathogens (Lage and Kenneth 2004). When exposed to pathogens, injury, or other stimuli, the immune system of P. clarkii responds by converting proPO into active phenoloxidase (PO) to counter stress and invasion (Cerenius et al. 2008). PO activity may arise via multiple pathways, including proPO and hemocyanin (HC), both of which play roles in immune responses (Wei et al. 2020). We found an increase in PO enzyme activity and proPO gene expression in P. clarkii, which reflects the activation of the prophenoloxidase system and enhances immune function. Similarly, the addition of MT to the diet reported by Yang et al. also improved the non-specific immunity of Cherax destructor (Yang et al. 2023).
The humoral immunity of crustaceans predominantly relies on the action of several enzymes, including lysozyme (LZM), alkaline phosphatase (AKP), and acid phosphatase (ACP) (Liu et al. 2020). LZM is a vital defense substance within the immune system of crustaceans that can indirectly exert bactericidal effects by stimulating the complement system and phagocytic cells (Ashouri et al. 2020). The variations in the activities of ACP and AKP can reflect the metabolic activities in crustaceans, influencing their immune functions and disease resistance (Lei et al. 2021). After supplementation with MT, the activities of LZM, AKP, and ACP, and the expression of the LZM gene were significantly increased, indicating that MT enhanced the immune defense of P. clarkii at the gene expression and enzyme activity level.
Nitric oxide (NO) is a highly reactive free radical generated endogenously by nitric oxide synthase (NOS) (Lai et al. 2024). NO can regulate immune cell activity and signaling, the modulation of inflammatory responses, and combat bacteria, viruses, and other pathogenic microorganisms (Christie et al. 2014; Gajbhiye and Khandeparker 2018; Y et al. 2000). In contrast to the control group, we found that adding MT significantly increased both the concentration and activity of NOS. Those data suggest that the addition of MT to the diet effectively regulated immune cell activity and signaling, which influenced the immune response of P. clarkii. Those findings were similar to the findings in a study of dietary arginine in the yellow grouper, Epinephelus awoara (Zhou et al. 2012).
Adding MT, known as a potent endogenous antioxidant, has demonstrated in various species its capability to augment an organism’s antioxidant capacity (Yang et al. 2023). ASA, also known as vitamin C, is an essential antioxidant (Liu et al. 2024). Total antioxidant capacity (T-AOC) serves as a measure indicating the comprehensive antioxidant capability of organisms, encompassing the collective effects of all antioxidants (such as vitamins, enzymes, polyphenolic compounds, etc.) against oxidative stress (Sies 2007). T-AOC can be used to characterize the overall antioxidant status of living organisms (Helmut 2007). We observed an increase in the levels of ascorbic acid (ASA), as well as the T-AOC within P. clarkii fed MT. Those data suggest that MT increased antioxidant levels in individuals.
To maintain homeostasis in the body, crustaceans prevent or repair oxidative damage by eliminating ROS through their powerful antioxidant defense system, involving the activities of antioxidant enzymes (Xu et al. 2018). We found that CAT and SOD gene expression increased after MT supplementation. Previous studies indicated that adding MT to the daily diet of Litopenaeus vannamei may enhance the activities of antioxidant enzymes (Li et al. 2023c). Those findings agree with the outcomes of our research. Thus, the supplementation of MT not merely elevated the activity of antioxidant enzymes but also heightened the non-enzyme system level in P. clarkii, thereby augmenting its antioxidant capacity.
Apoptosis is a dynamic process that preserves bodily homeostasis and enhances environmental adaptability in response to cellular stress (Zhao 2012). Nevertheless, an overabundance of apoptosis may result in chronic degeneration or immune dysfunction, whereas the inability of apoptosis might contribute to increased cellular dysfunction (Zheng et al. 2023). Cytochrome C (Cyt C) acts as an apoptosis-inducing factor by forming channels in the inner mitochondrial membrane and activating the apoptosis-executing protein caspase-3 to enter the cytoplasm and promote apoptosis (Guan et al. 2023). In the current research, the gene expression levels of Cyt C and caspase-3 decreased after MT supplementation and tissue sections exhibited a decrease in apoptosis. A study by Tian et al. on the effects of dietary supplementation with β-1, 3-glucan (immune enhancer) in Macrobrachium nipponense also revealed increased antioxidant capacity and decreased apoptosis (Tian et al. 2023a, b). Therefore, we speculated that dietary MT supplementation enhanced the antioxidant immune properties of crayfish and reduced oxidative stress, thereby reducing the occurrence of apoptosis.
As a highly complex ecosystem, the intestinal flora has a profound influence on host physiology, immunity, nutrition, and metabolism through its interactions with the host (Henao-Mejia et al. 2012; Ma et al. 2017). The equilibrium of gut microbiota (including richness and diversity) stands as a paramount factor affecting the health of aquatic animals (Yang et al. 2019; Zhang et al. 2024). Some studies have shown that MT reduces gut microbiota disruption in mice and prevents gut damage in fish (Li et al. 2023b; Miao et al. 2022). The present study identified a total of three core phyla in P. clarkii supplemented with different concentrations of MT, which was similar to the findings of previous reports (Chen et al. 2021). We found a significant increase in the abundance of Firmicutes and a decrease in the abundance of the Proteobacteria in the intestinal tract of P. clarkii after dietary supplementation with MT. Previous studies also found that organic acids and essential oils benefit the intestinal flora of L. vannamei and enhanced the immune response and resilience against diseases through the augmentation of Firmicutes and reduction in Proteobacteria abundance (He et al. 2017). Because many intestinal Proteobacteria are pathogenic to aquatic organisms (Ma et al. 2017). This could be attributed to the fact that MT enhances the body’s immune enzyme activity, thereby reducing the number of harmful bacteria in the gut, protecting the gut from damage by pathogens.
At the genus level, the relative abundance of Lactococcus, Citrobacter, and Candidatus Bacilloplasma were increased in P. clarkii fed MT. Lactobacilli have been found to maintain the balance of intestinal flora by prohibiting the translocation and adhesion of pathogenic bacteria, modulating immunomodulatory properties, and suppressing inflammatory responses in the gut (Chen et al. 2023; Dong et al. 2014). Citrobacter has an immune-protective impact in the intestinal tract and is capable of eliminating harmful bacteria from the intestinal lumen (Guo et al. 2020). Candidatus Bacilloplasma is an essential endogenous component of the gut microbiota of crustaceans (Kostanjšek et al. 2007; Sun et al. 2020). Therefore, after supplementation with MT, the abundance of beneficial bacteria in the gut of crayfish increased, leading to an increase in P. clarkii immune function and antibacterial ability. We found that the abundance of Acinetobacter johnsonii and Bdellovibrio sp. were also improved in P. clarkii fed MT. Acinetobacter is one of the dominant genera in the intestinal tract of healthy crustaceans, and helps to prevent infections from other pathogenic bacteria, such as Vibrio parahaemolyticus (Alfiansah et al. 2020; Song et al. 2020a). Bdellovibrio sp. is a specialized predator of Gram-negative bacteria that can parasitize bacteria and cause them to lyse; thus, Bdellovibrio spp. are considered potential probiotic alternatives to antibiotics (Yang et al. 2023a). Overall, MT supplementation altered the structure of the gut microbiota in crayfish, increased the abundance of immune-associated flora, and improved the immune response in the gut.
Conclusion
In this study, we assessed the effects of different concentrations of MT on the growth performance, antioxidant capacity, immune response, and intestinal-microbiota-related indices in P. clarkii. Broken-line model based on the dietary MT levels and SGR, FCR, and HSI indicated that MT addition to P. clarkii diets was more appropriate in the range of 154.09–157.09 mg/kg. Supplementing the diet with MT significantly increased the weight gain rate, enhanced antioxidant-immunity-related enzyme activities and gene expression, reduced apoptosis, and altered the structure of the intestinal flora in P. clarkii. Those findings provide new insights into the promotion of dietary MT supplementation in the field of aquaculture.
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Agapito MT, Herrero B, Pablos MI, Miguel JL, Recio JM (1995) Circadian rhythms of melatonin and serotonin-N-acetyltransferase activity in Procambarus clarkii. Comp Biochem Physiol Part A: Mol Integr Physiol 112(1):179–185
Ahmadi S, Taghizadieh M, Mehdizadehfar E, Hasani A, Khalili Fard J, Feizi H, Hamishehkar H, Ansarin M, Yekani M, Memar MY (2024) Gut microbiota in neurological diseases: melatonin plays an important regulatory role. Biomed Pharmacother 174:116487. https://doi.org/10.1016/j.biopha.2024.116487
Akbarian A, Kazerani HR, Mohri M, Raji AR, Jamshidi A, Golian A (2014) Exogenous melatonin improves growth performance, intestinal microbiota, and morphology in temporarily feed restricted broilers. Livest Sci 167:400–407
Alfiansah YR, Peters S, Harder J, Hassenrück C, Gärdes A (2020) Structure and co-occurrence patterns of bacterial communities associated with white faeces disease outbreaks in Pacific white-leg shrimp Penaeus vannamei aquaculture. Sci Rep 10:11980. https://doi.org/10.1038/s41598-020-68891-6
Amenyogbe E (2023) Application of probiotics for sustainable and environment-friendly aquaculture management - a review. Cogent Food & Agriculture 9:2226425. https://doi.org/10.1080/23311932.2023.2226425
Amri A, Kessabi K, Bouraoui Z, Sakli S, Gharred T, Guerbej H, Messaoudi I, Jebali J (2020) Effect of melatonin and folic acid supplementation on the growth performance, antioxidant status, and liver histology of the farmed gilthead sea bream (Sparus aurata L.) under standard rearing conditions. Fish Physiol Biochem 46:2265–2280
AOAC (1995) Official Methods of Analysis of Official Analytical Chemists International, 16th edn. Association of Official Analytical Chemists, Arlington, VA
Ashouri G, Soofiani NM, Hoseinifar SH, Jalali SAH, Morshedi V, Valinassab T, Bagheri D, Doan HV, Mozanzadeh MT, Carnevali O (2020) Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass ( Lates calcarifer ) juveniles. Aquaculture 518:734638–734638
Cai Y, Kang Y (2023) Gut microbiota and metabolites in diabetic retinopathy: Insights into pathogenesis for novel therapeutic strategies. Biomed Pharmacother 164:114994
Cai J, Yang J, Chen X, Zhang H, Zhu Y, Liu Q, Zhang Z (2021) Melatonin ameliorates trimethyltin chloride-induced cardiotoxicity: the role of nuclear xenobiotic metabolism and Keap1-Nrf2/ARE axis-mediated pyroptosis. BioFactors (Oxford, England) 48(2):481–497
Cerenius L, Lee BL, Söderhäll K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Chen J, Chen C, Tan Q (2018) Ontogenic changes in the digestive enzyme activities and the effect of different starvation duration on the digestive enzyme activities of larval red swamp crayfish (Procambarus clarkii). Aquac Res 49(2):676–683
Chen H, Wang Y, Zhang J, Bao J (2021) Intestinal microbiota in white spot syndrome virus infected red swamp crayfish (Procambarus clarkii) at different health statuses. Aquaculture 542:736826. https://doi.org/10.1016/j.aquaculture.2021.736826
Chen Y, Chen H, Gong F, Yang F, Jiang Q, Xu Y, Xia W (2022a) A comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res Int 159:111630
Chen Y, Chi S, Zhang S, Dong X, Yang Q, Liu H, Tan B, Xie S (2022b) Evaluation of Methanotroph (Methylococcus capsulatus, Bath) bacteria meal on body composition, lipid metabolism, protein synthesis and muscle metabolites of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 547:737517. https://doi.org/10.1016/j.aquaculture.2021.737517
Chen Y, Gao H, Zhao J, Ross RP, Stanton C, Zhang H, Chen W, Yang B (2023) Exploiting lactic acid bacteria for inflammatory bowel disease: A recent update. Trends Food Sci Technol 138:126–140. https://doi.org/10.1016/j.tifs.2023.06.007
Christie AE, Fontanilla TM, Roncalli V, Cieslak MC, Lenz PH (2014) Diffusible gas transmitter signaling in the copepod crustacean Calanus finmarchicus : identification of the biosynthetic enzymes of nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H 2 S) using a de novo assembled transcriptome. Gen Comp Endocrinol 202:76–86
Cruz MHC, Leal CLV, Cruz JF, Tan DX, Reiter RJ (2014) Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 82(7):925–932
Dong Y, Chen C, Ren J, Liu Z (2014) Immunomodulatory effects of lactic acid bacteria on gut-associated immune system: research progress. Chinese Journal of Microecology 26(2):5. https://doi.org/10.13381/j.cnki.cjm.201402027
Duan Y, Liu Q, Wang Y, Zhang J, Xiong D (2018) Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol 78:279–288
Fu C, Cui Z, Shi X, Liu J, Jiang Y, Zhang R (2022) Effects of dietary glyceryl monolaurate supplementation on growth performance, non-specific immunity, antioxidant status and intestinal microflora of Chinese mitten crabs. Fish Shellfish Immunol 125:65–73
Gajbhiye DS, Khandeparker L (2018) Involvement of inducible nitric oxide synthase (iNOS) in immune-functioning of Paphia malabarica (Chemnitz, 1782). Fish Shellfish Immunol 84:384–389
Gao W, Liu YJ, Tian LX, Mai KS, Liang GY, Yang HJ, Huai MY, Luo WJ (2010) Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquac Nutr 16:327–333
Glenny E, Liu J, Djukic Z, Pellizzon M, Carroll I (2020) The effect of dietary fiber modifications in purified diets relative to grain-based diets on gastrointestinal anatomy and intestinal microbial communities in mice. Curr Dev Nutr 4:685–685
Guan T, Feng J, Zhu Q, Wang L, Xie P, Wang H, Li J (2023) Effects of abamectin on nonspecific immunity, antioxidation, and apoptosis in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol 142:109137. https://doi.org/10.1016/j.fsi.2023.109137
Guo K, Ruan G, Fan W, Fang L, Wang Q, Luo M, Yi T (2020) The effect of nitrite and sulfide on the antioxidant capacity and microbial composition of the intestines of red swamp crayfish. Procambarus Clarkii Fish Shellfish Immunol 96:290–296
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2010) Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93(3):350–384
He W, Rahimnejad S, Wang L, Song K, Lu K, Zhang C (2017) Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol 70:164–173
Helmut S (2007) Total antioxidant capacity: appraisal of a concept. J Nutr 137:1493–1495
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482:179–185
Herrero MJ, Martínez FJ, Míguez JM, Madrid JA (2007) Response of plasma and gastrointestinal melatonin, plasma cortisol and activity rhythms of European sea bass (Dicentrarchus labrax) to dietary supplementation with tryptophan and melatonin. J Comp Physiol B 177:319–326
Koh A, Vadder FD, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345
Kostanjšek R, Štrus J, Avguštin G (2007) “Candidatus Bacilloplasma”, a novel lineage of mollicutes associated with the hindgut wall of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda). Appl Environ 73:5566–5573
Lage C, Kenneth S (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Lai Q, Yu C, Zhao F, Cheng Y, Zheng Q, Pan C, Lin Z, Yang S, Zhang Q, Chen J, Wang W (2024) Protection of Pacific white shrimp (Penaeus vannamei) against white spot syndrome virus infection by nitric oxide-generating compound S-nitrosoglutathione. Aquaculture 579:740204. https://doi.org/10.1016/j.aquaculture.2023.740204
Lei Z, Xiaoyu W, Libo H, Xinyu J, Chen L, Jie Z, Chao P, Xianliang Z, Li L, Xianghui K (2021) The related immunity responses of red swamp crayfish (Procambarus clarkii) following infection with Aeromonas veronii. Aquac Rep 21:100849
Li Y, Liu Z, Jiang Q, Ye Y, Zhao Y (2022) Effects of nanoplastic on cell apoptosis and ion regulation in the gills of Macrobrachium nipponense. Environ Pollut 300:118989. https://doi.org/10.1016/j.envpol.2022.118989
Li P, Hou L, Hu F, Gao Y, Si Y, Ren H (2023a) Effects of dietary Humulus scandens extract on growth performance, antioxidant responses and intestinal microflora of Yellow River carp (Cyprinus carpio). Aquac Rep 30:101566. https://doi.org/10.1016/j.aqrep.2023.101566
Li W, Wang Z, Cao J, Dong Y, Chen Y (2023b) Melatonin improves the homeostasis of mice gut microbiota rhythm caused by sleep restriction. Microbes Infect 25:105121. https://doi.org/10.1016/j.micinf.2023.105121
Li Y, Yang Y, Li S, Ye Y, Du X, Liu X, Jiang Q, Che X (2023c) Effects of dietary melatonin on antioxidant and immune function of the Pacific white shrimp (Litopenaeus vannamei), as determined by transcriptomic analysis. Comp. Biochem. Physiol. Part D: Genomics Proteomics 48:101146–101146
Li Y, Ye Y, Li S, Feng J, Liu X, Che X, Jiang Q, Chen X (2023d) Transcriptomic analysis of the antioxidant responses and immunomodulatory effects of dietary melatonin in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol 142:109173. https://doi.org/10.1016/j.fsi.2023.109173
Liu F, Qu YK, Geng C, Wang AM, Zhang JH, Chen KJ, Liu B, Tian HY, Yang WP, Yu YB (2020) Effects of hesperidin on the growth performance, antioxidant capacity, immune responses and disease resistance of red swamp crayfish ( Procambarus clarkii ). Fish Shellfish Immunol 99:154–166
Liu H, Qian K, Zhang S, Yu Q, Du Y, Fu S (2022) Lead exposure induces structural damage, digestive stress, immune response and microbiota dysbiosis in the intestine of silver carp (Hypophthalmichthys molitrix). Comp Biochem Physiol Part C: Toxicol Pharmacol 262:109–464
Liu A, To VPTH, Dumas A, Hernandez JM, Santigosa E (2024) Vitamin nutrition in shrimp aquaculture: A review focusing on the last decade. Aquaculture 578:740004. https://doi.org/10.1016/j.aquaculture.2023.740004
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Ma S, Sun Y, Wang F, Mi R, Wen Z, Li X, Meng N, Li Y, Du X, Li S (2017) Effects of tussah immunoreactive substances on growth, immunity, disease resistance against Vibrio splendidus and gut microbiota profile of Apostichopus japonicus. Fish Shellfish Immunol 63:471–479
Maciel FE, Ramos BP, Geihs MA, Vargas MA, Cruz BP, Meyer-Rochow VB, Vakkuri O, Allodi S, Monserrat JM, Nery LEM (2009) Effects of melatonin in connection with the antioxidant defense system in the gills of the estuarine crab Neohelice granulata. Gen Comp Endocrinol 165(2):229–236
Meng X, Cai H, Li H, You F, Jiang A, Hu W, Li K, Zhang X, Zhang Y, Chang X, Yang G, Zhou Z (2023) Clostridium butyricum-fermented Chinese herbal medicine enhances the immunity by modulating the intestinal microflora of largemouth bass (Micropterus salmoides). Aquaculture 562:738768. https://doi.org/10.1016/j.aquaculture.2022.738768
Meyer-Rochow VB (2001) The crustacean eye: dark/light adaptation, polarization sensitivity, flicker fusion frequency, and photoreceptor damage. Zool Sci 18(9):1175–1197
Miao Z, Miao Z, Liu M, Xu S (2022) Melatonin ameliorates imidacloprid-induced intestinal injury by negatively regulating the PGN/P38MAPK pathway in the common carp (Cyprinus carpio). Fish Shellfish Immunol 131:1063–1074
Michalak L, Morales-Lange B, Montero R, Horn SJ, Mydland LT, Øverland M (2023) Impact of biorefinery processing conditions on the bioactive properties of fucoidan extracts from Saccharina latissima on SHK-1 cells. Algal Res 75:103221. https://doi.org/10.1016/j.algal.2023.103221
Ou J, Li Y, Ding Z, Xiu Y, Wu T, Du J, Li W, Zhu H, Ren Q, Gu W, Wang W (2013) Transcriptome-wide identification and characterization of the Procambarus clarkii microRNAs potentially related to immunity against Spiroplasma eriocheiris infection. Fish Shellfish Immunol 35(2):607–617
Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature’s most versatile biological signal? FEBS J 273:2813–2838
Reiter RJ (1991) Melatonin: The chemical expression of darkness. Mol Cell Endocrinol 79(1–3):C153–C158
Reiter RJ, Tan D-X, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol Reprod 81(3):445–456
Reiter RJ, Rosales-Corral SA, Manchester LC, Tan DX (2013) Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci 14(4):7231–7272
Reiter RJ, Tan D-X, Korkmaz A, Rosales-Corral SA (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update 20(2):293–307
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61(3):253–278
Sainath SB, Swetha CH, Reddy PS (2013) What do we (need to) know about the melatonin in crustaceans? J Exp Zool Part A 319(7):365–377
Sébastien B, Valérie C, Syaghalirwa NM, Lambert J, Dubois M, Patrick K (2021) Ex vivo approach supports both direct and indirect actions of melatonin on immunity in pike-perch Sander lucioperca. Fish Shellfish Immunol 112:143–150
Sies H (2007) Total antioxidant capacity: appraisal of a concept12. J Nutr 137:1493–1495
Song M, Pan L, Zhang M, Huang F, Gao S, Tian C (2020a) Changes of water, sediment, and intestinal bacterial communities in Penaeus japonicus cultivation and their impacts on shrimp physiological health. Aquac Int 28:1847–1865
Song Y, Song X, Wu M, Pang Y, Shi A, Shi X, Niu C, Cheng Y, Yang X (2020b) The protective effects of melatonin on survival, immune response, digestive enzymes activities and intestinal microbiota diversity in Chinese mitten crab ( Eriocheir sinensis ) exposed to glyphosate. Comp Biochem Physiol Part C: Toxicol Pharmacol 238:108–845
Sun Y, Han W, Liu J, Liu F, Cheng Y (2020) Microbiota comparison in the intestine of juvenile Chinese mitten crab Eriocheir sinensis fed different diets. Aquaculture 515:734518. https://doi.org/10.1016/j.aquaculture.2019.734518
Tian J, Yang Y, Du X, Xu W, Zhu B, Huang Y, Ye Y, Zhao Y, Li Y (2023a) Effects of dietary soluble β-1,3-glucan on the growth performance, antioxidant status, and immune response of the river prawn (Macrobrachium nipponense). Fish Shellfish Immunol 138:108848. https://doi.org/10.1016/j.fsi.2023.108848
Tian J, Yang Y, Xu W, Du X, Ye Y, Zhu B, Huang Y, Zhao Y, Li Y (2023b) Effects of β-1,3-glucan on growth, immune responses, and intestinal microflora of the river prawn (Macrobrachium nipponense) and its resistance against Vibrio parahaemolyticus. Fish Shellfish Immunol 142:109142. https://doi.org/10.1016/j.fsi.2023.109142
Wang M, Qin Y, Liu Y, Yang H, Wang J, Ru S, Cui P (2023a) Short-term exposure to enrofloxacin causes hepatic metabolism disorder associated with intestinal flora dysbiosis in adult marine medaka (Oryzias melastigma). Mar Pollut Bull 192:114966. https://doi.org/10.1016/j.marpolbul.2023.114966
Wang M, Qin Y, Liu Y, Yang H, Wang J, Ru S, Cui P (2023b) Short-term exposure to enrofloxacin causes hepatic metabolism disorder associated with intestinal flora dysbiosis in adult marine medaka (Oryzias melastigma). Mar Pollut Bull 192:114–966
Wei K, Wei Y, Song C (2020) The response of phenoloxidase to cadmium-disturbed hepatopancreatic immune-related molecules in freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol 99:190–198
Wittig B, Zeitz M (2003) The gut as an organ of immunology. Int J Colorectal Dis 18:181–187
Wu P, Jiang WD, Jiang J, Zhao J, Liu Y, Zhang YA, Zhou XQ, Feng L (2016) Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 58:462–473
Xu W-N, Liu W-B, Yang W-W, Zhang D-D, Jiang G-Z (2014) Identification and differential expression of hepatopancreas microRNAs in red swamp crayfish fed with emodin diet. Fish Shellfish Immunol 39:1–7
Xu Z, Regenstein JM, Xie D, Lu W, Ren X, Yuan J, Mao L (2018) The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol 72:564–571
Y, L.C., S, Z.H., M, Y.S., R, J.Y., S, L.C. (2000) Nitric oxide synthase activity and immunoreactivity in the crayfish Procambarus clarkii. NeuroReport 11:1273–1276
Yang X, Xu M, Huang G, Zhang C, Pang Y, Cheng Y (2019) Effect of dietary L-tryptophan on the survival, immune response and gut microbiota of the Chinese mitten crab. Eriocheir Sinensis Fish Shellfish Immunol 84:1007–1017
Yang H, Cao Q, Zhu Z, Cao Y, Ji T, Wei W, Zhao H, Zhang Y (2023a) Bdellovibrio lyse multiple pathogenic bacteria and protect crucian carp Carassius auratus gibelio from Aeromonas veronii infections. Aquaculture 562:738741. https://doi.org/10.1016/j.aquaculture.2022.738741
Yang Y, Xu W, Du X, Ye Y, Tian J, Li Y, Jiang Q, Zhao Y (2023) Effects of dietary melatonin on growth performance, antioxidant capacity, and nonspecific immunity in crayfish. Cherax destructor Fish Shellfish Immunol 138:108846
Ye Y, Li S, Zhu B, Yang Y, Du X, Li Y, Zhao Y (2024) Effects of dietary melatonin on growth performance, nutrient composition, and lipid metabolism of Pacific white shrimp (Penaeus vannamei). Aquaculture 578:740095. https://doi.org/10.1016/j.aquaculture.2023.740095
Yi S, Li Y, Shi L, Zhang L, Li Q, Chen J (2018) Characterization of population genetic structure of red swamp crayfish, Procambarus clarkii. China Sci Rep 8(1):5586
Yu T, Zhang Y, Hu X, Meng W (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake. China Ecotoxicol Environ Saf 81:55–64
Yu Q, Fu Z, Huang M, Xu C, Wang X, Qin JG, Chen L, Han F, Li E (2021) Growth, physiological, biochemical, and molecular responses of Pacific white shrimp Litopenaeus vannamei fed different levels of dietary selenium. Aquaculture 535:736393. https://doi.org/10.1016/j.aquaculture.2021.736393
Zhan M, Xi C, Gong J, Zhu M, Shui Y, Xu Z, Xu G, Shen H (2022a) 16S rRNA gene sequencing analysis reveals an imbalance in the intestinal flora of Eriocheir sinensis with hepatopancreatic necrosis disease. Comp Biochem Physiol D: Genomics Proteomics 42:100988. https://doi.org/10.1016/j.cbd.2022.100988
Zhan M, Xi C, Gong J, Zhu M, Shui Y, Xu Z, Xu G, Shen H (2022b) 16S rRNA gene sequencing analysis reveals an imbalance in the intestinal flora of Eriocheir sinensis with hepatopancreatic necrosis disease. Comp Biochem Physiol Part D: Genomics Proteomics 42:100988
Zhang H, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57(2):131–146
Zhang Y, Qian C, Huang J, Li J, Jian X, Li Z, Cheng Y, Li J (2023) Suitable natural astaxanthin supplementation with Haematococcus pluvialis improves the physiological function and stress response to air exposure of juvenile red swamp crayfish (Procambarus clarkii). Aquaculture 573:739577
Zhang S, Liu S, Liu H, Li H, Luo J, Zhang A, Ding Y, Ren T, Chen W (2024) Stochastic assembly increases the complexity and stability of shrimp gut microbiota during aquaculture progression. Mar Biotechnol 26:92–102
Zhao H (2012) Extrinsic and Intrinsic Apoptosis Signal Pathway Review, in: Tobias MN (Ed.), Apoptosis and Medicine. IntechOpen, Rijeka, p. Ch. 1
Zhao L, Huang J, Wu S, Li Y, Pan Y (2022) Integrative analysis of miRNA and mRNA expression associated with the immune response in the intestine of rainbow trout (Oncorhynchus mykiss) infected with infectious hematopoietic necrosis virus. Fish Shellfish Immunol. 131:54–66
Zheng J, Zhao Y, Feng Y, Qian W, Zhang Y, Dong B, Liang Q (2023) c-Jun N-terminal kinase activation contributes to improving low temperature tolerance via regulating apoptosis in the Pacific white shrimp Penaeus vannamei. Fish Shellfish Immunol 139:108912. https://doi.org/10.1016/j.fsi.2023.108912
Zhernakova NI, Rybnikova SN (2008) The role of melatonin in pathogenesis of diseases of digestive system. Klin Med (Mosk) 86(4):14–18
Zhou QC, Zeng WP, Wang HL, Xie FJ, Tuo W, Zheng CQ (2012) Dietary arginine requirement of juvenile yellow grouper Epinephelus awoara. Aquaculture 350–353:175–182
Zhu L, Kong Y, Chang X, Feng J, Wang X, Hou L, Zhao X, Pei C, Kong X (2023) Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 562:738765
Funding
The study was supported by the Agriculture Research System of Shanghai, China (grant no. 202205) and the Young Elite Scientists Sponsorship Program by CAST (2022QNRC001).
Author information
Authors and Affiliations
Contributions
Yucong Ye: Conceptualization, Resources, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review & editing. Jiarong Huang: Conceptualization, Formal analysis, Investigation, Visualization, Writing - review & editing. Siwen Li: Conceptualization, Formal analysis, Writing - review & editing. Yiming Li: Conceptualization, Resources, Formal analysis, Visualization, Writing - review & editing, Project administration, Funding acquisition. Yunlong Zhao: Conceptualization, Resources, Formal analysis, Visualization, Writing - review & editing, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, Y., Huang, J., Li, S. et al. Effects of Dietary Melatonin on Antioxidant Capacity, Immune Defense, and Intestinal Microbiota in Red Swamp Crayfish (Procambarus clarkii). Mar Biotechnol 26, 623–638 (2024). https://doi.org/10.1007/s10126-024-10326-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-024-10326-8