Abstract
The effect of feeding rotifers enriched with taurine on the growth performance and survival of larval amberjack Seriola dumerili was investigated. Rotifers were enriched with a commercial taurine supplement at four levels (0, 200, 400, and 800 mg/l). The larvae were fed the enriched rotifers in triplicate from 3 days post-hatch for 7 days under static conditions. The average taurine contents of the rotifers were 1.5, 2.7, 4.2, and 7.2 mg/g dry matter, respectively. The growth of the fish fed rotifers enriched with the taurine supplement at 800 mg/l was significantly (P < 0.05) improved compared with that of the fish fed the rotifers without taurine enrichment. The survival rate improved proportionally up to a taurine supplement level of 400 mg/l, but no significant differences in survival were observed among treatments. The fraction of the larvae with inflated swim bladders did not vary significantly between treatments. Taurine content in the whole fish body increased with the taurine level in the rotifers. These results suggest that taurine enrichment of rotifers is an effective method of enhancing the growth of amberjack larvae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The amberjack Seriola dumerili is a commonly cultured species in southwest Japan, and aquaculture of this species together with that of the yellowtail Seriola quinqueradiata is dominant in that area [1]. However, the aquaculture of this species has been dependent on captured wild juveniles. In addition, ethical problems related to the importation of juveniles from other countries have caused concern [2, 3]. Although a stable supply of amberjack juveniles that are artificially produced in Japan is urgently needed to resolve these problems, the technology for rearing larvae of this species is still too underdeveloped to facilitate this goal [2, 4]. The rotifers Brachionus sp. are used as live food for amberjack larvae, and several attempts have been made to fortify these rotifers with essential fatty acids (EFA) [5, 6]. However, no study has focused on the fortification of rotifers with nutrients other than EFA.
Recent investigations on the nutritional requirements of marine fish have shown that taurine is an essential nutrient at various stages in the life cycles of fish [7–9]. Furthermore, several studies have indicated that fish species differ in their taurine pathways and capacities for taurine biosynthesis [10, 11]. For instance, yellowtail is inherently deficient in cysteine sulfinate decarboxylase, the limiting enzyme for taurine biosynthesis [10]. Thus, juvenile yellowtail fed a low-taurine diet showed inferior growth and survival [9], and broodstock yellowtail fed taurine-deficient diets exhibited poor spawning performance [12].
The taurine content of wild amberjack larvae was found to be higher than that of cultured larvae, and the levels in Artemia nauplii and especially rotifers were markedly lower than those in wild zooplankton samples [13]. In addition, several studies have indicated that taurine enrichment of rotifers and/or diets has positive effects on the growth of larval fish [14–18]. These findings suggest that the taurine content of the rotifers currently used for the larval rearing of amberjack is insufficient for the requirements of this species. However, the effects of taurine enrichment on the growth and survival of amberjack larvae have not yet been clarified.
In larval rearing, the contact of larvae with the tank bottom due to their poor ability to swim upward against gravity as well as their infrequent swimming behavior during the night are reported to be the main causes of larval mass mortality [19]. Mortality due to larval contact with the tank bottom is suggested to be associated with the lack of a functional swim bladder [20], and swim bladder inflation is considered to be critical to larval survival during rearing. Although relationships between the fraction of larvae with inflated swim bladders and either n-3 highly unsaturated fatty acid (n-3 HUFA) or DHA content in rotifers were observed in gilthead sea bream Sparus aurata [21] or amberjack [5], respectively, the effect of taurine on the fraction of larvae with inflated swim bladders has not yet been fully clarified in any fish.
The aim of this study was to investigate the effect of feeding rotifers enriched with various levels of taurine on the growth, survival, and swim bladder inflation of amberjack larvae.
Materials and methods
Rotifer enrichment and larval rearing
A feeding experiment was conducted at Shibushi Station, National Center for Stock Enhancement, Shibushi, Kagoshima, Japan. Brachionus rotundiformis, so-called S-type rotifers, were used for the experiment. The rotifers were stocked at a density of 500 individuals/ml in 200-l tanks (water volume 100 l) filled with 100 % seawater maintained at 25 °C. The rotifers were enriched for 12 h with 1.0 ml/l docosahexaenoic acid (DHA)-enriched Chlorella vulgaris (Super Fresh Chlorella V12, Chlorella Industry Co., Fukuoka, Japan) and then enriched for a further 9 h with 0, 200, 400, and 800 mg/l of a commercial taurine supplement (treatments Tau0, Tau200, Tau400, and Tau800, respectively; Aquaplus ET, Marubeni Nisshin Feed Co., Tokyo, Japan). Fertilized eggs of amberjack used for the experiment were obtained from reared broodstock at the Komame Branch, Stock Enhancement Technology Development Center, Otsuki, Kochi, Japan. In the feeding trial, 10,000 larvae at 1 day post-hatch (DPH) were added to each of twelve (four treatment groups in triplicate) 500-l black polyethylene tanks (water volume 500 l), and the trial was conducted without water exchange. At the start of the trial, samples of fish for chemical analyses were taken from the stock tank. Aeration was provided to each tank at 0.3 l/min through an air-stone. The photoperiod was set at 14 h light (6:00–20:00):10 h dark. Water temperature was not controlled, but the temperature was stable at 25 ± 0.7 °C during the trial. The DHA and taurine-enriched rotifers were added to the tanks from 3 to 10 DPH at around 8:00 and 14:00 to maintain a density of more than 10 individuals/ml. Rotifer density in the larval rearing tanks was checked by sampling 5 ml of the rearing water twice a day (6:00 and 13:00). To avoid starving the rotifers after they had been introduced into the larval rearing tanks, the DHA-enriched Chlorella was supplied to the tanks at a rate of 5 ml/tank twice a day (6:00 and 13:00). A surface skimmer was installed between 3 and 6 DPH to keep the surface free from lipidic films, which is a requisite for swim bladder inflation [22]. The total length was measured for 20 fish from each tank every 2 days during the feeding trial. The fraction of larvae with air-inflated swim bladders (%) was determined using a profile projector (V-12BSC, Nikon Corp., Tokyo, Japan) in the same 20 fish from each tank on 4 and 6 DPH. At the end of the trial (11 DPH), the amberjack larvae in each tank were counted and sampled for chemical analyses. Samples of enriched rotifers for chemical analyses were taken five times during the trial. The sampling of rotifers in the larval rearing tanks (tank rotifers) was achieved by siphoning off part of the rearing water at the end of the feeding trial. The rotifer and fish samples were washed with fresh water and frozen immediately at −80 °C until analyses.
Chemical analysis
The moisture contents of the samples were gauged by drying them for 10 h at 110 °C. Free amino acids (including taurine) in the samples were extracted in 0.6 N perchloric acid [23]. The free amino acid composition was determined by an automatic amino acid analyzer (L-8500, Hitachi, Tokyo, Japan) equipped with a packed column (ion exchange resin F2622SC, 4-mm i.d. × 150 mm, Hitachi). Lipids from rotifer samples were extracted by the chloroform–methanol (2:1, v/v) method [24], including 0.01 % butylhydroxytoluene. The preparation of fatty acid methyl esters and the conditions employed for fatty acid composition analysis using a gas–liquid chromatograph (GC-2010, Shimadzu, Kyoto, Japan) were the same as those described previously [6].
Statistical analysis
The effects of rotifer enrichment on the growth, survival, and swim bladder inflation of amberjack larvae as well as the chemical compositions of the rotifers and larval samples were compared using one-way analysis of variance (ANOVA), and the taurine and mean total free amino acid contents of the enriched and tank rotifers were compared using two-way ANOVA. Percentage data (survival and swim bladder inflation) were arcsine transformed prior to statistical analysis. The differences between treatment means were compared using Tukey’s test. The SPSS 11.0 software package (SPSS, Chicago, IL, USA) was used for all statistical analyses. In all of the statistical tests, differences with P < 0.05 were considered significant.
Results
Lipid content and total fatty acid composition of the rotifers
Although the crude lipid contents of the enriched rotifers, irrespective of the taurine enrichment level, were numerically higher than those of the tank rotifers, the differences were not statistically significant (P > 0.05) due to large variability in the crude lipid contents of the tank rotifers. There was also no significant difference between enriched and tank rotifers, and between rotifers with different taurine enrichments, in the proportions of major fatty acids such as eicosapentaenoic acid (EPA), DHA, and n-3 HUFA.
Taurine and total free amino acid contents of the rotifers
The taurine content of the enriched rotifers increased significantly (P < 0.05) as the taurine supplement was increased from 1.5 to 7.2 mg/g (Table 1). In the enriched rotifers, there was no significant difference in total free amino acid content among the treatments. Compared to the contents in the enriched rotifers, the contents of taurine in the tank rotifers were generally lower, except for the Tau0 treatment, although the differences increased as the level of taurine supplementation increased. In the tank rotifers, there was no significant difference in total free amino acid content among the treatments. Compared to the total free amino acid contents of the enriched rotifers, the contents of the tank rotifers were significantly lower.
Growth, survival, and swim bladder inflation of amberjack larvae
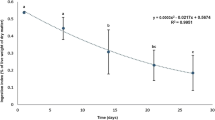
Survival rates were not significantly different among the treatments, but the survival rate in the Tau400 treatment group was the highest, followed by those in the Tau800, Tau200, and Tau0 groups (Fig. 1). The mean total length of the fish in the Tau800 treatment group was significantly higher than that of the fish fed the other rotifers at 10 DPH (Fig. 2). The fraction of larvae with inflated swim bladders did not significantly differ among treatments.
Taurine and total free amino acid contents of amberjack larvae
The taurine contents of the fish increased proportionally with the taurine levels of the rotifers (Table 2). The contents of serine, glutamic acid, and alanine showed opposite trends to the taurine content and were most abundant in fish of the Tau0 treatment group. There was no significant difference in total free amino acid content among the treatments, but the values tended to decrease as the content of taurine increased.
Discussion
In the present study, the mean total length of the fish in treatment Tau800 was significantly longer than those of the fish in the other treatments at the end of the feeding trial. Thus, we can conclude that taurine enrichment of rotifers effectively improved the growth of the amberjack larvae that fed on them, as also seen for other marine fish larvae such as those of red sea bream Pagrus major [14], Japanese flounder Paralichthys olivaceus [15], Pacific cod Gadus macrocephalus [16], and Senegalese sole Solea senegalensis [17]. Although growth is essentially muscle protein deposition [25], taurine is not incorporated into muscle proteins. Accordingly, its effects on amino acid metabolism are expected to be related to indirect regulatory and/or metabolic functions. The taurine content of the larvae is suggested to affect their retention of amino acids. In Senegalese sole, amino acid retention increased when the larval body had a higher taurine content [17]. In the present study, the taurine content was highest in fish fed the rotifers from the Tau800 group. Thus, the improved growth performance of fish fed this rotifer may be attributed to increased amino acid retention. By contrast, the contents of several free amino acids in fish fed the rotifers from the Tau800 group were lower than those of fish fed the rotifers that did not undergo taurine enrichment. At the start of exogenous feeding, marine fish larvae find complex proteins difficult to digest [26], so increasing the levels of free amino acids in their food will mean that more of these amino acids are available to the larvae for both protein synthesis and energy production [27]. However, the amounts of total free amino acid in the rotifers were similar irrespective of the taurine enrichment applied (Table 1). The relationship between low amounts of free amino acid in the tissue amino acid pool of the taurine-enriched fish and muscle protein deposition or energy production needs to be studied.
Tissue free amino acid compositions are affected by the dietary amino acid (protein-band) composition [28], and the contents of some free essential amino acids in fish tissues reflect the requirements of the fish [29]. In addition, shortly after a fish has taken in an excessive amount of taurine, the excess taurine is excreted in its urine [30]. Thus, the taurine level in fish tissue may be a useful indicator for estimating the taurine requirement. In this study, the taurine content of amberjack larvae increased dose-dependently with the taurine content of the rotifers from 3.2 to 7.4 mg/g dry matter, and did not reach a plateau. Although the taurine content of the tank rotifers increased with the level of taurine supplementation from 1.7 to 3.4 mg/g dry matter, the contents of wild zooplankton samples were 8.2 or 11.8 mg/g dry matter [13, 31]. These values are quite high compared to the taurine requirements (2–4 mg/g rotifer) of other fish species [14–16]. Therefore, amberjack larvae may require a higher taurine level in the rotifers that they feed on than the taurine levels in the tank rotifers used in this study.
In the present study, the contents of taurine in the tank rotifers were generally lower than those in enriched rotifers; in particular, tank rotifers that received the Tau800 treatment contained 47 % less taurine than the enriched rotifers that received the Tau800 treatment. Even though the taurine content of the tank rotifers in the Tau800 treatment group was 3.4 mg/g dry matter, the taurine contents of the tank rotifers in the Tau200 and 400 treatment groups were only 2.4 and 2.6 mg/g dry matter, which were not significantly different from the taurine content in the tank rotifers in the Tau0 treatment group. A positive correlation between dietary taurine level and growth rate has been suggested for Pacific cod [16] and turbot Scophthalmus maximus [32] larvae. In the present study, a significant improvement in amberjack larval growth was only observed in fish fed rotifers enriched with the highest level of taurine (Tau800). Thus, the lower growth of the amberjack larvae fed rotifers from the Tau200 and 400 treatment groups can be attributed to the lower taurine contents of the tank rotifers. Further detailed investigations of the taurine level in rotifers that optimizes the growth of amberjack larvae are necessary.
Dietary taurine is suggested to affect survival in certain fish species [9, 18]. In cobia Rachycentron canadum larvae, the survival rate of fish fed rotifers and Artemia nauplii enriched with taurine was higher than the survival rate of fish fed rotifers that had not been enhanced with taurine [18]. In the present study, the survival rates of amberjack larvae fed rotifers enriched with taurine up to the 400 mg/l level of enrichment seemed to improve compared to larvae fed rotifers without such taurine enrichment, although the effect was not significant. Similar results were observed in other marine fish larvae [14–17], which suggests that the effect of taurine on survival depends on the fish species and its life cycle. Moreover, there were also no significant differences in the fraction of larvae with inflated swim bladders among the treatments in this study, in contrast with the effectiveness of DHA or n-3HUFA enrichment of rotifers in this context [5, 21]. The DHA or n-3HUFA contents of both the enriched and the tank rotifers were similar among treatments (Table 3). This suggests that dietary taurine supplementation during the rotifer feeding stages of amberjack larvae had no effect on the fraction of larvae with inflated swim bladders.
In conclusion, the results of this study indicate that taurine enrichment of rotifers is important during the early stages of amberjack larvae as it improves their growth. Since the growth-promoting effect was observed only in fish fed rotifers enriched with the highest level of taurine, the optimum taurine contents in both enriched and tank rotifers that will maximize the growth of amberjack larvae during the period without rearing-water exchange should be clarified.
References
Takaoka O (2005) Greater amberjack. In: Kumai H (ed) Aquaculture system (in Japanese). Marine fish, vol 1. Koseisha Koseikaku, Tokyo, pp 31–44
Mushiake K (2006) Challenges to mass production of amberjack Seriola dumerili juveniles and improvements of rearing techniques for aquaculture (in Japanese with English abstract). Nippon Suisan Gakkaishi 72:1158–1160
Yoshinaga T, Kinami R, Hall KA, Ogawa K (2006) A preliminary study on the infection of anisakid larvae in juvenile greater amberjack Seriola dumerili imported from China to Japan as mariculture seedings. Fish Pathol 41:123–126
Shiozawa S, Takeuchi H, Hirokawa J (2003) Improved seed production techniques for the amberjack, Seriola dumerili (in Japanese with English abstract). Saibai Giken 31:11–18
Matsunari H, Hashimoto H, Oda K, Masuda Y, Imaizumi H, Teruya K, Furuita H, Yamamoto T, Hamada K, Mushiake K (2013) Effects of docosahexaenoic acid on growth, survival and swim bladder inflation of larval amberjack Seriola dumerili. Aquac Res. doi:10.1111/j.1365-2109.2012.03174.x
Matsunari H, Hashimoto H, Oda K, Masuda Y, Imaizumi H, Teruya K, Furuita H, Yamamoto T, Hamada K, Mushiake K (2012) Effect of different algae used for enrichment of rotifers on growth, survival and swim bladder inflation of larval amberjack Seriola dumerili. Aquac Int 20:981–992
Takeuchi T (2001) A review of feed development for early life stages of marine finfish in Japan. Aquaculture 200:203–222
Kim SK, Takeuchi T, Yokoyama M, Murata Y, Kaneniwa M, Sakakura Y (2005) Effect of dietary taurine levels on growth and feeding behavior of juvenile Japanese flounder Paralichthys olivaceus. Aquaculture 250:765–774
Takagi S, Murata S, Goto T, Endo M, Yamashita H, Ukawa M (2008) Taurine is an essential nutrient for yellowtail Seriola quinqueradiata fed non-fish meal diets based on soy protein concentrate. Aquaculture 280:198–205
Yokoyama M, Takeuchi T, Park GS, Nakazoe J (2001) Hepatic cysteinesulphinate decarboxylase activity in fish. Aquac Res 32:216–220
Goto T, Tiba K, Sukurada Y, Takagi S (2001) Determination of hepatic cysteinesulfinate decarboxylase activity in fish by means of OPA-prelabeling and reverse-phase high-performance liquid chromatographic separation. Fish Sci 67:553–555
Matsunari H, Hamada K, Mushiake K, Takeuchi T (2006) Effects of taurine levels in broodstock diet on reproductive performance of yellowtail Seriola quinqueradiata. Fish Sci 72:955–960
Yamamoto T, Teruya K, Hara T, Hokazono H, Hashimoto H, Suzuki N, Iwashita Y, Matsunari H, Furuita H, Mushiake K (2008) Nutritional evaluation of live food organisms and commercial dry feeds used for the seed production of amberjack Seriola dumerili. Fish Sci 74:1096–1108
Chen JC, Takeuchi T, Takahashi T, Tomoda T, Koiso M, Kuwada H (2004) Effect of rotifer enriched with taurine on growth and survival activity of red sea bream Pagrus major larvae (in Japanese with English abstract). Nippon Suisan Gakkaishi 70:542–547
Chen JC, Takeuchi T, Takahashi T, Tomoda T, Koiso M, Kuwada H (2005) Effect of rotifers enriched with taurine on growth in larvae of Japanese flounder Paralichthys olivaceus (in Japanese with English abstract). Nippon Suisan Gakkaishi 71:342–347
Matsunari H, Arai D, Koiso M, Kuwada H, Takahashi T, Takeuchi T (2005) Effect of feeding rotifers enriched with taurine on growth performance and body composition of Pacific cod larvae Gadus macrocephalus. Aquac Sci 53:297–304
Pinto W, Figueira L, Ribeiro L, Yúfera M, Dinis MT, Aragão C (2010) Dietary taurine supplementation enhances metamorphosis and growth potential of Solea senegalensis larvae. Aquaculture 309:159–164
Salze G, McLean E, Craig SR (2012) Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 362:44–49
Tanaka Y, Kumon K, Nishi A, Eba T, Nikaido H, Shiozawa S (2009) Status of the sinking of hatchery-reared larval Pacific bluefin tuna on the bottom of the mass culture tank with different aeration design. Aquac Sci 57:587–593
Takashi T, Kohno H, Sakamoto W, Miyashita S, Murata O, Sawada Y (2006) Diel and ontogenetic body density change in Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquac Res 37:1172–1179
Koven WM, Tandler A, Kissil GW, Sklan D, Friezlander O, Harel M (1990) The effect of dietary n-3 polyunsaturated fatty acids on growth, survival and swim bladder development in Sparus aurata larvae. Aquaculture 91:131–141
Papandroulakis N, Mylonas CC, Maingot E, Divanach P (2005) First results of greater amberjack (Seriola dumerili) larval rearing in mesocosm. Aquaculture 250:155–161
Ogata H, Murai T (1994) White muscle of masu salmon, Oncorhynchus masou masou, smolts possesses a strong buffering capacity due to a high level of anserine. Fish Physiol Biochem 13:285–293
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Carter CG, Houlihan DF (2001) Protein synthesis. In: Wright PA, Anderson AJ (eds) Fish physiology, vol 20. Academic, San Diego, pp 31–75
Rønnestad I, Conceição LEC, Aragão C, Dinis MT (2000) Free amino acids are absorbed faster and assimilated more efficiently than protein in postlarval Senegal sole (Solea senegalensis). J Nutr 130:2809–2812
Fyhn HJ (1989) First feeding of marine fish larvae: are free amino acids the source of energy? Aquaculture 80:111–120
Kaushik SJ, Luquet P (1980) Influence of bacterial protein incorporation and of sulphur amino acid supplementation to such diets on growth of rainbow trout, Salmo gairdneri Richardson. Aquaculture 19:163–175
Cowey CB, Walton MJ (1989) Intermediary metabolism. In: Halver JE (ed) Fish nutrition. Academic, New York, pp 259–329
Yokoyama M, Nakazoe J (1992) Accumulation and excretion of taurine in rainbow trout (Oncorhynchus mykiss), fed diets supplemented with methionine, cystine, and taurine. Comp Biochem Physiol 102A:565–568
Matsunari H, Takeuchi T, Murata Y, Takahashi M, Ishibashi N, Chuda H, Arakawa T (2003) Changes in the taurine content during the early growth stages of artificially produced yellowtail compared with wild fish (in Japanese with English abstract). Nippon Suisan Gakkaishi 69:757–762
Conceição LEC, van der Meeren T, Verreth JAJ, Evjen MS, Houlihan DF, Fyhn HJ (1997) Amino acid metabolism and protein turnover in larval turbot (Scophthalmus maximus) fed natural zooplankton or Artemia. Mar Biol 129:255–256
Acknowledgments
We express our sincere gratitude to Mr. H. Ueno, Shibushi Station, National Center for Stock Enhancement, and Messrs K. Tsuruoka, T. Katayama, Y. Hirata, T. Hayashi, A. Imai, and A. Yoshimura, Tokyo University of Marine Science and Technology, for assistance with the feeding experiment. This study was financially supported in part by a Research Project for Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries (grant no. 18003), Ministry of Agriculture, Forestry and Fisheries, Government of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsunari, H., Hashimoto, H., Iwasaki, T. et al. Effect of feeding rotifers enriched with taurine on the growth and survival of larval amberjack Seriola dumerili . Fish Sci 79, 815–821 (2013). https://doi.org/10.1007/s12562-013-0657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-013-0657-y