Abstract
The effect of algae with different DHA contents used for the enrichment of rotifers on the growth performance, survival, and swim bladder inflation of larval amberjack Seriola dumerili was investigated. Rotifers were enriched with freshwater Chlorella vulgaris containing three levels of DHA (rotifer containing DHA 0.04, 0.60, 1.32 g DHA 100 g−1 DM) and Nannochloropsis (0.04 g DHA 100 g−1 DM; 2.54 g EPA 100 g−1DM). The larvae were fed the enriched rotifers in triplicate from 3 days post-hatch for 7 days in static condition. The same algae used for rotifer enrichment were supplied to the larval tanks. Growth and survival rate of fish fed the rotifers enriched with Nannochloropsis were higher than those of fish fed the rotifers enriched with all three Chlorella treatments. Swim bladder inflation was lowest in fish fed the rotifers enriched with Nannochloropsis. The proportion of EPA was higher in fish fed the rotifers enriched with Nannochloropsis, while that of DHA increased proportionally with the DHA levels in the rotifers enriched with Chlorella. These results suggest that rotifers enriched with Nannochloropsis (EPA-rich rotifers) are effective to enhance growth and survival, but DHA instead of EPA is essential to improve the swim bladder inflation in amberjack larvae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An approach without water exchange during the first appropriate 10 days post-hatch has been proposed to improve the survival of marine fish larvae in mass seed production (Teruya et al. 2008, 2009). In this technique, the amount of nutrient-enriched rotifers supplied to fish larvae should be carefully adjusted and sometimes omitted because some of the previously introduced rotifers have survived and are able to reproduce in the larval-rearing tank (Yamamoto et al. 2009). To avoid the starvation of rotifers after being introduced into the larval-rearing tanks, algae such as Chlorella or Nannochloropsis used for enrichment of rotifers are added to the tanks (Yoshimatsu et al. 1995; Teruya et al. 2009). Although the nutritional value of rotifers in larval-rearing tanks without water exchange could be maintained by appropriate supplementation of algae (Yamamoto et al. 2009), the effect of the kind of algae supplied to the larval-rearing tanks on the growth and survival of larvae has not yet been clarified.
Marine fish require n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as essential fatty acids (EFA) for survival, normal growth, and development (Izquierdo 1996; Takeuchi 1997; Tocher 2010). Rotifers enriched with freshwater Chlorella (without DHA enrichment) are rich in 18:2n-6, and those with Nannochloropsis are particularly rich in EPA (Kobayashi et al. 2008). Therefore, freshwater Chlorella enriched with DHA and/or DHA-rich supplements are used for the enrichment of DHA in rotifers for seed production (Yamamoto et al. 2008). Between EPA and DHA in terms of EFA, EPA effectively improves survival as well as DHA, but does not improve fish vitality for larvae of yellowtail Seriola quinqueradiata (Furuita et al. 1996b) and striped jack Pseudocaranx dentex (Takeuchi et al. 1996). In a recent study, DHA in rotifers has been shown to improve not only growth and survival, but swim bladder inflation of amberjack Seriola dumerili larvae under small-scale flow-through rearing systems (Matsunari unpuble. data). However, there is no information on the effect of EPA on amberjack larvae.
The aim of this study was to investigate the effect of rotifers enriched and fed in the larval-rearing tank without water exchange either with Nannochloropsis or with Chlorella containing three levels of DHA on the growth, survival, and swim bladder inflation of amberjack larvae.
Materials and methods
Rotifer enrichment and larval rearing
The feeding experiment was conducted at Shibushi Station, National Center for Stock Enhancement, Shibushi, Kagoshima, Japan, from June to July, 2009. Brachionus rotundiformis, so-called S-type rotifers cultured with freshwater Chlorella were used for the experiment. Rotifers were stocked in 200 L tanks (water volume 100 L) at a density of 500 individuals mL−1 and enriched for 12 h in 100 % sea water maintained at 28 °C. Rotifers were enriched with four commercial algae: (1) freshwater Chlorella vulgaris (Fresh Chlorella V12, Chlorella Industry Co., Ltd., Tokyo, Japan) (treatment DHA 0), (2) DHA-enriched C. vulgaris (High grade Chlorella V12, HG, Chlorella Industry) (treatment DHA 0.5), (3) another DHA-enriched C. vulgaris (Super fresh Chlorella V12, SV, Chlorella Industry) (treatment DHA 1.0), and (4) Nannochloropsis (Yanmarine K-1, YK1, Chlorella Industry) (treatment Nanno). The Chlorella products were added at a rate of 1.0 mL L−1. The Nannochloropsis were added at a rate of 2.7 mL L−1, the volume of which was the same as 1.0 mL L−1 of the Chlorella products. The enrichment of rotifers is summarized in Table 1.
The fertilized eggs used for the experiment were obtained from reared broodstock at the Komame Branch, Stock Enhancement Technology Development Center, Otsuki, Kochi, Japan, on July 7, 2009. In the experiment, 10,000 larvae at 1 day post hatching (DPH) were stocked in each of twelve (four groups in triplicate) 500 L black polyethylene tanks (water volume 500 L) and reared until 10 DPH without water supply. At the start of the experiment, samples of fish for chemical analysis were taken from the stock tank. Aeration was provided to each tank at 0.3 L min−1 through an air-stone. Photoperiod was set at 14 h light (6:00–20:00): 10 h dark. Newly enriched rotifers were added to the tanks from 3 DPH at around 8:00 and 14:00 to maintain a density of more than 10 individuals mL−1. Rotifer densities in the larval-rearing tanks were checked by sampling 5 mL of rearing water twice a day (6:00 and 13:00). To avoid starvation of rotifers after being introduced into the larval-rearing tanks, the respective Chlorella (3.5 mL tank−1) or Nannochloropsis (9.5 mL tank−1) used for the enrichment was added twice daily as food for rotifers in each larval-rearing tank. The volume of these algae was the same. A surface skimmer was installed between 3 and 6 DPH to keep the surface free from lipidic films, which is a requisite for successful swim bladder inflation (Papandroulakis et al. 2005). The total length was measured for twenty fish from each tank on 4, 6, and 10 DPH. Then, the number of rotifers in the digestive tract of larvae was counted under a stereomicroscope (SMZ1500, Nikon Corp., Tokyo, Japan) for the same twenty fish. The larvae were pressed down under a cover glass on a slide glass in order to examine their gut contents. Samples of enriched rotifers were taken three times during the experiment. The presence of air inflated swim bladder (%) was also determined using a profile projector (V-12BSC, Nikon Corp., Tokyo, Japan) for the same twenty fish from each tank on 4 and 6 DPH. The sampling of rotifers that had survived with supplemental algae in the larval-rearing tanks (tank rotifers) was done by siphoning part of the rearing water from the larval-rearing tank at the end of feeding experiment (9:00 on 11 DPH). At the end of the rearing experiment, the amberjack larvae were counted in each tank. The rotifer and fish samples were washed with freshwater, frozen immediately, and stored at −80 °C until analysis. The water temperature, dissolved oxygen (DO), and pH of the rearing water were checked twice each day during the rearing period; mean values were 27.4–28.9 °C, 7.54–8.09 mg O2 L−1, 8.11–8.45, respectively, and there were no significant differences between treatments.
Chemical analysis
Lipids from rotifers and fish samples were extracted by the chloroform–methanol (2:1, v/v) method (Folch et al. 1957) containing 0.01 % butylhydroxytoluene. Polar and neutral lipids were separated with a silica cartridge (Sep-pak plus; Waters, Milford, MA, USA) as described by Juaneda and Rocquelin (1985). Fatty acid methyl esters (FAME) were prepared by transesterification with boron trifluoride in methanol according to the procedure of Miyashita et al. (1999) and were purified using the Sep-pak cartridge with diethyl ether/n-hexane (5:95). The FAME were analyzed using a gas–liquid chromatograph (GC-2010; Shimadzu, Kyoto, Japan) equipped with a hydrogen flame ionization detector and an Omegawax® 320 fused silica capillary column (30 m × 0.32 mm × 0.2 μm film thickness; Supelco, Bellefonte, PA, USA). The column temperature was initially held at 160 °C for 5 min, followed by an increase at a rate of 4 °C min−1 to a final temperature of 210 °C. The carrier gas was helium, and the pressure was 80 kPa. Individual FAME were quantified with an integrator (C-R7A plus; Shimadzu) and identified by comparison with known standards (GLC-68B, Funakoshi, Tokyo, Japan and known fish oil FAME as the secondary standard) and expressed as area percent of the total identified fatty acids. Fatty acid contents in the rotifers were calculated based on the lipid content and percentage of each fatty acid in the total fatty acids.
Statistical analysis
Data were subjected to one-way ANOVA, and the differences between treatment means were compared using Tukey’s test. When the data of enriched and tank rotifers were compared, Student’s t test was applied. For all statistical analyses, the SPSS 11.0 microcomputer software package (SPSS, Chicago, IL, USA) was used. In all statistical tests, differences at P < 0.05 were considered as significant.
Results
Rotifers density in larval-rearing tank
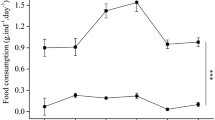
The density of rotifers in the larval-rearing tanks during the experiment is shown in Fig. 1. The density of rotifers in treatment DHA 0, 0.5, and Nanno in the rearing tank fluctuated between 12 and 22 rotifers mL−1. Since the density of rotifers in treatment DHA 1.0 decreased from 17 to 11 rotifers mL−1 around 5 DPH, newly enriched rotifers were added to the tanks to maintain a density of more than 10 rotifers mL−1 at 6, 7, and 8 DPH. To the other treatment groups, newly enriched rotifers were not added.
Lipid contents and fatty acid composition of enriched rotifers
The lipid contents and certain fatty acid compositions of the enriched rotifers are shown in Table 2. There were no significant differences in the lipid contents among the treatments. The proportions of linoleic acid (LA; 18:2n-6) and linolenic acid (LNA; 18:3n-3) were most abundant in the rotifers of treatment DHA 0. The proportion of EPA in the total lipids was highest in the rotifers of treatment Nanno, intermediate in rotifers of treatments DHA 0.5 and 1.0, and lowest in rotifers of treatment DHA 0. The proportions of DHA in the total lipids of rotifers of treatment DHA 0.5 and 1.0 were significantly higher compared to the rotifers of treatment DHA 0 and Nanno. The DHA/EPA of rotifers increased from 1.38 (DHA 0) to 1.91 (DHA 1.0), while the DHA/EPA of rotifers enriched with Nannochloropsis was 0.02.
Lipid contents and fatty acid composition of rotifers in larval-rearing tanks
The lipid contents and certain fatty acid compositions of tank rotifers are shown in Table 3. The total lipid content of tank rotifers in treatment Nanno was significantly higher compared with the tank rotifers enriched with the three Chlorella products. The proportions LA and LNA in the total lipids of rotifers of treatment DHA 0 were most abundant. The proportion of EPA and n-3 LC-PUFA in the total lipids of rotifers was highest in treatment Nanno. The proportion of DHA in the total lipids was highest in the rotifers of treatment DHA 1.0, intermediate in rotifers of treatments DHA 0.5 and 0, and lowest in rotifers of treatment Nanno. Compared to the total lipid contents of the enriched rotifers, the contents of the tank rotifers significantly decreased (P < 0.05) (Fig. 2). The proportions of EPA in the tank rotifers were significantly higher relative to the enriched ones, except for treatment Nanno. The proportions of DHA of rotifers in treatment DHA 0.5, 1.0, and Nanno were not significantly different between the enriched and tank rotifers, while the proportion of tank rotifers of treatment DHA 0 was significantly higher compared to the enriched ones. The DHA/EPA of tank rotifers in the Chlorella-treated groups was approximately 1.0, while the DHA/EPA of tank rotifer fed with Nannochloropsis was 0.01.
Growth, feeding, survival, and swim bladder inflation of amberjack larvae
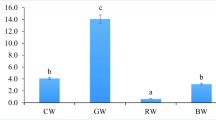
The growth of fish was not significantly different between the treatments (Table 4). There were no significant differences in the number of rotifers found in the digestive tract among the treatment, but the value at 10 DPH of fish in treatment DHA 0 was lower, although not significantly, compared to values of fish fed the other rotifers (Fig. 3). The survival rate of fish fed the rotifers enriched with Nannochloropsis was significantly higher than that of fish fed the rotifers enriched with the Chlorella products. Among the rotifers enriched with the Chlorella products, the survival rates of fish fed the rotifers in treatment DHA 0.5 and 1.0 tended to be improved compared to the rate in treatment DHA 0, although significant differences were not found among them. The swim bladder inflation of fish was not significantly different between the treatments, but the value was numerically highest in treatment DHA 0.5, followed by treatment DHA 1.0, DHA 0, and Nanno.
Lipid contents and fatty acid composition of larvae
The lipid contents and compositions of certain fatty acids of the polar lipids of larvae are shown in Table 4. There were no significant differences in the total lipid contents among the treatments. In fish of treatment Nanno, the proportion of EPA in the polar lipid fraction was significantly higher, and that of DHA was significantly lower than those of fish in the Chlorella-treatment groups. The levels of DHA in the polar lipid fraction increased proportionally with the DHA levels of the tank rotifers (Table 5).
Discussion
In the present study, the rotifer density in the larval-rearing tanks of treatment DHA 1.0 decreased around 5 DPH. The productivity of rotifers varies according to the environmental conditions such as temperature and DO level. Low levels of DO (<0.8 mg L−1) and sudden drop of DO (from 6.1 to 2.3 mg L−1) have been reported to induce impaired productivity of rotifers (Yamasaki et al. 1987; Koiso and Hino 2006). In the present study, DO was more than 6.0 mg L−1 and a sudden drop of DO was not observed. On the other hand, the density of rotifers in the larval-rearing tanks of treatment DHA 0, 0.5, and Nanno were maintained between 12 and 22 ind. mL−1. The low productivity of rotifers observed in treatment DHA 1.0 may be due to the higher levels of DHA in the Chlorella. In the rotifer of treatment DHA 1.0, not only the density but also the activity (swimming frequency of rotifers) decreased. In many cases in Japan, DHA-enriched fresh water Chlorella is supplied to the larval-rearing tank for preventing the rotifers in the tank from starvation (Yoseda et al. 2008; Hamasaki et al. 2009). There are several reports dealing with the nutritional value of rotifers enriched with different algae (Watanabe et al. 1983; Reitan et al. 1993). However, the effect of the kind of algae on the activity and productivity of rotifers has rarely been assessed. Detailed investigations of the different algae used for enrichment of rotifers on activity and productivity of rotifers are necessary.
Feeding density of rotifers is suggested to affect the growth performance in certain fish species. In bluefin tuna Thunnus thynnus larvae, the optimal rotifer density in the tank was estimated to be from 10 to 30 ind. mL−1 for the initial feeding (Sawada et al. 2000). In grouper Epinephelus suillus larvae, rotifer intake and growth of fish fed rotifers at differing densities (5, 10, and 20 ind. mL−1) were not significantly different between the treatments (Duray et al. 1996). In the present study, the density of rotifers in the larval-rearing tanks of all treatment was maintained from 10 to 25 ind. mL−1. In addition, there were no significant differences in the number of rotifers in the digestive tract among the treatment. Thus, the differences in the density of rotifers have no impact on larval performance.
The nutritional value of rotifers in larval-rearing tanks without water exchange can be maintained by appropriate supplementation of algae (Yamamoto et al. 2009). This observation was reconfirmed in the present study; the proportions of EPA and DHA of the tank rotifers reflect the proportions of the algae (Nannochloropsis and DHA-enriched Chlorella) supplemented to the larval-rearing tanks. On the other hand, the proportions of EPA and DHA of rotifers in treatment DHA 0 significantly increased compared to the enriched ones, although the Chlorella contains no EPA and DHA (Kobayashi et al. 2008). A similar trend was also observed in the continuous-culture rotifers; the proportion of DHA of rotifers fed DHA-unenriched Chlorella in the harvest tank increased relative to that in the cultivation tank (Kotani et al. 2009). Rotifers have low synthetic capability of LC-PUFA from precursor molecules. Rotifers fed on baker’s yeast for several generations contained LC-PUFA, although the yeast contained mainly palmitoleic acid and oleic acid and was completely devoid of LC-PUFA (Lubzens et al. 1985). Thus, the increase in EPA and DHA levels of rotifers fed the fatty acids-unenriched Chlorella in larval-rearing tank in the present study is probably attributable to a result of LC-PUFA synthesis and elongation from the precursors by the rotifers.
The highest growth and survival rates were observed in amberjack larvae fed rotifers of treatment Nanno (EPA-rich rotifers), compared to larvae fed rotifers fed with other Chlorella products. An increase in dietary n-3 LC-PUFA level improved the larval growth of gilthead sea bream Sparus aurata (Rodríguez et al. 1994) and Japanese flounder Paralichthys olivaceus (Izquierdo et al. 1992; Furuita et al. 1999). The contents of n-3 LC-PUFA in the tank rotifers treated with Nannochloropsis were 3.12 g 100 g−1 (EPA 2.28 g 100 g−1), while those with the three Chlorella products were from 0.58 to 1.75 g 100 g−1 dry matter basis (DM). The optimum n-3 LC-PUFA level in rotifers for maximum survival rate in amberjack larvae reared in a flow-through system have been estimated to be approximately 2.5 g 100 g−1 DM (EPA 0.5 g 100 g−1, DHA 1.5 g 100 g−1) (Matsunari unpuble. data). Thus, EPA effectively improved growth and survival as well as DHA in amberjack larvae as shown in other marine fish larvae such as red sea bream Pagrus major (Watanabe et al. 1989), yellowtail (Furuita et al. 1996b), and striped jack (Takeuchi et al. 1996). The requirement of n-3 LC-PUFA is determined not only as its absolute amount in the diet (food), but also as the relative proportion of DHA and EPA. The ratio of dietary DHA to EPA has been suggested to affect the normal growth and development in certain fish species (Watanabe and Kiron 1994; Rodríguez et al. 1997). In the present study, there was no correlation between the DHA/EPA and growth or survival rate of larval amberjack. However, there was a large divergence with the proportions of EPA, DHA, and n-3 LC-PUFA of rotifers in each treatment. Therefore, the ratio of dietary DHA/EPA was not used for evaluating the nutritional value of EFA in rotifers enriched with Nannochloropsis.
In seed production, two different phenomena have been indicated as the causes of mass mortality (Miyashita 2006). The first phenomenon is the adhesion of larvae to the water surface. The other is the larval contact with the tank bottom due to their weak upward swimming ability against the gravitational force and infrequent swimming behavior during the night-time (Papandroulakis et al. 2005; Takashi et al. 2006). Mortality due to the latter phenomenon has been associated with the lack of a functional swim bladder (Kitajima et al. 1993). Initial swim bladder inflation is achieved via ingesting air at the water surface during a brief but finite period, when the pneumatic duct connecting the gut and the swim bladder is open (Rieger and Summerfelt 1998). In larval amberjack, swim bladder inflation has been suggested to be achieved in a similar way (Teruya et al. 2009). In the present study, the swim bladder inflation of fish fed rotifers treated with Nannochloropsis, although not statistically different from the others, was inferior to that of other fish fed rotifers treated with Chlorella. The n-3 LC-PUFA level in rotifers for improvement of swim bladder inflation in amberjack larvae reared in a flow-through system have been estimated to be approximately 2.0 g 100 g−1 DM (Matsunari unpubl. data). Although the content of n-3 LC-PUFA in the tank rotifers treated with Nannochloropsis was 3.12 g 100 g−1 DM, the swim bladder inflation was not improved in amberjack larvae fed rotifers of treatment Nanno. On the other hand, the DHA level of tank rotifers in the Chlorella-treated groups was from 0.20 to 0.75, while that of the rotifers enriched with Nannochloropsis was 0.03. The swim bladder inflation of amberjack larvae fed rotifers enriched with DHA was superior to that of fish fed rotifers without DHA enrichment (Matsunari unpubl. data). In terms of DHA, the rotifers of treatment Nanno would not be sufficient to satisfy the requirement for improvement of swim bladder inflation. In larval red sea bream and yellowtail, EPA is inferior to DHA as an EFA from the viewpoint of vitality (Watanabe et al. 1989; Furuita et al. 1996a, b). Thus, the lower swim bladder inflation of amberjack larvae fed rotifers with Nannochloropsis (rotifers contain little DHA) could be attributable to the dietary deficiency of DHA, resulting in having difficulties in remaining beneath the surface or penetrating the water surface due to their poorer vitality.
The results of the present study suggest that the rotifers enriched with Nannochloropsis (EPA-rich rotifers) are effective for enhancing growth and survival, but are not able to improve the swim bladder inflation in amberjack larvae during the post-hatch period under static water conditions.
References
Duray MN, Estudillo CB, Alpasan LG (1996) The effect of background color and rotifer density on rotifer intake, growth and survival of the grouper (Epinephelus suillus) larvae. Aquaculture 146:217–224
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Furuita H, Takeuchi T, Toyota M, Watanabe T (1996a) EPA and DHA requirements in early juvenile red sea bream using HUFA enriched Artemia nauplii. Fish Sci 62:246–251
Furuita H, Takeuchi T, Watanabe T, Fujimoto H, Sekiya S, Imaizumi K (1996b) Requirements of larval yellowtail for eicosapentaenoic acid, docosahexaenoic acid, and n-3 highly unsaturated fatty acid. Fish Sci 62:372–379
Furuita H, Konishi K, Takeuchi T (1999) Effect of different levels of eicosapentaenoic acid docosahexaenoic acid in Artemia nauplii on growth, survival and salinity tolerance of larvae of the Japanese flounder, Paralichthys olivaceus. Aquaculture 170:59–69
Hamasaki K, Tsuruoka K, Teruya K, Hashimoto H, Hamada K, Hotta T, Mushiake K (2009) Feeding habits of hatchery-reared larvae of greater amberjack Seriola dumerili. Aquaculture 288:216–225
Izquierdo MS (1996) Essential fatty acid requirements of cultured marine fish larvae. Aquac Nutr 2:183–191
Izquierdo MS, Arakawa T, Takeuchi T, Haroun R, Watanabe T (1992) Effect of n-3 HUFA levels Artemia on growth of larval Japanese flounder (Paralichthys olivaceus). Aquaculture 105:73–82
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non-phosphorous lipids from rat heart using silica cartridges. Lipids 20:40–41
Kitajima C, YamaneY MatsuiS, Kihara Y, Furuichi M (1993) Ontogenetic change in buoyancy in the early stage of red sea bream. Nippon Suisan Gakkaishi 59:209–216
Kobayashi T, Nagase T, Hino A, Takeuchi T (2008) Effect of combination feeding of Nannochloropsis and freshwater Chlorella on the fatty acid composition of rotifer Brachionus plicatilis in a continuous culture. Fish Sci 74:649–656
Koiso M, Hino A (2006) Effect of sudden drop of dissolved oxygen on population growth and feeding in the rotifer Brachionus plicatilis. Aqua Sci 54:37–41
Kotani T, Genka T, Fushimi H, Hayashi M, Dierckens K, Sorgeloos P (2009) Effect of cultivation methods on nutritional enrichment of euryhaline rotifer Brachionus plicatilis. Fish Sci 75:975–984
Lubzens E, Marko A, Tietz A (1985) De novo synthesis of fatty acids in the rotifer, Brachionus plicatilis. Aquaculture 47:27–37
Miyashita S (2006) Surfacing and bottoming death in seedling production. Nippon Suisan Gakkaishi 72:947–948 (in Japanese with English abstract)
Miyashita K, Inukai N, Ota T, Sasaki S, Ota T (1999) Antioxidant activity of water extracts from fish eggs on PC liposomes. Nippon Suisan Gakkaishi 65:488–494 (in Japanese with English abstract)
Papandroulakis N, Mylonas CC, Maingot E, Divanach P (2005) First results of greater amberjack (Seriola dumerili) larval rearing in mesocosm. Aquaculture 250:155–161
Reitan KI, Rainuzzo JR, Øie G, Olsen Y (1993) Nutritional effects of algal addition in first feeding of turbot (Scophthalmus maximus L.) larvae. Aquaculture 118:257–275
Rieger PW, Summerfelt RC (1998) Microvideography of gas bladder inflation in larval walleye. J Fish Biol 53:93–99
Rodríguez C, Pérez JA, Lorenzo A, Izquierdo MS, Cejas J (1994) n-3 HUFA requirement of larval gilthead seabream Sparus aurata when using high levels of eicosapentaenoic acid. Comp Biochem Physiol 107A:693–698
Rodríguez C, Pérez JA, Díaz M, Izquierdo MS, Fernández-Palacios H, Lorenzo A (1997) Influence of the EPA/DHA ratio in rotifers on gilthead seabream (Sparus aurata) larval development. Aquaculture 150:77–89
Sawada Y, Miyashita S, Aoyama M, Kurata M, Mukai Y, Okada T, Murata O, Kumai H (2000) Rotifer size selectivity and optimal feeding density of bluefin tuna, Thunnus thynnus, larvae. Aquac Sci 48:169–177
Takashi T, Kohno H, Sakamoto W, Miyashita S, Murata O, Sawada Y (2006) Diel and ontogenetic body density change in Pacific bluefin tuna, Thunnus orientalis (Temminck and Schlegel), larvae. Aquac Res 37:1172–1179
Takeuchi T (1997) Essential fatty acid requirements of aquatic animals with emphasis on fish larvae and fingerlings. Rev Fish Sci 5:1–25
Takeuchi T, Masuda R, Ishizaki Y, Watanabe T, Kanematsu M, Imaizumi K, Tsukamoto K (1996) Determination of the requirement of larval striped jack for eicosapentaenoic acid and docosahexaenoic acid using enriched Artemia nauplii. Fish Sci 62:760–765
Teruya K, Yoseda K, Oka M, Nishioka T, Nakano S, Mori K, Sugaya T, Hamasaki K (2008) Effects of photoperiod on survival, growth and feeding of seven band grouper Epinephelus septemfaciatus larvae. Nippon Suisan Gakkaishi 74:645–652 (in Japanese with English abstract)
Teruya K, Hamasaki K, Hashimoto H, Katayama T, Hirata Y, Tsuruoka K, Hayashi T, Mushiake K (2009) Ontogenetic changes of body density and vertical distribution in rearing tanks in greater amberjack Seriola dumerili. Nippon Suisan Gakkaishi 75:54–63 (in Japanese with English abstract)
Tocher DR (2010) Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac Res 41:717–732
Watanabe T, Kiron Y (1994) Prospects in larval fish dietetics. Aquaculture 124:223–251
Watanabe T, Kitajima C, Fujita S (1983) Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34:115–143
Watanabe T, Izquierdo MS, Takeuchi T, Satoh S, Kitajima C (1989) Comparison between eicosapentaenoic and docosahexaenoic acids in term of essential fatty acid efficacy in larval red sea bream. Nippon Suisan Gakkaishi 55:1635–1640
Yamamoto T, Teruya K, Hara T, Hokazono H, Hashimoto H, Suzuki N, Iwashita Y, Matsunari H, Furuita H, Mushiake K (2008) Nutritional evaluation of live food organisms and commercial dry feeds used for the seed production of amberjack Seriola dumerili. Fish Sci 74:1096–1108
Yamamoto T, Teruya K, Hara T, Hokazono H, Kai I, Hashimoto H, Furuita H, Matsunari H, Mushiake K (2009) Nutritional evaluation of rotifers in rearing tanks without water exchange during seed production of amberjack Seriola dumerili. Fish Sci 75:697–705
Yamasaki S, Secor DH, Hirata H (1987) Population growth of two types of rotifer (L and S) Brachionus plicatilis at different dissolved oxygen levels. Nippon Suisan Gakkaishi 53:1303
Yoseda K, Yamamoto K, Asami K, Chimura M, Hashimoto K, Kosaka S (2008) Influence of light intensity on feeding, growth, and early survival of leopard coral grouper (Plectropomus leopardus) larvae under mass-scale rearing conditions. Aquaculture 279:55–62
Yoshimatsu T, Hayashi M, Toda K, Furuichi M, Kitajima C (1995) Preliminary experiment on the requirement of larval redlip mullet for essential fatty acids, and the supplemental effect of Nannochloropsis to rearing water. Nippon Suisan Gakkaishi 61:912–918 (in Japanese with English abstract)
Acknowledgments
We express our sincere gratitude to Mr. H. Ueno, Shibushi Station, National Center for Stock Enhancement, for assistance with the feeding experiment. This study was financially supported in part by Research Project for Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries (Grant No. 18003), Ministry of Agriculture, Forestry and Fisheries, Government of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsunari, H., Hashimoto, H., Oda, K. et al. Effect of different algae used for enrichment of rotifers on growth, survival, and swim bladder inflation of larval amberjack Seriola dumerili . Aquacult Int 20, 981–992 (2012). https://doi.org/10.1007/s10499-012-9522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-012-9522-8