Abstract
Bacteria cause deformation and blemishing in pearls, and we investigate this relationship. We examined pearls derived from Pinctada margaritifera, Pinctada maxima, and Pinctada fucata, and determined the location of bacteria using histopathological and immunohistochemical techniques and 16S ribosomal RNA (rRNA) sequence analyses. The most remarkable change was the inflammatory reaction located between the pearl nucleus and the nacreous layer, composed of hemocytic infiltration with melanization, periostracum, and fibrous aragonite-like structures. These anomalous changes were limited to abnormal sites, and such inflammatory reaction sites are a major factor in the formation of pearl abnormalities. Bacteria were detected from the inflammatory sites and are suspected as the causative agent. Most of these bacteria were anaerobic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pearl industry is a high-value global market based on gemstones produced by pearl oysters. The major oyster species used in oceanic pearl culture are from the family Pteriidae and include the Akoya pearl oyster Pinctada fucata, the South Sea pearl oyster Pinctada maxima, and the black-lip pearl oyster Pinctada margaritifera. Cultured pearls are produced by grafting a round nucleus and a piece of mantle pallial tissue from a donor oyster into the gonad of a recipient pearl oyster. There, the mantle tissue grows and wraps around the nucleus, becoming a pearl sac, and secretes a nacre lining on the nucleus [1, 2]. Pearl quality, which is directly related to value, is measured by a variety of parameters, such as iridescence, shape, presence/absence of blemishes, size, color, and surface complexion [1, 2]. Deformation (shape) and blemishes are major abnormalities which affect the quality and commercial value of pearls. To avoid producing deformed and blemished pearls, Japanese pearl farms employ “shitate,” a treatment based on the combination of two procedures applied before the mantle graft operation. The first procedure, which results in a better grafting operation, is known as “ran-nuki” and induces the release of gametes by “shock” treatments, including changes in temperature, salinity, or depth. The second procedure, known as “yokusei,” stresses the oyster by crowding them in a basket where there is reduced oxygen, food, and current speed for a month. In addition, “yojou,” wherein oysters are suspended in gentle currents, is employed as a treatment after mantle graft. This procedure gently revives the oyster’s health and reduces pearl anomalies [1, 2]. Slight modifications of these procedures are used in black pearl and white South Sea pearl farming. All of these procedures were developed based on past investigations of physiologically induced pearl deformation and blemishes [3–5]. Previous studies on the causes of pearl deformation and blemishes have focused on technical concerns during handling and grafting of the mantle, as well as surgical scalpel maneuvering [6, 7]. Although reducing deformation and blemish rates has been a priority, lack of knowledge regarding the true source of these problems has hampered success [8]. As a result, Akoya pearl farms have reported deformation and blemish rates exceeding 90 % of the total crop [9].
During nucleation, antibiotics have been experimentally administered to enhance pearl quality [2, 10, 11]. Furthermore, it has been reported that pearl blemishes are caused by a melanin-like deposit resulting from reaction towards foreign materials [12]. These observations, together with the foul odor noted in oysters with deformed pearls, may indicate a bacterial origin for pearl anomalies. To date, few studies have reported a cause–effect relationship between bacteria and pearl anomalies [7]. Our intent is to demonstrate the existence of any type of bacteria at the deformation and blemish site, and further examine the specific relationship between bacteria and pearl anomalies.
Materials and methods
Samples
Three different species of oysters from different locations were used in this study. Black pearls (diameter 10–15 mm) derived from black-lip pearl oyster Pinctada margaritifera from Fiji, South Sea pearls (diameter 9–18 mm) from gold-lip pearl oyster Pinctada maxima from Indonesia, and Akoya pearls (diameter 8–9 mm) from Akoya pearl oyster Pinctada fucata from Japan were used. Pearls were divided into three different groups: round regular shape, deformed shape, and blemished (n = 10, each).
Pearl preparation and bacterial DNA collection
Pearl surfaces were washed with DNAoff (Takara Bio, Shiga, Japan) and with milli-Q water. Under aseptic conditions, a portion of pearl axis (2–3 mm thick) was sectioned with a microcutter (MC-201N; Maruto, Tokyo, Japan). During the sectioning process the powder produced was collected and preserved in 100 % ethanol for bacterial DNA collection.
Histological procedures
The sections obtained were decalcified and fixed with 0.5 M ethylenediaminetetraacetic acid (EDTA) with 2 % formaldehyde for 10 days at 37 °C. After formaldehyde removal, they were embedded in paraffin and cut. The sections were then stained with hematoxylin and eosin as per usual procedures. The sections were then processed by potassium permanganate and oxalic acid bleaching, Fontana–Masson staining, and Schmorl stain for confirmation of melanin. Immunohistological staining using antibacterial peptidoglycan antibody MAB995 (Millipore, Tokyo, Japan) followed for detecting bacterial locations.
16S rRNA analysis for bacterial DNA
DNA extraction and PCR
During the sectioning process, powder including bacterial DNAs was collected and preserved in 100 % ethanol. Bacterial DNAs from each pearl were extracted from the powder using a QIAamp DNA Stool Mini Kit (Qiagen, Tokyo, Japan) following the manufacturer’s instructions. For polymerase chain reaction (PCR), the following universal primer sets were employed: 27F, 5′AGAGTTTGATCMTGGCTCAG (M = A/C); 1492R, 5′GGTTACCTTGTTACGACTT [13] for bacterial 16S rRNA, with polymerase KOD FX Neo (Toyobo, Osaka, Japan), following the manufacturer’s instructions. PCR was initiated with denaturation at 95 for 15 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 54 °C for 1 min, and elongation at 72 °C for 2 min, and finalized with extension at 72 °C for 10 min. The PCR products were pooled for each species, P. margaritifera, P. maxima, and P. fucata, for the clone library. After the clone library was constructed, sequences were performed by the conventional method.
Clone library analysis
To analyze bacterial flora, the 16S rDNA sequences (mostly around 600 base pairs from the 5′ end) were assigned to major phylogenetic groups based on a BLAST search against GenBank.
Results
Gross observations
Gross observation showed that pearl abnormalities could be classified into the following four categories: round pearls without blemish (Fig. 1a), round pearls with blemish (Fig. 1b), deformed pearls with or without blemish (Fig. 1c), and pearls having a nonnacreous outer layer partially above the surface (Fig. 1d). In the cross-sections of all the anomalies, there were black/brown inclusions at the affected site (Fig. 1e).
Histological analysis
There were three types of pearl with no blemish. The first type includes a nacreous lining tightly surrounding the nucleus but with nothing between the nacre and the nucleus (Fig. 2a). The second type possesses a thin periostracum formation wholly or partially in contact with the surface of the nucleus, and a thin, fibrous aragonite-like formation on the outer part of the acidophilic periostracum (Fig. 2b). The fibrous aragonite-like formation usually exists between the prismatic layer and nacreous layer of the shell [14]. The third type includes an inflammatory reaction, manifested by a hemocytic infiltration with black to brown pigmentation in contact with nucleus, the periostracum layer, and the fibrous aragonite-like formation, in sequence from the nucleus outward. The nacreous layer ultimately covered this inflammatory reaction (Fig. 2c). Although this reaction surrounds wholly and/or partially in contact with the surface of the nucleus, the blemish was not visible when the outermost nacreous layer was thick. In blemished pearls, the inflammatory reaction is visible due to a thin nacreous layer.
Microscopic observation of normal and abnormal pearls. a Nacreous layer tightly surrounds the nucleus with nothing between the nacre and the nucleus. b Thin periostracum formation wholly or partially in contact with surface of nucleus, and thin fibrous aragonite-like formation on the outer part of the periostracum. c Site of inflammatory reaction, consisting of the hemocytic infiltration with black/brown pigmentation in contact with nucleus, the periostracum layer, and the fibrous aragonite-like formation, in sequence from the nucleus to the outer part. The outermost nacreous layer covers this inflammatory reaction. d Blemished round pearl. This inflammatory reaction site is sufficiently thin that there is no influence on shape. e Deformation containing a thick layer of black/brown substance with hemocyte infiltration and periostracum. f Deformation with vacant cavity in between the inflammatory reaction site. g Deformation with a thick layer of periostracum and/or fibrous aragonite-like structure. h Deformation composed of a multilayered inflammatory reaction site. i Deformation with the inflammatory reaction site on the outside of a previously secreted nacreous layer. j Seed pearls attached to a round pearl covered with periostracum and a fibrous aragonite-like structure, entirely surrounded by the outermost nacreous layer. k The nonnacreous outer layer wholly or partially above the surface is the inflammatory reaction site. l Positive reaction of immunohistological staining with antibacterial peptidoglycan antibody on the inflammation site. Na nacre, Nu nucleus, P periostracum, IS inflammation site, FA fibrous aragonite-like structure, Pr prismatic layer, C cavity, non-NA nonnacreous structure, arrow positive reaction on immunohistochemistry
At the inflammatory reaction site, the black/brown substance in the pigmentation was bleached by potassium permanganate and oxalic acid. Fontana–Masson staining colored the substance to deep black, despite some remaining brown. Most of the pigmentation was positive to Schmorl stain, although some remained brown. Accordingly, the majority of the black/brown substances was determined to be melanin [15], whereas the tests also indicated the presence of a pigment other than melanin on the periostracum. The unaffected region of the nonblemished third pearl type and blemished pearls had histological appearance similar to the first or second type. In round pearls, this inflammatory reaction site was not present or was sufficiently thin to have no influence on shape (Fig. 2d). However, deformed pearls displayed differences within each condition of the inflammatory reaction site. Six types of deformation were found: (1) deformation containing a thick layer of black/brown substance with hemocyte infiltration under the periostracum (Fig. 2e), (2) deformation with a vacant cavity between the inflammatory reaction site (Fig. 2f), (3) deformation with a thick layer of periostracum and/or a fibrous aragonite-like structure (Fig. 2g), (4) deformation composed of a multilayered inflammatory reaction sites (Fig. 2h), and (5) deformation with the inflammatory reaction site on the outside of a previously formed nacreous layer once secreted (Fig. 2i). Here, in some cases a prismatic structure was also found under the fibrous aragonite-like layer. The sixth type of deformation had aberrant seed pearls attached on the precedent pearl and covered with periostracum and the fibrous aragonite-like structure, and in addition the whole body was surrounded by the outermost nacreous lining (Fig. 2j). Furthermore, the unaffected region of the deformed pearl had the same appearance as the first or second type of unblemished pearls. As shown in Fig. 1d, the area of nonnacreous outer layer wholly or partially above the surface was the inflammatory reaction site.
Immunohistological analysis
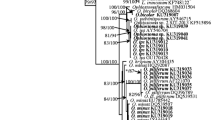
Hematoxylin–eosin stain observation showed very fine cocci and rod-shaped structures frequently seen on the inflammatory site. Most of these structures stained positive on immunohistological staining with antibacterial peptidoglycan antibody (Fig. 2l). These positive reactions varied in strength and size (Fig. 3).
Bacterial flora detected by 16S rRNA analysis
The bacterial flora inside of the pearl was species-specific to each of the oysters, P. margaritifera, P. maxima, and P. fucata. The BLAST analysis found 23 species of 96 clones in P. margaritifera (Table 1), 22 species of 91 clones in P. maxima (Table 2), and 23 species of 93 clones in P. fucata (Table 3); in all instances there was more than 97 % similarity with known published sequence including uncultured bacteria. In the library of pearls from P. margaritifera, the sequences closely related to uncultured bacterium clone 305G6 (16/96 clones), Vibrio mediterranei isolate AP11 (14/96 clones), Serratia sp. S3.MAC.008 (7/96 clones), and Clostridium sp. AN-AS8 (5/96 clones) were frequent. The sequences closely related to uncultured Firmicutes bacterium clone 1HP1-B14 (10/91 clones), Vibrio harveyi strain FA-1 (8/91 clones), Vibrio rotiferianus strain BV1 (7/91 clones), uncultured bacterium clone F-40 (7/91 clones), and uncultured bacterium clone C2_10.1_1 (7/91 clones) were frequent in the library of pearls from P. maxima. The library of pearls from P. fucata frequently presented the sequences closely related to Leuconostoc citreum strain SJL810 (26/93 clones), Lactococcus plantarum strain NBRC 100936 (11/93 clones), Lactococcus lactis strain Cb3-1 (10/93 clones), Citrobacter freundii strain C3-1 (5/93 clones), and Haemophilus influenzae strain M7453 (5/93 clones). The partial sequences of bacterial 16S rRNA obtained using 27F/1492R have been archived in DDBJ under accession numbers AB739705–AB739984.
Discussion
Studies to date indicate that deformation and blemishes result from debris such as eggs, aggregated hemocytes, and exfoliated tissues due to physiological and technical effects [3, 4, 9], or are caused by dysfunction of the pearl sac [16, 17]. Our histological observations, however, show that the most remarkable change was within an inflammatory reaction, suggesting involvement of irritating agents in pearl anomalies. Immunohistological analysis also found bacteria only at the sites of deformation and blemishes. However, bacteria were not consistently found in all abnormal parts of pearls, except in one instance. Consequently, strong correlation between deformation/blemishes and bacteria is supported by our results. However, it is unclear if all the bacteria detected inside the pearl are related to deformation and blemishing, since the clone library method detected bacteria that were both alive and dead. However, in one instance of the sixth type, we suspect that the deformed/blemished pearl was not caused by the bacteria but by the aberration of a seed pearl. Further bacteriological study should be done to clarify the details of bacterial contribution to abnormalities.
Our findings on blemishing reveal that this process is caused by a black/brown substance which is mainly melanin and visible through the nacreous lining when the nacre is thin or less pigmented. The symptoms of brown ring disease of bivalves are known to be due to black pigmentation on the inner surface of the shells caused by bacteria [18, 19]. In mollusks, this black pigmentation is a consequence of an inflammatory reaction against foreign material with subsequent melanization [20, 21]. In blemished pearls, therefore, melanin deposition must be taking place followed by the inflammatory reaction against bacteria. However, pigments other than melanin were also found. Since it is known that the dark-brown matter formed in pearl and shell of the pearl oyster includes flavoxanthin [22], it is possible that these pigments were either secreted by the oyster or result directly from presence of bacteria.
Several types of deformation were observed. The most prominent observations of the histological analyses were the cavity and inflammatory reaction site in the midnacreous layer. The bacterial analysis also confirmed great bacterial species diversity, suggesting that there may be various kinds of irritants produced in varying quantities inside the pearl, e.g., gases, acids, or toxic metabolites. Such irritants probably induce the cavity, inflammatory response, or dysfunction of the pearl sac to varying degrees. As a result, the various combinations of histological and physiological damage are reflected in the differing types and levels of pearl deformation.
The bacteria detected from deformed and blemished pearls were anaerobic, allowing them to survive and proliferate inside the pearl. During the nucleation procedure, there are many opportunities for bacterial contamination, since the surgical room is usually in an open system and the graft is not usually conducted under sterile conditions. The location of inflammation around the nucleus suggests that bacteria were introduced during the surgical procedure. In addition, some bacteria might invade the pearl sac after the oyster is settled in the ocean, since there were unusual cases where the inflammatory reaction site was present on the outside of previously secreted nacreous layer (Fig. 2i). Our results clearly link various bacteria with the pearl anomalies, and reinforce the need for sterilization during the graft process. We also must caution against the habitual use of antibiotics for pearl quality improvement, as this is an abuse of antibiotics [2, 10, 11]. Further research is needed to find the most suitable sterilization mode as well as dosages of appropriate disinfectants.
Reference
Wada K (1999) Science of the pearl. Shinju Shinbunsha, Tokyo (in Japanese)
Taylor J, Strack E (2008) Pearl production. In: Southgate PC, Lucas L (eds) The pearl oyster. Elsevier, Oxford, pp 273–302
Uemoto H (1961) Physiological studies on nuclear insertion operation of pearl oyster I-III. Bull Natl Pearl Res Lab 6:619–635 (in Japanese)
Uemoto H (1962) Physiological studies on the nuclear insertion operation of pearl oyster IV. On control of physiological condition after operation. Bull Natl Pearl Res Lab 8:896–903 (in Japanese)
Atsumi T, Ishikawa T, Inoue N, Ishibasi R, Aoki H, Nishikawa H, Kamiya N, Komaru A (2011) Improvement of the production of high-quality pearls by keeping post-operative pearl oysters Pinctada fucata in low-salinity seawater. Nippon Suisan Gakkaishi 77:68–74 (in Japanese)
Hasuo M (1959) The influence on quality of cultured pearls caused by the extent of friction on the mantle piece of pearl oyster Pinctada martensii (Dunker) on pearl formation. Bull Natl Pearl Res Lab 5:494–498 (in Japanese)
Aoki S (1959) Some experiments on the nuclear insertion in the pearl-culture of oyster (Pinctada fucata martensii) IV. On the quality and shape of the cultured pearl in relation to the site of the pearl-formation. Bull Natl Pearl Res Lab 5:516–540
Norton J, Lucas J, Turner I, Mayer R (2000) Approaches to improve cultured pearl formation in Pinctada margaritifera through use of relaxation, antiseptic application and incision closure during bead insertion. Aquaculture 184:1–17
Aoki H, Hayawaki Y, Yamamoto M, Ito T, Takeuchi A, Deguchi A, Koga F, Nishikawa K, Nomura K, Oyama K, Yamashita M, Iwaki H (2007) Comparison Japanese and hybrid of pearl oyster Pinctada fucata in characteristic of farming and pearl quality. Zenshinren Gijutsukenkyukai Kaiho 21:1–5 (in Japanese)
Miyauchi I (1962) The study on the pearl quality improvement. Suisan Zoshoku 9:207–214 (in Japanese)
Taniguchi T, Zenitani B (1963) On the use of antibiotics for operation of pearl mother-shells and its effect on the quality of pearl produced. Bull Fac Fish Nagasaki Univ 14:30–34 (in Japanese)
Miyashita T, Takagi R (2011) Tyrosinase causes the blue shade of an abnormal pearl. J Molluscan Stud 77:312–314
Galkiewicz JP, Kellogg CA (2008) Cross-kingdom amplification using bacteria-specific primers: complications for studies of coral microbial ecology. Appl Environ Microbiol 74:7828–7831
Dauphin Y, Ball AD, Cotte M, Cuif J, Meibom A, Salomé M, Susini J, Williams CT (2008) Structure and composition of the nacre-prisms transition in the shell of Pinctada margaritifera (Mollusca, Bivalvia). Anal Bioanal Chem 390:1659–1669
Lillie RD, Fullmer HM (1976) Endogenous pigments. In: Histopathologic technic and practical histochemistry. McGraw-Hill Inc., New York, pp 485–527
Aoki S (1966) Comparative histological observations on the pearl-sac tissues forming nacreous, prismatic and periostracal pearls. Nippon Suisan Gakkaishi 32:1–10
Machii A (1959) Studies on the histology of the pearl-sac IV. On the pearl-sac tissue of so-called “kokuhan” (black spotted). Bull Natl Pearl Res Lab 5:407–410
Paillard C, Korsnes K, Chevalier PL, Boulay CL, Harkestad L, Eriksen AG, Willassen E, Bergh Ø, Bovo C, Skår C, Mortensen S (2008) Vibrio tapetis-like strain isolated from introduced Manila clams Ruditapes philippinarum showing symptoms of brown ring disease in Norway. Dis Aquat Organ 81:153–161
Paillard C (2004) A short-review of brown ring disease, a vibriosis affecting clams, Ruditapes philippinarum and Ruditapes decussatus. Aquat Living Resour 17:467–475
Renwrantz L, Schmalmack W, Redel R (1996) Conversion of phenoloxidase and peroxidase indicators in individual haemocytes of Mytilus edulis specimens and isolation of phenoloxidase from haemocyte extract. J Comp Physiol B 165:647–658
Luna-González A, Maeda-Martίnez AN, Vargas-Albores F, Ascencio-Valle F, Robles-Mungaray M (2003) Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bivalve molluscs. Fish Shellfish Immunol 15:275–282
Yano I (1975) An acetone soluble pigment in dark brown matter formed in both the nacres of the shell and the pearl of the pearl oyster, Pinctada fucata (Gould). Bull Natl Pearl Res Lab 19:2149–2151 (in Japanese)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogimura, T., Futami, K., Katagiri, T. et al. Deformation and blemishing of pearls caused by bacteria. Fish Sci 78, 1255–1262 (2012). https://doi.org/10.1007/s12562-012-0545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-012-0545-x