Abstract
Antibacterial properties of crude extract from rhubarb and its major bioactive compounds against Aeromonas hydrophila were assayed. Major bioactive compounds (anthraquinone derivatives) in rhubarb collected from different cultivation areas were determined by ultra-performance liquid chromatography (UPLC); the antibacterial activity [minimum inhibitory concentration (MIC)] of rhubarb was positively related to the anthraquinone content (r = 0.9306, P < 0.01). The MIC values of five anthraquinones against A. hydrophila were found to be in the range 50–200 μg/ml. Action-mode studies showed that anthraquinones (emodin) inhibits cellular functions by binding to cell DNA after penetrating the cell membrane, resulting in cell death. The present study suggests that anthraquinones extracted from rhubarb have potential use as antimicrobials for control of A. hydrophila.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aeromonas hydrophila is a Gram-negative rod-shaped bacterium belonging to the Aeromonidae, a family that is widely distributed in fresh water, sewage-contaminated water, sludge, soil, and foods. A. hydrophila is an important bacterial fish pathogen and is associated with several diseases of fish, such as hemorrhagic septicemia, fin and tail rot, and epizootic ulcerative syndrome [1, 2]. These diseases have caused high mortality in freshwater fish, resulting in extensive losses around the world. Antibiotics and chemotherapeutics used to control these diseases can result in the development of drug-resistant bacteria, environmental pollution, and residues in fish. With the increasing demand for organic aquaculture, there has been growing interest in using natural products in aquaculture to prevent diseases for their lesser side-effects than antibiotics [3–6].
Rhubarb is an important Chinese herbal medicine (called Dahuang) and has been widely used as a plant medicine for treatment of blood stagnation, constipation, and mental and renal disorders, as well as a purgative agent, in China for a long time. In rhubarb, anthraquinone derivatives [emodin, chrysophanol, rhein, aloe-emodin, and physcion (Fig. 1) and their glucosides] are thought to be the major active components, having many different biological and pharmacological properties such as antioxidant [7], antibacterial [8], antiviral [9], antifungal [10], antiatherosclerotic [11], and anticancer activities [12]. Due to their biological effects, increasing attention is being paid to these compounds. Recent literature has shown that rhubarb can promote nonspecific immune system functions in fish and prawns to prevent pathogenic infection, mitigate the negative effects of crowding stress, and promote growth [6, 13]. None of these previous studies, however, screened antibacterial activity of rhubarb and its major components against A. hydrophila in vitro. Meanwhile, there have been few reports and discussion on the mechanisms of action of antimicrobial components.
Therefore, the aims of the present work are: (1) to investigate the antibacterial activities of crude extract from rhubarb and its major components against A. hydrophila, (2) to detect the contents of five anthraquinones in rhubarb collected from different cultivation areas, and (3) to investigate the mechanism of action of anthraquinones against A. hydrophila.
Materials and methods
Microorganisms and chemicals/reagents
Aeromonas hydrophila TPS-30, BSK-10 were obtained from Zhejiang Institute of Freshwater Fisheries, and A. hydrophila IB101, JG101, 4LNS301, CCH201, LNB101, CG101 were obtained from the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences. Eight rhubarb samples were purchased in Chinese markets near production areas of Rheum species. Emodin, chrysophanol, rhein, aloe-emodin, and physcion (with purity >99%) were obtained from Kemiou Chemical Reagent Company (Shanghai, China). A Spin Column Genomic DNA isolation kit was purchased from Bio Basic Inc., Canada. A Cell Apoptosis PI detection kit was purchased from Nanjing KeyGen Biotech. Co. Ltd., China. Ethidium bromide (EB) was obtained from Amersco Inc. (Solon, OH, USA). UPLC-grade methanol was purchased from Sigma–Aldrich (St. Louis, MO, USA). All other reagents (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were of analytical grade.
Extraction of anthraquinones
Dried rhubarb was ground into fine powder and passed through a sieve (60 mesh). Rhubarb powder (10.0 g) was mixed with 100 ml 80% ethanol, and extraction was carried out at 80°C for 2 h and repeated three times. The extracts were combined, filtered, and then concentrated using a rotary evaporator at 40°C under vacuum and lyophilized using a freeze-dryer (LGJ-10D; Four-Ring Science Instrument Beijing Co., Ltd., China). The freeze-dried sample of crude extract was stored at 4°C until use.
UPLC analysis
Ultra-performance liquid chromatography (UPLC) analyses were carried out using an UPLC apparatus equipped with a Waters Acquity PDA detector (Waters, USA) and an Acquity UPLCTM BEH C18 column (100 mm × 2.1 mm, particle size 1.7 μm; Waters, USA). The column oven temperature was fixed at 45°C. The eluents were: A, water 0.1% formic acid; B, acetonitrile/methanol (20:80, v/v). The gradient program was as follows: 10–30% B (15 min), 30–100% B (18 min) at constant flow of 0.3 ml/min. The peaks of the anthraquinone compounds were monitored at 280 nm. UV–Vis absorption spectra were recorded online from 200 to 600 nm during UPLC analysis.
Extraction of A. hydrophila genomic DNA
Genomic DNA from A. hydrophila TPS-30 was extracted using the Spin Column Genomic DNA isolation kit. The purity of the extracted DNA was checked by the absorbance ratio A 260/A 280 (OD260/OD280 = 1.83). DNA concentration was determined from the absorbance at 260 nm (A 260 = 1.0 OD for 50 μg/ml) using a UV-2100 spectrophotometer (UNIC) [14].
Antibacterial activity (MIC)
The antimicrobial activities of the rhubarb extracts and its major components (anthraquinone derivatives) were determined by using a twofold microdilution broth method [15]. A. hydrophila were grown to mid-log phase in LB broth for 16 h at 37°C. Twofold serial dilutions of 80 μl of test samples were transferred to test-tubes to final concentrations of 6400, 3200, 1600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, and 0 μg/ml, which were previously filled with 1900 μl LB medium. Bacterial suspension (20 μl) was then added to each test-tube to final concentration of 106 colony-forming units (CFU)/ml. Test-tubes were incubated at 37°C for 24 h. After incubation, microbial growth was determined by estimating the increased turbidity of each well, measured at 630 nm using a UV-2100 spectrophotometer microplate reader (UNIC). The MIC was calculated from the highest dilution showing complete inhibition of the tested strain. All analysis was carried out in triplicate, and the median value of each triplicate was used for data analysis.
Bacterial membrane permeability
Aeromonas hydrophila TPS-30 was grown to mid-log phase in LB for 16 h at 37°C, and cells were collected, washed, and resuspended in 1 ml deionized water (absorbance at 630 nm was adjusted to 0.2). The emodin sample solution (10 μl) of 2 MIC concentrations was added in test-tubes, and incubated at 37°C for various times. Then, the cell suspensions were centrifuged at 10000 rpm for 10 min, and the supernatants were diluted at 100-fold [16]. The amount of released K+ was measured by atomic absorption spectrometer (Spectr AA 220; VARIAN, USA).
Transmission electron microscopy
Exponential-phase bacteria were treated with 2 MIC of emodin for 4 h at 37°C. Cells were harvested by centrifugation and washed twice with deionized water. After treatment, the bacterial pellets were fixed with 2.5% buffered glutaraldehyde for 1 h. The cells were then post-fixed in 1% buffered osmium tetroxide for 1 h, stained en bloc with 1% uranyl acetate, dehydrated in graded ethanol concentrations, and subsequently embedded in spur resin. The buffer used was 0.1 M sodium cacodylate (pH 7.4). Thin sections were prepared on Formvar copper grids and stained with 2% uranyl acetate and lead citrate [17]. Penicillin was used as positive controls, and double-distilled water as negative controls. Microscopy was performed with a transmission electron microscopy (H-7000; Hitachi, Japan) under standard operating conditions.
Flow cytometric analysis
Membrane integrity after emodin treatment was determined by flow cytometric analysis using propidium iodide (PI) as a probe [18]. A. hydrophila TPS-30 was grown to log phase in LB and mixed with emodin at concentration of 2 MIC for 4 h at 37°C. Cells were washed three times with phosphate-buffered saline (PBS), and resuspended at concentration of 106 CFU/ml in the same buffer. The emodin-treated cells were incubated in PI solution (50 μg/ml final concentration) for 30 min at 37°C, followed by removal of unbound dye through excessive washing with PBS. PI was excited at 488 nm using an argon laser, and the resulting fluorescence emission was collected through a 660-nm long-pass filter. Penicillin was used as positive controls, and double-distilled water as negative controls. Flow cytometry analysis was conducted using a FACScan instrument (Calibur, BO, USA).
Fluorescence measurement
The fluorescence spectrum of emodin in the absence and presence of A. hydrophila genomic DNA was measured using a F-7000 fluorophotometer (Hitachi, Japan) at room temperature with excitation at 290 nm (kex = 290 nm). The change of fluorescence spectra from 360 to 560 nm was measured as increasing concentrations (0, 5.0, 10.0 and 20 μg/ml) of DNA were added to emodin with a fixed concentration (of 200 μg/ml) at room temperature [19]. Tris–HCl buffer (10 mM, pH 7.2) was used as blank solution for all samples.
Competitive binding of emodin and EB with bacterial DNA
Fluorescence measurements of competitive binding assays were carried out using a F-7000 fluorophotometer (Hitachi, Japan). DNA was dissolved in 2 ml Tris–HCl buffer (10 mM, pH 7.2) to final concentration of 10 μg/ml; 10 μl EB (2 μM) solution was added to the DNA solution, and the EB–DNA solution was placed in a thermostated water bath at 37°C for 10 min. Varying concentrations of emodin (0, 50, 100, and 200 μg/ml) were then added to the EB–DNA solution, and the fluorescence spectra were measured for each test solution after 30 min of incubation at 37°C. The solutions were excited at 535 nm, and spectra were recorded from 550 to 720 nm [20, 21]. Tests were performed in a 1-cm-path-length quartz cell.
KI fluorescence quenching
The experiment was performed according to methods described by Guo et al. [19] and Song et al. [22], with some modifications. The concentration of emodin was adjusted to 200 μg/ml; potassium iodide (KI) was dissolved in Tris–HCl buffer (10 mM, pH 7.2) to final concentrations of 0, 1, 2, 4, 6, 8, and 10 mmol. Varying concentrations of KI were then added to emodin-containing solutions in the absence or presence of DNA (10 μg/mL). Emission spectra were scanned from 360 to 560 nm with fixed excitation wavelength of 290 nm. Data were plotted according to the Stern–Volmer equation [23]
where F 0 and F are the fluorescence intensities in the absence and the presence of the DNA, respectively. K SV is the Stern–Volmer quenching constant, and [Q] is the concentration of quencher.
Results
Determination of anthraquinones in rhubarb
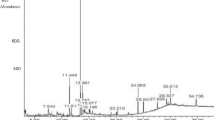
Ultra-performance liquid chromatography analysis results of the crude extract of rhubarb are shown in Table 1 and Fig. 2. The total contents of five anthraquinones obtained from rhubarb from different cultivation areas ranged from 5.87 ± 0.30 to 24.86 ± 0.81 mg/g. The antibacterial activity (MIC) of rhubarb was positively related to the anthraquinone content (r = 0.9306, P < 0.01).
Antibacterial activity of anthraquinones
The antibacterial activities (MIC) of five anthraquinones are shown in Table 2. The MIC values of the five anthraquinones against A. hydrophila ranged from 50 to 200 μg/ml, the general order of their antibacterial activity being: emodin = rhein = aloe-emodin > physcion = chrysophanol. Considering the antibacterial activity and content of emodin, emodin was chosen as a candidate anthraquinone derivatives for study of the antibacterial mechanism.
Bacterial membrane permeability
The effect of emodin on the membrane permeability of A. hydrophila was investigated by measuring the amount of potassium ions released from emodin-treated cells. When the bacterial membrane is damaged, to a certain extent, small ions such as potassium and phosphate tend to leach out, and cytoplasmic constituents released from the cell were monitored. Figure 3 shows that a significant potassium efflux from bacteria cells was induced after incubation, and the K+ efflux increased with increasing incubation time from 0.5 to 4 h; when time was increased further, only slight changes was observed. The increase in the amount of K+ released from A. hydrophila after treatment provides evidence that emodin probably acted on the plasma membrane by increasing permeabilization, causing ion leakage from the cell.
Transmission electron microscopy
Transmission electron microscopy was used to observe the morphological changes of bacterial cells treated with emodin. The electron micrographs are displayed in Fig. 4. Control cells of nontreated bacteria remained intact and showed a smooth surface (Fig. 4a). However, after 4 h of treatment, the cells showed important morphological changes such as breakage of cell wall and membrane, and leakage of cellular cytoplasmic contents was also observed (Fig. 4b, c), which was similar to in previous studies [25].
Flow cytometric analysis
To investigate whether the antibacterial effect of emodin was induced by damage to the plasma membrane, the cells were incubated with emodin and PI. PI is a fluorochrome that intercalates into nucleic acid as a viability marker, which is supposed to penetrate cells and stain them only when membrane integrity is lost [26]. Detection of internal PI in single cells was analyzed via flow cytometry. As shown in Fig. 5, in the absence of emodin, 97.15% of untreated control cells showed no PI fluorescence signal (Fig. 5a), indicating viable cells excluding the PI dye. However, when treated with emodin and penicillin, 71.65% and 81.5% of A. hydrophila cells were labeled fluorescently after 4 h of incubation, respectively (Fig. 5b, c), thereby indicating that emodin induced PI influx into the cells.
Fluorescence spectra study
The spectrophotometric titrations of emodin with A. hydrophila genomic DNA, in the concentration range of 0–20 μg/ml, provided information about the emodin–DNA interaction mode. As shown in Fig. 6, emodin has strong intrinsic fluorescence; in the absence of DNA, the wavelength maximum of emodin was about 425 nm when excited at 290 nm. When increasing concentrations (5.0, 10, and 20 μg/ml) of DNA were added to the emodin solution, the fluorescence intensity of emodin gradually decreased. This could be due to intercalation of emodin into the base pairs of the DNA helix, resulting in electric charge transfer and change of excited electronic states, which would lead to lower fluorescence [19]. An emission decrease is widely recognized as an indication of interactions between drugs and DNA [27].
Competitive binding of emodin and EB with bacterial DNA
To confirm the mode of interaction of DNA with emodin, a competitive binding experiment was carried out using EB as a probe. EB is one of the most sensitive fluorescent probes that can bind with DNA. Fluorescence of free EB is low, but intense fluorescence is emitted after binding with DNA, due to intercalation between adjacent base pairs within the double helical structure of DNA. This enhanced fluorescence can be quenched when it coexists with a reagent molecule that undergoes a similar reaction. This can be used to monitor the binding mode, thereby indicating the ability of a compound to prevent intercalation of EB into DNA [28]. Accordingly, the experiment was carried out by titrating the EB–DNA system with emodin. When the concentration of emodin was increased, a remarkable fluorescence decrease of the EB–DNA system was observed at the maximum of 590 nm (Fig. 7). This phenomenon indicated that EB was partially replaced by emodin in the EB–DNA system, and EB was released from a hydrophobic environment into the water solution. The result suggested that emodin binds to DNA in the intercalating mode [21, 29].
KI fluorescence quenching
The I− ion is a dynamic quenching agent, and the mode of action between small molecules and DNA can be determined by evaluating the effect of the I− ion on the quenching of fluorescence of small molecules. When a small molecule inserts into the bases of DNA, these bases (in the DNA double helix structure) together with the negatively charged phosphor-diester skeleton, inhibit the action of the anion quencher located close to small molecules, resulting in a weakening of the quenching effect of the I− ion [30].
Figure 8 shows Stern–Volmer plots of the KI quenching effect in the absence and presence of DNA. Quenched fluorescence yielded Y = 1.0151 + 0.069X, r = 0.998 and Y = 1.0015 + 0.045X, r = 0.998, respectively. The quenching constant of I− to emodin was 0.069 L/mmol, but the quenching constant of the KI–emodin system in the presence of DNA was 0.045 L/mmol. From Fig. 8, it can be seen that, in the absence DNA, increasing the KI concentration caused efficient quenching of the fluorescence of emodin in a concentration-dependent manner. In the presence of DNA, however, KI showed less effective quenching of emodin fluorescence than that observed in the absence of DNA. This phenomenon suggests that emodin binds to DNA, possibly in the intercalating mode. The intercalation leads to a decrease in the collision frequency of quenching molecules, so DNA plays a protective role [22].
Discussion
Analysis of the antibacterial activity of rhubarb showed that the crude extract exhibited excellent antibacterial activity against A. hydrophila and the antibacterial activity (MIC) of rhubarb was positively related to the anthraquinone content (r = 0.9306, P < 0.01), which indicated that anthraquinones was a major antibacterial component in rhubarb against the growth of A. hydrophila. However, based on their average MICs against A. hydrophila (50–200 μg/ml) (Table 2), five anthraquinones showed different antibacterial activities. Comparisons of the activities of the five anthraquinones revealed that the effects of emodin, rhein, and aloe-emodin against all bacterial strains were higher than those of physcion and chrysophanol. This was consistent with the results of previous reports [8, 24], which suggested that the antibacterial activity of these anthraquinone derivatives might be related to the type of substituent groups on the molecular structure. All of these anthraquinone derivatives have the same hydroxyanthraquinone nucleus composed of two ketone groups at C9 and C10 and two hydroxyl groups at C1 and C8, while different groups are substituted at C3 and C6 of the phenyl ring (Fig. 1). Three anthraquinones (rhein, emodin, and aloe-emodin) have polar substituent carboxyl, hydroxyl, and hydroxymethyl groups at C3, C6, and C3, respectively. It was reported that the presence of polar functional group (carboxyl, hydroxyl, and hydroxymethyl) can increase antibacterial activity [8, 24]. Although physcion and chrysophanol also have hydroxyl groups at C1 and C8 (Fig. 1), the apolar methyl and weakly polar methoxyl in chrysophanol and physcion might weaken their antibacterial activity.
Emodin, one of the important bioactive compounds in rhubarb, has shown a wide variety of pharmacological activities, such as anti-inflammatory [31], antioxidant [7], antimicrobial [8], and antitumor activities [32]. Among their wide biological activity, only in a few cases has their molecular mechanism been elucidated. In particular, the antibacterial activity and mechanisms of action of emodin against A. hydrophila have been little reported. To learn about the possible mechanism of antibacterial activity against A. hydrophila, here we investigated the morphology of treated cells and the molecular mechanism of emodin–DNA interactions. Several possible mechanisms of action were proposed.
Damage to the bacterial cell wall and cytoplasmic membrane might indicate loss of structural integrity and of the membrane’s ability to act as a permeable barrier. In our experiments, FACScan analysis showed that emodin increased the plasma membrane permeability for influx of ONPG into cells (Fig. 5), and caused large leakage of potassium ions from treated cells (Fig. 3). Moreover, morphological changes and leakage of cytoplasmic contents were also demonstrated by electron micrographs of A. hydrophila cells treated with emodin (Fig. 4). All results elucidated that emodin increased membrane permeabilization and caused leakage of intracellular contents. Cell death might be the result of cell contents leakage or the initiation of autolytic processes [33].
The assays reported herein and previous reports [25, 34] indicated that emodin can bind and insert into the cell membrane, leading to loss of cytoplasmic membrane integrity. What then does emodin do inside the cell? Can it act on intracellular targets in bacteria? Previous studies have demonstrated that anthraquinone derivatives of Chinese rhubarb could inhibit macromolecular synthesis in cells [35, 36], so it was also hypothesized to target intracellular processes in bacteria beyond membrane permeabilization. Therefore, we investigated the molecular mechanism of the bactericidal activity of emodin on A. hydrophila genomic DNA. Interestingly, fluorescence spectroscopic studies showed that emodin could bind with the phosphate group of DNA and intercalate into the base pairs of the DNA helix, suggesting that DNA may be a target for the antibacterial activity of this anthraquinone, which might affect replication and transcription, repress expression, and even lead to cell death [36].
In addition, the antimicrobial activity of emodin might involve other modes of action. Previous studies have demonstrated that anthraquinone derivatives could inhibit the activities of nicotinamide adenine dinucleotide (NADH) oxidase and succinate oxidase of mitochondria [37]. Therefore, we propose that emodin might inhibit electron transfer of the respiratory chain, substrate oxidation, and dehydrogenation processes in the bacteria. As a result, such inhibition could lead to uncoupling of oxidative phosphorylation, restraining of active transport, and loss of pool metabolites [33, 36].
References
Larsen JL, Jensen NJ (1977) An Aeromonas species implicated in ulcer-disease of the cod (Gadus morhua). Nordisk Vet Med 29:199–211
Lu CP (1992) Pathogenic Aeromonas hydrophila and the fish diseases caused by it. J Fish China 16:282–288 (in Chinese)
Ardó L, Yin G, Xu P, Váradi L, Szigeti G, Jeney Z, Jeney G (2008) Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275:26–33
Harkrishnan R, Balasundaram C (2008) In vitro and in vivo studies of the use of some medicinal herbals against the pathogen Aeromonas hydrophila in goldfish. J Aquat Anim Health 20:165–176
Nya EJ, Austin B (2009) Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32:963–970
Xie J, Liu B, Zhou QL, Su YT, He YJ, Pan LK, Ge XP, Xu P (2008) Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var Jian. Aquaculture 281:5–11
Iizuka A, Iijima OT, Kondo K, Itakura H, Yoshie F, Miyamoto H, Kubo M, Higuchi M, Takeda H, Matsumiya T (2004) Evaluation of rhubarb using antioxidative activity as an index of pharmacological usefulness. J Ethnopharmacol 91:89–94
Wang J, Zhao H, Kong W, Jin C, Zhao Y, Qu Y, Xiao X (2010) Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 17:684–689
Hsiang CY, Hsieh CL, Wu SL, Lai IL, Ho TY (2001) Inhibitory effect of anti-pyretic and anti-inflammatory herbs on herpes simplex virus replication. Am J Chin Med 29:459–467
Agarwal SK, Singh SS, Verma S, Kumar S (2000) Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol 72:43–46
Liu YF, Yan FF, Liu Y, Zhang C, Yu HM, Zhang Y, Zhao YX (2008) Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. Phytother Res 22:935–942
Huang Q, Lu GD, Shen HM, Chung MCM, Ong CN (2007) Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev 27:609–630
Liu B, Xie J, Ge XP, Xu P, Wang AM, He YJ, Zhou QL, Pan LK, Chen RL (2010) Effects of anthraquinone extract from Rheum officinale Bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol 29:49–57
Rupesh KR, Deepalatha S, Krishnaveni M, Venkatesan R, Jayachandran S (2006) Synthesis, characterization and in vitro biological activity studies of Cu-M (M = Cu2+, Co2+, Ni2+, Mn2+, Zn2+) bimetallic complexes. Eur J Med Chem 41:1494–1503
Naghmouchi K, Drider D, Khead E, Lacroix C, Prevost H, Fliss I (2006) Multiple characterizations of Listeria monocytogenes sensitive and insensitive variants to divergicin M35, a new pediocin-like bacteriocin. J Appl Microbiol 100:29–39
Hao G, Shi YH, Tang YL, Le GW (2009) The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptide 30:1421–1427
Friedrich CL, Rozek A, Patrzykat A, Hancock REW (2001) Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J Biol Chem 276:24015–24022
Jang WS, Kim HK, Lee KY, Kim SA, Han YS, Lee IH (2006) Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. Febs Lett 580:1490–1496
Guo JB, Zhang GW, Chen XX, Wang JJ (2008) Studies on the Interaction between D-(+) catechin and DNA. J Anal Sci 24:507–511 (in Chinese)
Tang YL, Shi YH, Zhao W, Hao G, Le GW (2009) Interaction of MDpep9, a novel antimicrobial peptide from Chinese traditional edible larvae of housefly, with Escherichia coli genomic DNA. Food Chem 115:867–872
Khan S, Nami SAA, Siddiqi KS, Husain E, Naseem I (2009) Synthesis and characterization of transition metal 2,6-pyridinedicarboxylic acid derivatives, interactions of Cu(II) and Ni(II) complexes with DNA in vitro. Spectrochim Acta A 72:421–428
Song YM, Kang JW, Wang ZH, Lu XQ, Gao JH, Wang LF (2002) Study on the interactions between CuL2 and morin with DNA. J Inorg Biochem 91:470–474
Kumar CV, Asuncion EH (1993) DNA binding studies and site selective fluorescence sensitization of an anthryl probe. J Am Chem Soc 115:8547–8553
Chen QH, Zheng WF, Su XL, Lai WS (1962) Studies on chinese rhubarb I. Preliminary study on the antibacterial activity of anthraquinone derivatives of chinese rhubarb (Rheum palmatum L.). Acta Pharm Sin 9:757–762 (in Chinese)
Shan B, Cai YZ, Brooks JD, Corke H (2008) Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem 109:530–537
Ananta E, Heinz V, Knorr D (2004) Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol 21:567–577
Trommel JS, Marzilli LG (2001) Synthesis and DNA binding of novel water-soluble cationic methylcobalt porphyrins. Inorg Chem 40:4374–4383
Lepecq JB, Paoletti C (1967) A fluorescent complex between ethidium bromide and nucleic acid. J Mol Biol 27:87–106
Song YM, Kang JW, Zhou J, Wang ZH, Lu XQ, Wang LF, Gao JZ (2000) Study on the fluorescence spectra and electrochemical behavior of ZnL2 and morin with DNA. Spectrochim Acta A 56:2491–2497
Kumar CV, Asuncio EH (1992) Sequence dependent energy transfer from DNA to a simple aromatic chromophore. J Chem Soc Chem Commun 6:470–472
Kuo YC, Meng HC, Tsai WJ (2001) Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res 2:73–82
Wang CG, Yang JQ, Liu BZ, Jin DT, Wang C, Zhong L, Zhu D, Yan Wu (2010) Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur J Pharmacol 627:33–41
Denyer SP (1990) Mechanisms of action of biocides. Int Biodeter 2–4:89–100
Alves DS, Pérez-Fons L, Estepa A, Micol V (2004) Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem Pharmacol 68:549–561
Wang SR, Chen QH (1977) Studies of chinese rhubarb XIII. Interaction of anthraquinone derivatives of chinese rhubarb with DNA. Acta Biochem Biophys Sin 1:95–98 (in Chinese)
Li DD, Su XL, Chen QH (1964) Studies of chinese rhubarb VIII. Mechanism of antibiotic action of anthraquinone derivatives (2) effect on the metabolism of nitrogen containing compounds in S. aureus. Acta Biochem Biophys Sin 2:151–160 (in Chinese)
Chen CL, He BF, Chen QH (1988) Biochemical study of chinese rhubarb inhibiton of anthraquinone derivatives on NADH oxidase and succinate oxidase of mitochondrion. J Chin Biochem 1:36–41 (in Chinese)
Acknowledgments
This research work was jointly supported by the project of the Key Open Laboratory for Genetic Breeding of Aquatic Animals and Aquaculture Biology from the Ministry of Agriculture (BZ2009-24) and the earmarked fund for Modern Agro-industry Technology Research System, China (nycytx-49).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, C., Wang, H., Lv, W. et al. Antibacterial properties of anthraquinones extracted from rhubarb against Aeromonas hydrophila . Fish Sci 77, 375–384 (2011). https://doi.org/10.1007/s12562-011-0341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0341-z