Abstract
Berries have been implicated as the probable vehicle of infection in multiple outbreaks of norovirus and hepatitis A virus (HAV). These foods often receive minimal or no processing and may be exposed to virus contamination at each stage of production. In an increasingly globalized world, berries have a wide distribution and can give rise to the spread of diseases in distant parts of the world. With the aim of describing the virological quality of the berries cultivated in Argentina, a total of 184 soft fruits of different varieties (strawberries, blueberries, raspberries, blackberries, currants, pomegranate arils, cassis, and elder) were collected during the periods 2016–2018 and 2020. Viral particles were eluted and concentrated by polyethylene glycol precipitation according to ISO 15216-2:2019 guidelines. Genome detection of norovirus (NoV) genogroups I (GI) and II (GII), HAV, rotavirus, and enterovirus was performed by real-time RT-PCR with TaqMan probes. Positive samples were amplified by conventional RT-PCR and the amplicons were purified and sequenced in both directions. Phylogenetic analysis was performed using the Neighbor-Joining method based on the evolutionary model Kimura-2-parameters. NoV GII.6 was detected in 1/184 (0.5%) of the soft fruits, corresponding to a raspberry sample obtained during the fall of 2017. No presence of other human enteric viruses was found in the other berries analyzed. The collected data are the first in Argentina in relation to the prevalence of enteric viruses in berries and is useful as reference data for a risk assessment of soft fruits as vehicles of foodborne pathogenic viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruits and vegetables have important health benefits, however, sometimes these foods that are eaten raw contain microorganisms like viruses, parasites, bacteria, or fungi, which can cause damage to the health of those persons who eat them. The World Health Organization (WHO) estimated that each year around 600 million people in the world fall ill (almost 1 in 10 inhabitants) from eating contaminated food and that 420,000 die due to foodborne hazards, resulting in a global burden of foodborne diseases of 33 million Disability Adjusted Life Years (DALYs) (WHO, 2015). The majority of foodborne illnesses result from viral pathogens, such as norovirus, succeeded by bacterial and parasite pathogens (Gould et al., 2013; WHO, 2015). The burden of foodborne diseases is supported by individuals of all ages, but particularly by children under 5 years of age and by people living in low-income subregions of the world (WHO, 2015). Foodborne illness may be caused by contaminated fruits and vegetables. In the United States (US), from 2004 to 2013, 36% of cases of foodborne illnesses resulted from consumption of contaminated produce (Fischer et al., 2015). Moreover, data reported to the US Centers for Disease Control and Prevention's Foodborne Disease Outbreak Surveillance System during 1998–2013 showed that the percentage of outbreaks attributed to raw produce (among outbreaks with a food) increased from 8% during 1998–2001 to 16% during 2010–2013; being raw produce outbreaks most commonly attributed to vegetable row crops (38%), fruits (35%), and seeded vegetables (11%) (Bennett et al., 2018). An actualized literature review of healthcare-associated foodborne outbreaks revealed that frequently reported implicated foods were vegetables and fruits (25%), and these food matrices were the food vehicles associated with the highest number of case-patients (1308/3802 case-patients; 35%) (Boone et al., 2021).
In recent years, the consumption of berries such as raspberries, blueberries, blackberries, and currants has substantially increased (Evans & Ballen, 2017; Farruggia et al., 2016; Li et al., 2017). Berries come from a variety of plant types (low-growing, ground-based bushes, small shrubs, or as aerial crops from taller plants) that are grown under a variety of agricultural systems. Because soft fruits have high juice content and are fragile, they are sensitive to handling and damage, and therefore, they are usually harvested by hand, rather than by mechanization. Moreover, berries are generally consumed fresh and are not subject to further processing to eliminate pathogenic microorganisms such as viruses (Gao et al., 2019; Ryu et al., 2015). Therefore, good agricultural practices and hazard analysis and critical control point (HACCP) systems are essential in the berry producing industry to minimize the risk of microbial contamination and ensure food safety.
Viral contamination of berries may occur at several points on the farm-to-fork pathway, starting from the use of contaminated water for irrigation, contact with human feces during cultivation (wastewaters) or contaminated harvesting equipment, from unhygienic practices of berry pickers in the production field, packing shed or supply chain, and also from consumers while on the shelf prior to consumption (Bozkurt et al., 2021). Even though enteric viruses cannot grow in or on foods, they are environmentally stable, can survive adverse conditions, and are resistant to the extreme pH and enzymes of the gastrointestinal tract (D’Souza et al., 2007). Moreover, human enteric viruses are highly infectious and it has been estimated that 1–10 viral particles are enough to infect humans. Therefore, contamination of foods with microscopic amounts of infected feces can cause outbreaks and illnesses (Atmar et al., 2014; Purcell et al., 2002).
In recent decades, berries (both fresh and frozen) have been implicated as the probable vehicle of infection in multiple outbreaks of hepatitis A virus (HAV) and human norovirus (NoV) belonging to genogroups I (GI) and II (GII) (CDC, 2018). Other enteric viruses such as rotavirus (RV) and enteroviruses (EV), that represent a great impact on health, have also been detected in food; however, there are still no reports of foodborne outbreaks due to these agents (Maunula et al., 2013; Parada-Fabian et al., 2016).
In an increasingly globalized world, fresh produce is widely distributed and can lead to the spread of disease to distant parts of the planet (Nasheri et al., 2019). In order to promote good health, access to safe and nutritious food in sufficient quantities is essential.
Argentina produces a total of 58,700 tons of berries per year. The main berry varieties produced are strawberries (36,000 tons) and blueberries (20,000 tons), and to a lesser extent raspberries, blackberries, and currants, among others (RSA-CONICET, 2016). These fruits are mostly produced for export to other countries in America, Europe, and Asia (RSA-CONICET, 2016). In this context, the objective of this study was to determine the virological quality of various types of berries cultivated in Argentina, through the detection and characterization of enteric viruses with an impact on human health.
Materials and Methods
Background and Sample Collection
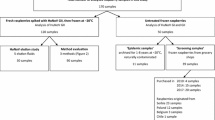
During January 2016–December 2017 and January–December 2020, a total of 184 samples of berries of different species: Fragaria × ananassa (strawberries, n = 75), Vaccinium myrtillus (blueberries, n = 68), Rubus idaeus (raspberries, n = 10), Morus rubra (blackberries, n = 12), Ribes rubrum (currants, n = 8), Punica granatum (pomegranate arils n = 6), Ribes nigrum (cassis, n = 3), and Sambucus canadensis (elder, n = 2), were collected from production plants (n = 157) or retail markets (n = 27) throughout Argentina. Table 1 depicts the Argentine regions and provinces where the samples were collected. All the samples were directly transported to the laboratory of Food Microbiology (CIATI) in individual sterile containers at 4–8 °C, where they were processed for viral elution and concentration within 24 h.
Elution and Concentration of Viruses from Berries
Viral extraction from berry samples was done from 25 g of product following the guidelines of the ISO 15216-2: 2019 standard with slight modifications and using feline calicivirus (FCV), a non-enveloped, positive sense RNA genome virus (Etherington et al., 2006) member of the Caliciviridae family, as a process control virus. Each soft fruit sample was processed by duplicate. The FCV stock (Generon S.p.A, San Prospero, Italy) used had a titer of approximately 2 × 106 PFU/mL. Briefly, 25 g of berries were placed into a mesh filter bag, 10 μL of FCV and 40 mL of Tris Glycine Beef Extract buffer (TGBE; Sigma-Aldrich, USA) solution supplemented with 1140 U of Aspergillus aculeatus pectinase (Novozym® 33,095, Argentina) were added. The samples were shaken at 60 rpm for 20 min at 25 °C (pH was kept at 9.0–9.5, adjusted with 1 N NaOH every 10 min). Then, the filtered liquid was transferred into a centrifuge tube and centrifuged at 10,000×g for 30 min at 4 °C. The supernatant was transferred into a clean tube, the pH was adjusted to 7.0 with 1 N HCl, and polyethylene glycol (PEG) 6000 and NaCl were added to the supernatant until a final concentration of 10% PEG 6000 (Sigma, USA) and 0.3 M NaCl was attained. The samples were shaken at 60 rpm for 60 min at 4 °C, followed by centrifugation at 10,000×g for 30 min at 4 °C, and the pellet was centrifuged again at 10,000×g for 5 min to compact. Then the pellet was resuspended in 500 μL PBS and transferred to a chloroform-resistant centrifuge tube with 500 μL chloroform-butanol. The mixture was incubated at room temperature for 5 min and then centrifuged at 10,000×g for 15 min at 4 °C. The aqueous phase was transferred to a fresh tube and retained for RNA extraction.
Virus Genome Detection and Characterization
Nucleic Acid Extraction
200 μL of the viral concentrates were subjected to RNA extraction using the commercial kit Direct-zol RNA MiniPrep (Zymo Research, CA, USA) to obtain 25 μL of RNA extract, according to the manufacturer's instructions. Extracted RNA was stored at − 80 °C until testing.
Enteric Virus Detection
RNA was detected using standardized one-step real-time RT-PCR procedures with primers and Taqman probes specific for the following enteric viruses: norovirus genogroups I (GI) and II (GII) (da Silva et al., 2007; Kageyama et al., 2003; Loisy et al., 2005; Svraka et al., 2007), HAV (Costafreda et al., 2006), RV (Zeng et al., 2008), EV (Read & Kurtz, 1999), and FCV (Di Pasquale et al., 2010). TaqMan RT-PCR assays were carried out in 20–25 μL of a reaction mixture comprising 5 μL of extracted RNA and 15–20 μL of master mix. Commercial kits were used in all cases: WHATfinder Norovirus Type I ID (Generon S.p.A., Italy) for norovirus GI; WHATfinder Norovirus Type II ID (Generon S.p.A., Italy) for norovirus GII; WHATfinder HAV ID (Generon S.p.A., Italy) for HAV detection; G-DiaNota™ (Diagenode, Belgium) for NoV GI, NoV GII, and RV; O-DiaENT™ (Diagenode, Belgium) for EV; and WHATfinder Recovery Efficiency Kit (Generon S.p.A., Italy) for FCV. Real-time RT-PCR amplifications were run in a Stratagene Mx3005P system (Stratagene, CA, USA) in a 96-well format under the following conditions: (i) 10 min at 50 °C for reverse transcription, 95 °C for 3 min for initial denaturation and Taq activation, then followed by 45 cycles of amplification with denaturation at 95 °C for 10 s, and annealing and extension at 60 °C for 45 s, for the kits from Generon S.p.A.; and 30 min at 50 °C for reverse transcription, 95 °C for 10 min for initial denaturation then followed by 45 cycles of amplification with denaturation at 95 °C for 15 s, and annealing and extension at 55 °C for 30 s and 68 °C for 30 s, for the kits from Diagenode.
Positive and negative controls were included in each real-time RT-PCR run. All the amplification reactions were run in duplicate in two independent assays. When no signal of the process control virus was observed, a ten-fold dilution of the cDNA was analyzed. A sample that had a cycle threshold (Ct) value below 43, with no evidence of amplification in the negative control (threshold not reached after 45 cycles) was considered positive.
Extraction Efficiency of FCV
The process control FCV was quantified from a standard curve generated using undiluted process control virus RNA and ten-fold serial dilutions of the viral RNA stock (10–1–10–3), according to the indications of ISO 15216-2:2019 standard. The Cq values for the process control FCV assay from each of the tested samples were used to estimate the process control virus recoveries by reference to the process control virus RNA standard curve.
Norovirus Characterization and Sequence Analysis
Norovirus positive sample was re-amplified by RT-hemi-nested PCR with primers targeting ORF1/ORF2 junction and 5’ region of VP1 protein gene (Kitajima et al., 2010). Extracted RNA was reverse-transcribed into cDNA using random hexamer primers and M-MLV reverse transcriptase (Promega, Wisconsin, USA) according to the manufacturer's instructions. The first PCR was performed in 10 μL of reaction volume containing 2 μL of cDNA, GoTaq Buffer 5×, 10 μM of COG2F and G2SKR primers, 10 mM of dNTPs and 0.5 U of GoTaq DNA polymerase (Promega, Wisconsin, USA). The amplification was performed under the following conditions: initial denaturation at 94 °C for 3 min, followed by 40 cycles of amplification with denaturation at 94 °C for 30 s, primer annealing at 50 °C for 30 s, extension reaction at 72 °C for 1 min, and then a final extension at 72 °C for 7 min. The second PCR was performed in 10 μL of reaction volume containing 1 μL of the first PCR product, GoTaq Buffer 5×, 10 μM of G2SKF and G2SKR primers, 10 mM of dNTPs, and 0.5 U of GoTaq DNA polymerase (Promega, Wisconsin, USA). PCR amplification with 35 cycles was performed under conditions identical to those for the first PCR. The RT-hemi-nested-PCR was run in a Mastercycler® gradient (Eppendorf, Hamburg, Germany).
Hemi-nested PCR amplicon for norovirus GII was purified and sequenced in both directions with primers G2SKF/G2SKR, using the dideoxynucleotide chain terminator method with Big Dye TM terminator version 3.1 cycling conditions on an automated sequencer (model 3730XL; Applied Biosystems, Foster City, CA, USA) by Macrogen Inc. (Seoul, South Korea). The editing, alignment, and comparison of the nucleotide sequence obtained were performed using the MEGA-X program (Molecular Evolutionary Genetic Analysis) version 10.1 (Kumar et al., 2018). The consensus sequence obtained was compared with the sequences of standard strains published in the GenBank, using the BLAST program. Multiple sequence alignment was performed with the Clustal W program. Phylogenetic relationships were calculated using the Kimura-2-parameter method (Kimura, 1980) as a model of substitution. The statistical significance of the inferred phylogenies was estimated using the Neighbor-Joining method to construct the phylogenetic tree with a bootstrap of 1000 pseudo-replicate data sets. The GenBank accession number for the sequence obtained in this study was MW420919.
Results
Viral contamination was detected in 1 out of 184 (0.5%) of the berry samples analyzed, corresponding to a raspberry sample collected in April 2017 from a production plant located in the province of Buenos Aires, Argentina. Norovirus GII was identified in the contaminated raspberry sample. No presence of norovirus GI, HAV, RV, or EV was detected in the strawberry, blueberry, raspberry, blackberry, currant, pomegranate aril, cassis, and elder samples analyzed (Table 2). The process control FCV was recovered from all the viral concentrates with a mean efficiency of 23 ± 12%, revealing that no sample showed total inhibition of PCR and that there was no total loss of the viral particles at any point in the process. In those cases where the efficiency was lower than 1%, dilutions were made, and after that, in all cases FCV was recovered with efficiencies higher than 1%. Thus, the performance of the technique complied with the criteria described in the ISO 15216-2:2019 standard, which stipulates higher than 1% extraction efficiency.
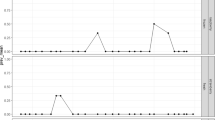
The phylogenetic analysis of the norovirus strain detected in the raspberry sample revealed that it belongs to genogroup II genotype 6 (Fig. 1).
Phylogenetic tree based on the capsid-gene nucleotide sequence of norovirus GII (251 nt) strain. The tree was constructed by the Neighbor-Joining method and the Kimura-2-parameter model. Bootstrap values above 70% are given at branch nodes. The scale bar represents 20% genetic distance. The strain isolated in this study is indicated by a black circle (GenBank accession number MW420919)
Discussion
Control and prevention of viral diseases transmitted by foods that are eaten raw is challenging. In relation to the berries producing industry, the first step for the prevention of viral transmission is to know the virological quality of the fruits in the preharvest or harvest stage in order to implement control measures to reduce viral contamination.
The results obtained from our study revealed a low prevalence of enteric viruses in berries produced in Argentina (0.5%). Worldwide, there is limited data regarding the prevalence of enteric viruses in raw soft fruits. Belgium and France were the first countries to provide data about the prevalence of norovirus on fresh soft red fruits, reporting a prevalence of 34.5% and 6.7%, respectively (Baert et al., 2011). However, sequence confirmation was not successful for the majority of the samples tested. A recent study by Cook et al. (2019) reported the prevalence of NoV in 2.3% and 3.6% of the fresh and frozen raspberries marketed in the United Kingdom, respectively. Also, 15.2% of the tested soft fruits in Mexico were positive for rotavirus (Parada-Fabian et al., 2016). De Keuckelaere et al. (2015) found norovirus in 8.6% of the tested raspberries from the Netherlands. Furthermore, Li et al. (2018) detected foodborne viruses (NoV GI, NoV GII, and HAV) on 0.3% of berry samples from different countries including Germany, Bulgaria, France, Poland, Switzerland, Czech Republic, USA, Spain, Russia, and Turkey, and also recorded human adenovirus presence in 0.9% of the tested samples. Prevalence differences between the studies might be explained by the relatively low number of samples tested in some studies and the difficulties linked to low numbers of heterogeneously distributed viruses which can lead to false-negative results (Butot et al., 2014). This might be overcome by improving the efficiencies of viral extraction and detection techniques.
Although the worldwide prevalence of enteric viruses in berries is relatively low, numerous reports demonstrated the association of viral foodborne outbreaks with the consumption of contaminated berries (Bozkurt et al., 2021; Gao et al., 2019; Torok et al., 2019). During the period 2008–2018, Nasheri et al. (2019) reported a total of 12 outbreaks of HAV and 40 outbreaks of norovirus related to frozen berries. Overall, norovirus infections comprised a higher number of total cases, except in 2013, when multiple outbreaks of HAV associated with different frozen fruits were reported in Europe and North America. Italy experienced a large HAV-1A outbreak with approximately 1800 reported cases, associated with frozen berries in 2013 (Scavia et al., 2017). Also, during 2013, an outbreak occurred in the USA with 165 confirmed cases of HAV-1B that was attributed to imported frozen pomegranate arils (Collier et al., 2014). Moreover, during the same year, cases of HAV infections were reported simultaneously in three countries of the European Union due to the consumption of frozen soft fruits (Macori et al., 2018). Reports revealed that, in general, the vehicles for most HAV outbreaks were frozen strawberries and frozen pomegranate arils, whereas frozen raspberries were indicated as the source of the majority of the norovirus outbreaks (Nasheri et al., 2019).
In our study, only norovirus GII.6 was detected in Argentinean berries, corresponding to a raspberry sample. This result is in agreement with the worldwide higher prevalence of norovirus in this soft fruit matrix. During the last 2–3 decades, symptomatic infection in humans has been mostly linked to a single norovirus genotype, GII.4 (Eden et al., 2013). However, more recently, novel non-GII.4 strains have been observed as predominant genotypes in children hospitalized due to acute gastroenteritis (Diakoudi et al., 2019). In this context, in the last years there have been some gastroenteritis outbreaks associated with norovirus GII.6 strains (Luo et al., 2015; Sharma et al., 2020; Zhang et al., 2020), and also some places reported the sustained circulation of norovirus GII.6 strain in the population (Diakoudi et al., 2019). Therefore, the prevalence of norovirus GII.6 requires attention.
Numerous risk factors are considered to be responsible for the contamination of berries, such as the type of cultural techniques (i.e., traditional soil or hydroponic cultivations) and the type of irrigation (i.e., lowered well-water or flooded surface channel water) (Macori et al., 2018). In addition, it has been reported that the main source of contamination of berries with enteric viruses could be the lack of appropriate hygiene facilities and unsafe hygiene practices, as the harvesting of small fruits mostly takes place by hand, a demonstrated vehicle by which human pathogenic viruses enter the berry fruit chain (El-Senousy et al., 2015; Macori et al., 2018; Parada-Fabian et al., 2016). Therefore, in the berries production process there are key stages that will result in a reduction in viral contamination, for example controlling the irrigation water quality, the management of the manure and the worker's health and hygiene.
Most of the samples analyzed in this manuscript correspond to soft fruits collected at the farm level. A follow-up study looking at the prevalence of viruses on soft fruits on farms will be interesting in order to ascertain whether viral contamination originates at the production site. Moreover, it would also be interesting to increase the number of samples obtained from retail markets, including fruit stalls on the street, in order to evaluate the prevalence of enteric viruses under these conditions.
The results obtained in the present study showed a low viral contamination of soft fruits produced in Argentina, indicating that probably good farming practices and HACCP are followed at the different production units throughout the country. Anyway, it must not be ruled out that viral loss during extraction and detection techniques and also low viral concentrations in the samples might be causes of low viral detection in the Argentinean soft fruits analyzed.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Atmar, R. L., Opekun, A. R., Gilger, M. A., Estes, M. K., Crawford, S. E., Neill, F. H., Ramani, S., Hill, H., Ferreira, J., & Graham, D. Y. (2014). Determination of the 50% human infectious dose for Norwalk virus. Journal of Infectious Diseases, 209(7), 1016–1022.
Baert, L., Mattison, K., Loisy-Hamon, F., Harlow, J., Martyres, A., Lebeau, B., Stals, A., Van Coillie, E., Herman, L., & Uyttendaele, M. (2011). Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? International Journal of Food Microbiology, 151(3), 261–269.
Bennett, S. D., Sodha, S. V., Ayers, T. L., Lynch, M. F., Gould, L. H., & Tauxe, R. V. (2018). Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiology and Infection, 146(11), 1397–1406.
Boone, I., Rosner, B., Lachmann, R., D’Errico, M. L., Iannetti, L., Van der Stede, Y., Boelaert, F., Ethelberg, S., Eckmanns, T., Stark, K., Haller, S., & Wilking, H. (2021). Healthcare-associated foodborne outbreaks in high-income countries: A literature review and surveillance study, 16 OECD countries, 2001 to 2019. Eurosurveillance Weekly, 26(41), 2001278.
Bozkurt, H., Phan-Thien, K. Y., van Ogtrop, F., Bell, T., & McConchie, R. (2021). Outbreaks, occurrence, and control of norovirus and hepatitis a virus contamination in berries: A review. Critical Reviews in Food Science and Nutrition, 61(1), 116–138.
Butot, S., Zuber, S., & Baert, L. (2014). Sample preparation prior to molecular amplification: 325 Complexities and opportunities. Current Opinion in Virology, 4, 66–70.
Centers for Disease Control and Prevention (CDC). (2018). National Outbreak Reporting System (NORS). Retrieved February 26, 2021, from https://wwwn.cdc.gov/norsdashboard/
Collier, M. G., Khudyakov, Y. E., Selvage, D., Adams-Cameron, M., Epson, E., Cronquist, A., Jervis, R. H., Lamba, K., Kimura, A. C., Sowadsky, R., Hassan, R., Park, S. Y., Garza, E., Elliott, A. J., Rotstein, D. S., Beal, J., Kuntz, T., Lance, S. E., Dreisch, R., Wise, M. E., Nelson, N. P., Suryaprasad, A., Drobeniuc, J., Holmberg, S. D., Xu, F., & Hepatitis A Outbreak Investigation Team. (2014). Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: An epidemiological case study. The Lancet Infectious Diseases, 14(10), 976–981.
Cook, N., Williams, L., & D’Agostino, M. (2019). Prevalence of Norovirus in produce sold at retail in the United Kingdom. Food Microbiology, 79, 85–89.
Costafreda, M. I., Bosch, A., & Pinto, R. M. (2006). Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environment Microbiology, 72(6), 3846–3855.
D’Souza, D. H., Moe, C. L., & Jaykus, L. (2007). Foodborne viral pathogens. In M. P. Doyle & L. R. Beuchat (Eds.), Food microbiology: Fundamentals and frontiers. ASM Press.
da Silva, A. K., Le Saux, J. C., Parnaudeau, S., Pommepuy, M., Elimelech, M., & Le Guyader, F. S. (2007). Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: Different behaviors of genogroups I and II. Applied and Environment Microbiology, 73(24), 7891–7897.
De Keuckelaere, A., Li, D., Deliens, B., Stals, A., & Uyttendaele, M. (2015). Batch testing for noroviruses in frozen raspberries. International Journal of Food Microbiology, 192, 43–50.
Diakoudi, G., Lanave, G., Catella, C., Medici, M. C., De Conto, F., Calderaro, A., Loconsole, D., Chironna, M., Bonura, F., Giammanco, G. M., Bányai, K., Tohma, K., Parra, G. I., Martella, V., & De Grazia, S. (2019). Analysis of GIIP7 and GII6 noroviruses circulating in Italy during 2011–2016 reveals a replacement of lineages and complex recombination history. Infection Genetics and Evolution, 75, 103–991.
Di Pasquale, S., Paniconi, M., De Medici, D., Suffredini, E., & Croci, L. (2010). Duplex real time PCR for the detection of hepatitis A virus in shellfish using Feline Calicivirus as a process control. Journal of Virological Methods, 163(1), 96–100.
Eden, J.-S., Tanaka, M. M., Boni, M. F., Rawlinson, W. D., & White, P. A. (2013). Recombination within the pandemic norovirus GII.4 lineage. Journal of Virology, 87(11), 6270–6282.
El-Senousy, W. M., Ragab, A. M., & Handak, E. M. (2015). Prevalence of rotaviruses groups A and C in Egyptian children and aquatic environment. Food and Environmental Virology, 7(2), 132–141.
Etherington, G. J., Ring, S. M., Charleston, M. A., Dicks, J., Rayward-Smith, V. J., & Roberts, I. N. (2006). Tracing the origin and co-phylogeny of the caliciviruses. Journal of General Virology, 87(5), 1229–1235.
Evans, E. A., & Ballen, F. H. (2017). An overview of US blueberry production, trade, and consumption, with special reference to Florida. Food and Resource Economics, 952, 1–8.
Farruggia, D., Crescimanno, M., Galati, A., & Tinervia, S. (2016). The quality perception of fresh berries: An empirical survey in the German market. Agr Agr Sci Proc, 8, 566–575.
Fischer, N., Bourne, A., & Plunkett, D. (2015). Outbreak alert! 2015: A review of foodborne illnesses in the US from 2004–2013. Center for Science in the Public Interest.
Gao, X., Wang, Z., Wang, Y., Liu, Z., Guan, X., Ma, Y., Zhou, H., Jiang, Y., Cui, W., Wang, L., & Xu, Y. (2019). Surveillance of norovirus contamination in commercial fresh/frozen berries from Heilongjiang Province, China, using a TaqMan real-time RT-PCR assay. Food Microbiology, 82, 119–126.
Gould, L. H., Walsh, K. A., Vieira, A. R., Herman, K., Williams, I. T., Hall, A. J., Cole, D., & Centers for Disease Control and Prevention. (2013). Surveillance for foodborne disease outbreaks—United States, 1998–2008. MMWR Surveillance Summaries, 62(2), 1–34.
ISO 15216-2. (2013). Microbiology of food and animal feed—Horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR—Part 2: Method for qualitative detection.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., Takeda, N., & Katayama, K. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2), 111–120.
Kitajima, M., Oka, T., Haramoto, E., Takeda, N., Katayama, K., & Katayama, H. (2010). Seasonal distribution and genetic diversity of genogroups I, II, and IV noroviruses in the Tamagawa River, Japan. Environmental Science & Technology, 44(18), 7116–7122.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549.
Li, Y., Sun, H., & Chen, L. (2017). The blueberry industry of China: The past 10 years and the future. Acta Hortic (wagening), 1180, 531–536.
Li, D., Butot, S., Zuber, S., & Uyttendaele, M. (2018). Monitoring of foodborne viruses in berries and considerations on the use of RT-PCR methods in surveillance. Food Control, 89, 235–240.
Loisy, F., Atmar, R. L., Guillon, P., Le Cann, P., Pommepuy, M., & Le Guyader, F. S. (2005). Real-time RT-PCR for norovirus screening in shellfish. Journal of Virological Methods, 123(1), 1–7.
Luo, L. F., Qiao, K., Wang, X. G., Ding, K. Y., Su, H. L., Li, C. Z., & Yan, H. J. (2015). Acute gastroenteritis outbreak caused by a GII.6 norovirus. World Journal of Gastroenterology, 21(17), 5295–5302.
Macori, G., Gilardi, G., Bellio, A., Bianchi, D. M., Gallina, S., Vitale, N., Gullino, M. L., & Decastelli, L. (2018). Microbiological parameters in the primary production of berries: A pilot study. Foods, 7(7), 105.
Maunula, L., Kaupke, A., Vasickova, P., Soderberg, K., Kozyra, I., Lazic, S., van der Poel, W. H., Bouwknegt, M., Rutjes, S., Willems, K. A., Moloney, R., D’Agostino, M., de Roda Husman, A. M., von Bonsdorff, C. H., Rzezutka, A., Pavlik, I., Petrovic, T., & Cook, N. (2013). Tracing enteric viruses in the European berry fruit supply chain. International Journal of Food Microbiology, 167(2), 177–185.
Nasheri, N., Vester, A., & Petronella, N. (2019). Foodborne viral outbreaks associated with frozen produce. Epidemiology and Infection, 147, e291.
Parada-Fabian, J. C., Juarez-Garcia, P., Natividad-Bonifacio, I., Vazquez-Salinas, C., & Quinones-Ramirez, E. I. (2016). Identification of enteric viruses in foods from Mexico city. Food and Environmental Virology, 8(3), 215–220.
Purcell, R. H., Wong, D. C., & Shapiro, M. (2002). Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. Journal of Infectious Diseases, 185(11), 1668–1671.
Read, S. J., & Kurtz, J. B. (1999). Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. Journal of Clinical Microbiology, 37(5), 1352–1355.
Red de Seguridad Alimentaria (RSA-CONICET). (2016). Foodborne viruses. Technical report. https://rsa.conicet.gov.ar/wp-content/uploads/2016/09/Informe-virus-transmitidos-por-alimentos.pdf
Ryu, S., You, H. J., Kim, Y. W., Lee, A., Ko, G. P., Lee, S. J., & Song, M. J. (2015). Inactivation of norovirus and surrogates by natural phytochemicals and bioactive substances. Molecular Nutrition & Food Research, 59, 65–74.
Scavia, G., Alfonsi, V., Taffon, S., Escher, M., Bruni, R., Medici, D., Pasquale, S. D., Guizzardi, S., Cappelletti, B., Iannazzo, S., Losio, N. M., Pavoni, E., Decastelli, L., Ciccaglione, A. R., Equestre, M., Tosti, M. E., Rizzo, C., & National Italian Task Force On Hepatitis A. (2017). A large prolonged outbreak of hepatitis A associated with consumption of frozen berries, Italy, 2013–14. Journal of Medical Microbiology, 66(3), 342–349.
Sharma, S., Hagbom, M., Carlsson, B., NederbyÖhd, J., Insulander, M., Eriksson, R., Simonsson, M., Widerström, M., & Nordgren, J. (2020). Secretor status is associated with susceptibility to disease in a large GII.6 norovirus foodborne outbreak. Food and Environmental Virology, 12(1), 28–34.
Svraka, S., Duizer, E., Vennema, H., de Bruin, E., van der Veer, B., Dorresteijn, B., & Koopmans, M. (2007). Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. Journal of Clinical Microbiology, 45(5), 1389–1394.
Torok, V. A., Hodgson, K. R., Jolley, J., Turnbull, A., & McLeod, C. (2019). Estimating risk associated with human norovirus and hepatitis A virus in fresh Australian leafy greens and berries at retail. International Journal of Food Microbiology, 309, 108327.
World Health Organization (WHO). (2015). World Health Organization. Estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. www.who.int: WHO Press.
Zhang, X. F., Chen, J. R., Song, C. L., Xie, D. J., Tan, M., Wang, L., Koroma, M. M., Hou, Y. Z., Dong, Z. P., Yu, J. R., Duan, W. T., Zhao, D. D., Du, J. R., Zhu, L., & Dai, Y. C. (2020). Characterization of a hospital-based gastroenteritis outbreak caused by GII.6 norovirus in Jinshan China. Epidemiology & Infection, 148, 289.
Zeng, S. Q., Halkosalo, A., Salminen, M., Szakal, E. D., Puustinen, L., & Vesikari, T. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Journal of Virological Methods, 153(2), 238–240.
Funding
This work was supported by the National Agency for Scientific and Technical Promotion (PICT 2018-04224). J.M.O. and P.B. are members of the researcher career program of CONICET, Argentina. V.E.P. is a recipient of a CONICET fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oteiza, J.M., Prez, V.E., Pereyra, D. et al. Occurrence of Norovirus, Rotavirus, Hepatitis a Virus, and Enterovirus in Berries in Argentina. Food Environ Virol 14, 170–177 (2022). https://doi.org/10.1007/s12560-022-09518-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-022-09518-z