Abstract
The objective of this study is to compare the prevalence of rotaviruses groups A and C in Egyptian children and aquatic environment. From 110 stool specimens of children with acute diarrhea and using RT-PCR, 35 samples (31.8 %) were positive for human rotavirus group A and 15 samples (13.6 %) were positive for human rotavirus group C. From 96 samples collected from Zenin wastewater treatment plant over a 2-year period (November 2009–October 2011) and using RT-PCR, rotavirus group A was detected in (4/24) 16.7 %, (5/24) 20.8 %, (4/24) 16.7 %, and (4/24) 16.7 %, while rotavirus group C was detected in (2/24) 8.3 %, (3/24) 12.5 %, (3/24) 12.5 %, and (0/24) 0 % in raw sewage, after primary sedimentation, after secondary sedimentation, and after final chlorination, respectively. Moreover, from 96 samples collected from El-Giza water treatment plant over a 2-year period (November 2009–October 2011), rotavirus group A was detected in (7/24) 29.2 %, (6/24) 25 %, (5/24) 20.8 %, and (3/24) 12.5 %, while rotavirus group C was detected in (3/24) 12.5 %, (1/24) 4.2 %, (1/24) 4.2 %, and (0/24) 0 % in raw Nile water, after sedimentation, after sand filtration, and after final chlorination, respectively. Using SYBR Green real-time RT-PCR, the number of human rotavirus group A genome or infectious units was higher than rotavirus group C. VP6 sequence analysis of the RT-PCR positive rotavirus group C samples revealed that four clinical specimens and three environmental samples showed similar sequences clustered with Moduganari/Human Nigerian strain AF 325806 with 98 % homology, and two clinical specimens and one environmental sample showed similar sequences clustered with Dhaka CB/Human Bangladesh strain AY 754826 with 97 % homology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteric viruses, like rotaviruses group A (GARV), noroviruses (NoV), human astroviruses (HAstV), enteric adenoviruses (EAdV), and others are known to be the major cause of diarrheal disease worldwide (Wilhelmi et al. 2003). As of January 2012, the World Health Organization estimates that globally 453,000 child deaths occurred during 2008 due to rotavirus infection (http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/), while many fatal cases are caused by other enteric viruses as well, especially in developing countries. The high economic impact of sporadic gastroenteritis cases and gastroenteritis outbreaks is now recognized, and thus prevention of enteric virus infection is one of the main goals in healthcare prevention programs (Ramani and Kang 2009; Santosham et al. 2010). Till now, the only available and highly effective vaccine on the market is targeted to GARV. This prevention measure is already successfully implemented in many countries around the world (http://www.who.int/vaccine_safety/committee/topics/rotavirus/rotashield/en/).
Drinking water can be contaminated with enteric viruses mainly via defect sewage collection system, where wastewater can pass into the groundwater or into a defective drinking water supply (Laine et al. 2011). At the rural areas, not all households are connected to the sewage collection systems but rather to septic tanks from which sewage may trickle into the soil and groundwater. Contamination of surface water or groundwater is also possible through fertilization of agriculture surfaces with animal or human excrement from which enteric viruses could be washed into the surface or groundwater. The further use of contaminated water may constitute a potential source of enteric virus infections (Maunula 2007). The importance of animal slurry is not minor since there are evidences proving the zoonotic potential of some enteric viruses, like GARV (Cook et al. 2004).
Rotaviruses (family Reoviridae, genus Rotavirus) are common enteric pathogens of humans and animals. Rotaviruses are classified into seven (sero) groups, designated A to G (Estes 2001). Rotaviruses of all serogroups share the capsid morphology and the unique genome structure that composed of 11 segments of double-stranded RNA. However, rotaviruses among serogroups share no genetic and antigenic relatedness (Saif and Jiang 1994). The three most important human rotavirus serogroups (A, B, and C) have remarkably different epidemiologic features and public health importance (Parashar et al. 2009). Rotaviruses group C were first detected from an Australian infant hospitalized with diarrhea in 1973 (Rodger et al. 1982). Since then, rotaviruses group C have been recognized as a cause of gastroenteritis in all age groups, accounting for either sporadic cases or large outbreaks of gastroenteritis in closed and semi-closed communities (Szucs et al. 1987; Saif and Jiang 1994; Jiang et al. 1995; Gabbay et al. 1999; Sánchez-Fauquier et al. 2003; Phan et al. 2004; Schnagl et al. 2004; Kuzuya et al. 2005). The observed differences in the epidemiological features for different groups of rotaviruses may be accounted by different mechanisms of transmission or by their different stabilities in the environment and their overall ecology. Rotavirus infections are thought to be acquired primarily via the fecal-oral route, including person-to-person contact (Offit and Clark 2000), consumption of contaminated water or food (Bosch 1998), and by zoonotic transmission (Gentsch et al. 2005). The air-borne transmission has been also hypothesized (Offit and Clark 2000).The incidence of rotavirus group C diarrhea in children is generally considered low (Szucs et al. 1987; Nilsson et al. 2000; Cunliffe et al. 2001; Sánchez-Fauquier et al. 2003; Bányai et al. 2006). The low number of identified cases does not permit us to understand the ecology and epidemiology, including the source and mode of transmission of rotavirus group C in a variety of settings and areas in the world. These shortcomings prompted us to investigate this issue from the view point of environmental virology. The objective of this study is to compare the prevalence of rotaviruses groups A and C in Egyptian clinical specimens and environmental samples.
Materials and Methods
Sewage Samples
A total of 96 sewage samples were collected monthly over a 2-year period (November 2009–October 2011) from Zenin wastewater treatment plant (WWTP) which uses an activated sludge treatment with a capacity of 330,000 m3/day. The samples were collected from raw wastewater, after primary sedimentation, after secondary sedimentation, and after final chlorination (final effluents). Four liters of raw sewage and of the other treated sewage samples were collected and transferred to clean sterile bottles and transported to the laboratory within 3 h after collection for examination.
Water Samples
A total of 96 water samples were collected monthly over a 2-year period (November 2009–October 2011) from El-Giza water treatment plant (WTP). The treatment facilities in the treatment plant are pre-oxidation with Cl2, coagulation-settling, sedimentation, filtration (rapid sand filters), and final Cl2 addition. Twenty liters of water sample were collected including inlet water (Nile water), after sedimentation, after sand filtration, and outlet water after final chlorination (drinking water).
Clinical Specimens
One hundred and ten stool specimens were collected monthly over a 1-year period (May 2011–April 2012) from Abo El-Reech hospital in Greater Cairo. Stool specimens were collected from children (<5 years old) suffering from acute diarrhea. Specimens were collected in clean containers and transferred to the laboratory within 3 h after collection for examination.
Concentration of Sewage and Water Samples
Sewage and water samples were concentrated by filtration through negatively charged nitrocellulose membranes (ALBET-Spain, 0.45 μm pore size, and 142 mm diameter filter series) after addition of AlCl3 to a final concentration of 0.5 mM and acidification to pH 3.5 and after passing through Whatmann No. 1 filter paper. The viruses adsorbed to the membrane were eluted with 75 ml of 0.05 M glycine buffer, pH 9.5 (using HCl 5 N) containing 3 % beef extract (Lab-Limco powder, OXOID, UK) (Smith and Gerba 1982; Rose et al. 1984). All samples were reconcentrated using an organic flocculation method (Kattzenelson et al. 1976). Briefly and according to they, the eluate was acidified to pH 3.5 using HCl (5 N) and centrifuged at 3,000 rpm for 15 min, the supernatant was discarded, and the pellet was dissolved in 1 ml of Na2HPO4 (0.14 N, pH 9). Samples were neutralized and kept at −70 °C until used.
Concentration of Clinical Specimens
Approximately, 0.1 g of stool specimens was weighed, diluted 1:10 in nuclease-free H2O, and vortexed for 30 s. Specimens were clarified by centrifugation at 7,000 rpm for 10 min at room temperature.
Viral Nucleic Acid Extraction
Viral RNA was extracted from 140 μl of the supernatant using BIOZOL Total RNA Extraction reagent (BIOFLUX—Japan) and according to the manufacturer’s instructions and to a 30 μl final volume.
RT-PCR of a Fragment of the VP6-Coding Gene of Rotaviruses Group A
The primers used for RT-PCR were the forward VP6-F 5′-GACGGNGCNACTACATGGT-3′ and the reverse VP6-R 5′-GTCCAATTCATNCCTGGTGG-3′ primers 1 μm for each and according to Iturriza-Gomara et al. (2002) using 200 U of M-MLV reverse transcriptase enzyme (Promega—USA) in a total volume of 10 μl and 1.5 U of Taq DNA polymerase (Biobasic—Canada) in a total volume of 50 μl. Nested PCR amplification of the target rotavirus VP6 fragment was performed using the forward primer, VP6-NF 5′-GCTAGAAATTTTGATACA-3′, and the reverse primer, VP6-NR 5′-TCTGCAGTTTGTGAATC-3′ (1 μm for each), and according to Gallimore et al. (2006) to amplify 155 bp fragment. PCR products (10 μl) were analyzed by electrophoresis on 3 % agarose gels (Panreac—Spain).
Quantification of Rotavirus Group A Genome Copies Using Real-Time RT-PCR Method
Real-time RT-PCR was performed for positive samples in the previous RT-PCR screening. The RT was done according to Iturriza-Gomara et al. (2002) using primers (VP6-F and VP6-R). Real-time PCR was done using power SYBR green PCR master mix (Applied Biosystem—UK) in a total volume of 25 μl. Amplification was performed as described previously (Kang et al. 2004) in a real-time PCR thermal cycler (OneStep, Applied Biosystem). The specificity of the reactions was determined by melting curve analysis of the amplicons. The rotavirus genome copy number was determined by comparison with a standard curve generated with serial dilutions (10−1 to 10−6) of a positive control plasmid (pCR2.1-TOPO, Invitrogen—USA).
Quantification of Rotavirus Group C Genome Copies Using Real-Time RT-PCR Method
Real-time RT-PCR was done for positive samples in previous RT-PCR screening using power SYBR green PCR master mix according to Logan et al. (2006) in VP7 region.
Cell Culture RT-PCR (CC-RT-PCR) for Quantification of Infectious Rotavirus Particles
Rotavirus CC-RT-PCR assay was performed according to Abad et al. (1997), El-Senousy et al. (2007), and Ghazy et al. (2008). The assay was performed on suspensions of infected MA104 cells. Primers VP6-F and VP6-R were used. The RT-PCR method was the same as described previously. The detection limit in this tissue culture assay using 100 μl of inoculum is 1 × 101 CC-RT-PCR units/ml, where CC-RT-PCR unit is the reciprocal end point dilution detectable by CC-RT-PCR.
Confirmation of the RT-PCR Positivity of Rotavirus Groups A and C by Amplimer Sequencing
The RT-PCR products of selected positive samples for rotavirus group A (four raw sewage, four Nile water samples, and eight clinical specimens) and positive samples of rotavirus group C covering all seasons were sequenced. Fifty to one hundred μl of the RT-PCR products were purified using a high-pure PCR products purification kit (Qiagen) following the manufacturer’s instructions. Sequencing was performed on 1–7 μl of the purified products with an ABI prism Big dye termination cycle sequencing ready reaction kit (Applied Biosystem) using the same primers as in the PCR and following the manufacturer’s instructions. The DNA was sequenced with an ABI prism 310 automated DNA sequencer. Sequence data from both strands of the PCR products were aligned and compared using the Clustalw and Blast programs (European Bioinformatics Institute).
Statistical Analysis
Mean and standard deviation were calculated to determine the removal efficiency of treatment processes in Zenin WWTP and El-Giza WTP, and also to determine the difference between the number of genome copies and infectious units for rotaviruses.
Results
Detection of Human Rotaviruses in Clinical Specimens
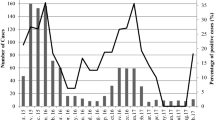
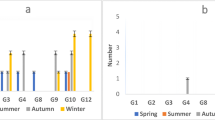
From stool specimens of 110 children with acute diarrhea (43 females 39.1 %, 67 males 60.9 %), aged from 0 to 3.5 years, from Abo El-Rish hospital in Greater Cairo over a 1-year period (May 2011–April 2012), 35 (31.8 %) specimens were positive for human rotavirus group A and 15 specimens (13.6 %) were positive for human rotavirus group C (Fig. 1). Ten specimens contained both rotavirus groups A and C (9 %).
Molecular Detection of Human Rotaviruses Groups A and C in Wastewater Samples Using RT-PCR
In Zenin WWTP, the human rotavirus group A RNA was detected in (4/24) 16.7 %, (5/24) 20.8 %, (4/24) 16.7 %, and (4/24) 16.7 % in raw sewage, after primary sedimentation, after secondary sedimentation, and final effluent samples, respectively (Table 1). The human rotavirus group C RNA was detected in (2/24) 8.3 %, (3/24) 12.5 %, (3/24) 12.5 %, and (0/24) 0 % in raw sewage, after primary sedimentation, after secondary sedimentation, and final effluent samples, respectively (Table 2).
Number of Human Rotaviruses Groups A and C Genomes and Infectious Units in Positive RT-PCR Samples of Zenin WWTP
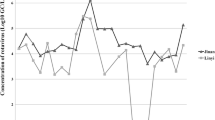
The number of human rotavirus group A genome ranged from 7 × 103 to 2 × 107 RNA copies/liter (copies/l), while the number of rotavirus group A infectious units ranged from 1 × 102 to 5 × 105 CC-RT-PCR units/liter (units/l) in raw sewage samples. For rotavirus group A genome, 1 log10 reduction was observed after primary sedimentation, 1–2 log10 reduction was observed after secondary sedimentation, and 2–3 log10 reduction was observed after final chlorination. For rotavirus group A infectious units, 0–1 log10 reduction was observed after primary sedimentation, 1–2 log10 reduction was observed after secondary sedimentation, and 1–3 log10 reduction was observed after final chlorination. On the other hand, 0–3 log10 differences were observed between rotavirus group A genome and infectious units (Table 3). The number of human rotavirus group C genome ranged from 8 × 102 to 1 × 103 RNA copies/l in raw sewage samples, while the number of rotavirus group C infectious units was 0 CC-RT-PCR units/l in all the samples (Table 4).
Molecular Detection of Human Rotaviruses Groups A and C in Water Samples Using RT-PCR
In El-Giza WTP, the human rotavirus group A RNA was detected in (7/24) 29.2 %, (6/24) 25 %, (5/24) 20.8 %, and (3/24) 12.5 % in Nile water, after sedimentation, after sand filtration, and after final chlorination, respectively (Table 5). Rotavirus group C RNA was detected in (3/24) 12.5 %, (1/24) 4.16 %, (1/24) 4.16 %, and (0/24) 0 % in Nile water, after sedimentation, after sand filtration, and after final chlorination, respectively (Table 6).
Number of Human Rotaviruses Groups A and C Genomes and Infectious Units in Positive RT-PCR Samples of El-Giza WTP
The number of human rotavirus group A genome ranged from 9 × 10 to 4 × 105 RNA copies/l, while the number of rotavirus group A infectious units ranged from 2 × 10 to 1 × 104 CC-RT-PCR units/l in Nile water samples. For rotavirus group A genome, 1 log10 reduction was observed after sedimentation, 0–1 log10 reduction was observed after sand filtration, and 1–3 log10 reduction was observed after final chlorination. For rotavirus group A infectious units, 0–1 log10 reduction was observed after sedimentation, 0–1 log10 reduction was observed after sand filtration, and 1–3 log10 reduction was observed after final chlorination. On the other hand, 0–2 log10 differences were observed between rotavirus group A genome and infectious units (Table 7). The number of human rotavirus group C genome ranged from 8 × 10 to 1 × 102 RNA copies/l in Nile water samples, while the number of rotavirus group C infectious units was 0 CC-RT-PCR units/l in all the samples (Table 8).
Sequence Analysis
Sequence analysis of PCR products of positive rotavirus group A samples showed sequences clustered with rotavirus group A (VP6) strain RVA/Human-wt/ETH/MRC-DPRU1843/2009/G1P[8] with 98 % homology. Rotavirus group C strains detected in seven (four clinical and three environmental) out of ten samples showed similar sequences and clustered with Moduganari/Human Nigerian strain AF 325806 with 98 % homology. Also, three samples (two clinical and one environmental) showed similar sequences clustered with Dhaka CB/Human Bangladesh strain AY 754826 with 97 % homology.
Discussion
The rotaviruses group A are considered the most important cause of severe dehydrating diarrheal illness in infants and young children worldwide, especially in the developing countries (Kapikian and Chanock 1996; Leclerc et al. 2002). In this study, rotavirus group A was more frequent than rotavirus group C in both Egyptian clinical specimens and environmental samples qualitatively using RT-PCR method and quantitatively using real-time RT-PCR method. This result was clear in clinical specimens where rotavirus group A was detected in 31.8 % of the specimens, while rotavirus group C was detected in 13.6 % of the specimens. Also, rotavirus group A was detected in (4/24) 16.7 % of raw sewage samples, while rotavirus group C was detected in (2/24) 8.3 % of the same raw sewage samples. In Nile water samples, rotavirus group A was detected in (7/24) 29.2 % of the samples, while rotavirus group C was detected in (3/24) 12.5 % of the same samples. Quantitatively, rotavirus group A genome copy numbers ranged from 7 × 103 to 2 × 107 genome copies/l in raw sewage samples, while rotavirus group C genome copy numbers ranged from 8 × 102 to 1 × 103 genome copies/l in the same samples. Also, rotavirus group A infectious units ranged from 1 × 102 to 5 × 105 CC-RT-PCR units/l in raw sewage samples, while complete absence of rotavirus group C infectious units was observed in the same samples. On the other hand, rotavirus group A genome copy numbers ranged from 9 × 10 to 5 × 105 genome copies/l in Nile water samples, while rotavirus group C genome copy numbers ranged from 8 × 10 to 1 × 102 genome copies/l in the same samples. Also, rotavirus group A infectious units ranged from 2 × 10 to 1 × 104 CC-RT-PCR units/l in Nile water samples, while complete absence of rotavirus group C infectious units was observed in the same samples. The higher prevalence of rotavirus group A in the raw sewage is a direct result to its higher prevalence in diarrheal specimens. In a previous study concerning the frequency of enteric viruses in the Egyptian aquatic environment, rotavirus group A was the most prevalent among RNA enteric viruses in raw sewage, treated effluents, Nile water, and drinking water of Greater Cairo (El-Senousy et al. 2004). In recent studies, rotavirus group A still has high prevalence in sewage and water of Cairo and Nile Delta (El-Senousy and El-Mahdy 2009; El-Senousy et al. 2013a; El-Senousy et al. 2013c). From the results, we could easily notice that the peak of incidence of group C rotavirus in clinical specimens and environmental samples was in cooler months (autumn and winter), which is similar to the peak of incidence of rotavirus group A. The presence of rotavirus group C in addition to the high prevalence of rotavirus group A represents a health risk to the Egyptian community. Although RotaTeq (live, oral, pentavalent vaccine, MERCK) and Rotarix (human monovalent, live attenuated vaccine containing one rotavirus strain of G1P[8] specificity, GlaxoSmithKline) are not included in the mandatory Egyptian vaccine programs, they are available in the private clinics but it is still expensive for the majority of the Egyptians. The cohort and seroepidemiological studies indicated that virtually all children are infected with rotaviruses group A by 5 years of age (Velazquez et al. 1996; Offit and Clark 2000), while the rate of infection with rotaviruses group C is much lower and antibody prevalence usually peaks at an age of 40–50 years or later (James et al. 1997; Steele and James 1999; Kuzuya et al. 2001).
Zenin WWTP which uses an activated sludge treatment technology, the most prevalent treatment technology in Egypt, was chosen as a model for Egyptian WWTPs using an activated sludge treatment technology. The means of rotavirus group A genome and infectious units removals were 1 log10 and 0.75 ± 0.5 log10, respectively, after the primary sedimentation of Zenin WWTP. The means of rotavirus group A genome and infectious units removals were 1.5 ± 0.6 log10 and 1.25 ± 0.5 log10, respectively, after the secondary sedimentation of Zenin WWTP. The means of rotavirus group A genome and infectious units removals were 2.25 ± 0.5 log10 and 1.75 ± 1 log10, respectively, after the final chlorination of Zenin WWTP. The means of rotavirus group A genome and infectious units removals were 3.75 ± 0.5 log10 and 3 ± 1.2 log10, respectively, after the whole treatment processes of Zenin WWTP. In El-Giza, WTP, the means of rotavirus group A genome and infectious units removals were 1 log10 and 0.86 ± 0.4 log10, respectively, after the sedimentation of El-Giza WTP. The means of rotavirus group A genome and infectious units removals were 0.67 ± 0.5 log10 and 0.5 ± 0.5 log10, respectively, after the sand filtration of El-Giza WTP. The means of rotavirus group A genome and infectious units removals were 1.8 ± 0.8 log10 and 1.8 ± 0.8 log10, respectively, after the final chlorination of El-Giza WTP. The means of rotavirus group A genome and infectious units removals were 2.86 ± 1.1 log10 and 2.57 ± 1.1 log10, respectively, after the whole treatment processes of El-Giza WTP. In the same time, it was not easy to estimate the capability of each treatment step of rotavirus group C removal either in water or wastewater treatment plants. Also, complete absence of rotavirus group C genome copies in treated sewage effluents (after chlorination) and in drinking water samples (after chlorination) was observed. It may return to the low number of positive raw sewage and Nile water samples and the low number of viral genome copies in these samples. On the other hand, complete absence of the infectious units of rotavirus group C in all positive samples was observed. It may be because of the very low number of the viral genome. Infectious units of enteric viruses are usually 2–3 log10 lower than genome copies in Egyptian sewage or Nile water samples (El-Senousy et al. 2007; El-Senousy et al. 2013a). Another reason may be the low sensitivity of MA104 cell line to the infectivity of rotavirus group C. Rotavirus group A genome copy numbers in the final chlorinated sewage effluents ranged from 9 × 10 to 9 × 103 genome copies/l, while the number of infectious units in the same samples ranged from 1 × 10 to 6 × 10 infectious units/l. Current wastewater treatments do not ensure complete virus removal (Blatchley et al. 2007; Bosch 2007; El-Senousy et al. 2013b); hence, viruses become environmental contaminants in numbers high enough to represent a public health threat, although low enough to pose serious difficulties for their detection (El-Senousy et al. 2013b). The high prevalence of rotavirus group A in Nile water samples is a direct consequence of either direct viral pollution of different human activities like waste of steamers or contamination of the river with treated and untreated sewage. Rotavirus group A RNA was detected three times in drinking water samples with genome copy numbers ranged from 8 × 10 to 8 × 102 genome copies/l, but no infectious units were recorded in all samples. Detection of genome copies in drinking water samples even without infectivity may result from some defects in the treatment processes of the water treatment plant. Few studies about rotaviruses group C in water and wastewater were published. In a recent study in Brazil, rotavirus group C was detected in 32 of 35 (91.4 %) influent samples and 20 of 35 (57.1 %) effluent samples. The detection rates of group C rotavirus varied among the treatment plants; 17 of 17 raw samples and 9 of 17 treated samples were positive in plant A, while the detection rate was 5 of 6 for both the influent and effluent samples from plant B and 4 of 6 for both the raw and treated sewage samples from plant C. In plant D, 6 of 6 raw samples and 3 of 6 treated samples were positive (Meleg et al. 2008).
The similarity between the sequences of rotavirus group C VP6 in both clinical specimens and environmental samples confirmed the circulation of this virus in the Egyptian community; however, this is the first report to detect, quantify, and genotype rotavirus group C in Egyptian clinical or environmental samples. Seven samples (four clinical and three environmental) showed similar sequences clustered with Moduganari\Human Nigerian strain AF 325806 with 98 % homology, and three samples (two clinical and one environmental) showed similar sequences clustered with Dhaka CB\ Human Bangladesh strain AY 754826 with 97 % homology. The similarity with African or south east Asian strains may be because of the circulation of viruses between different countries in the same or in the different continents by travelers and tourists.
More studies concerning the molecular epidemiology of rotaviruses groups B and C in addition to group A in Egyptian clinical specimens and environmental samples are still needed.
References
Abad, F. X., Pintó, R. M., Villena, C., Gajardo, R., & Bosch, A. (1997). Astrovirus survival in drinking water. Applied and Environment Microbiology, 63, 3119–3122.
Banyai, K., Jiang, B., Bogdán, A., Horváth, B., Jakab, F., Meleg, E., et al. (2006). Prevalence and molecular characterization of human group C rotaviruses in Hungary. Journal of Clinical Virology, 37, 317–332.
Blatchley, E. R, I. I. I., Gong, W. L., Alleman, J. E., Rose, J. B., Huffman, D. E., Otaki, M., & Lisle, J. T. (2007). Effects of wastewater disinfection on waterborne bacteria and viruses. Water Environment Research, 79, 81–92.
Bosch, A. (1998). Human enteric viruses in the water environment: A minireview. International Microbiology, 1, 191–196.
Bosch, A. (2007). Human viruses in water (1st ed.). Amsterdam: Elsevier.
Cook, N., Bridger, J., Kendall, K., Iturriza-Gómara, M., El-Attar, L., & Gray, J. (2004). The zoonotic potential of rotavirus. Journal of Infection, 48, 289–302.
Cunliffe, N. A., Dove, W., Jiang, B., Thinwda Cert, B. D., Broadhead, R. L., Molyneux, M. E., & Hart, C. A. (2001). Detection of group C rotavirus in children with acute gastroenteritis in Blantyre, Malawi. The Pediatric Infectious Disease Journal, 20, 1088–1090.
El-Senousy, W. M., Barakat, A. B., Ghanem, H. E., & Kamel, M. A. (2013a). Molecular epidemiology of human adenoviruses and rotaviruses as candidate viral indicators in the Egyptian sewage and water samples. World Applied Sciences Journal, 27, 1235–1247.
El-Senousy, W. M., Costafreda, M. I., Pintó, R. M., & Bosch, A. (2013b). Method validation for norovirus detection in naturally contaminated irrigation water and fresh produce. International Journal of Food Microbiology, 167, 74–79.
El-Senousy, W. M., & El-Mahdy, E. M. (2009). Detection and genotyping of rotaviruses in water treatment plants of El-Dakahlia Governorate. Egyptian Journal of Biotechnology, 31, 25–34.
El-Senousy, W. M., Guix, S., Abid, I., Pintó, R. M., & Bosch, A. (2007). Removal of astrovirus from water and sewage treatment plants, evaluated by a competitive reverse transcription-PCR. Applied and Environment Microbiology, 73, 164–167.
El-Senousy, W.M., Pintó, R.M., & Bosch, A. (2004). Epidemiology of human enteric viruses in the Cairo water environment. (Paper presented at the 1st International Conference of Environmental Research Division on Sustainable Development Environmental Challenges Facing Egypt. National Research Centre, Cairo, Egypt).
El-Senousy, W. M., Shahein, Y. E., Barakat, A. B., Ghanem, H. E., El-Hakim, A. E., & Ameen, S. M. (2013c). Molecular cloning and immunogenicity evaluation of rotavirus structural proteins as candidate vaccine. International Journal of Biological Macromolecules, 59, 67–71.
Estes, M. K. (2001). Rotaviruses and their replication. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, & S. E. Straus (Eds.), Fields virology (4th ed., Vol. 2, pp. 1747–1785). Philadelphia: Lippincott Williams and Wilkins.
Gabbay, Y. B., Jiang, B., Oliveira, C. S., Mascarenhas, J. D., Leite, J. P., Glass, R. I., & Linhares, A. C. (1999). An outbreak of group C rotavirus gastroenteritis among children attending a day-care center in Belem, Brazil. Journal of Diarrhoeal Diseases Research, 2, 69–74.
Gallimore, C. I., Taylor, C., Genney, A. R., Cant, A. J., Galloway, A., Iturriza-Gomara, M., & Gray, J. J. (2006). Environmental monitoring for gastroenteric viruses in a pediatric primary immunodeficiency unit. Journal of Clinical Microbiology, 44, 395–399.
Gentsch, J. R., Laird, A. R., Bielfelt, B., Griffin, D. D., Bányai, K., Ramachandran, M., et al. (2005). Serotype diversity and reassortment between human and animal rotavirus strains: Implications for rotavirus vaccine programs. Journal of Infectious Diseases, 192, 146–159.
Ghazy, M. M. E., El-Senousy, W. M., Abdel-Aatty, A. M., Hegazy, B., & Kamel, M. (2008). Performance evaluation of a waste stabilization pond in a rural area in Egypt. American Journal of Environmental Sciences, 4, 316–326.
http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/. Accessed Oct 2014.
http://www.who.int/vaccine_safety/committee/topics/rotavirus/rotashield/en/. Accessed 30 Nov 2010.
Iturriza Gomara, M., Wong, C., Blome, S., Desselberger, U., & Gray, J. (2002). Molecular characterization of VP6 genes of human rotavirus isolates: Correlation of genogroups with subgroups and evidence of independent segregation. Journal of Virology, 76, 6596–6601.
James, V. L. A., Lambden, P. R., Caul, E. O., Cooke, S. J., & Clarke, I. N. (1997). Seroepidemiology of human group C rotavirus in the UK. Journal of Medical Virology, 52, 86–91.
Jiang, B., Dennehy, P. H., Spangenberger, S., Gentsch, J. R., & Glass, R. I. (1995). First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. Journal of Infectious Diseases, 172, 45–50.
Kang, G., Iturriza-Gomara, M., Wheeler, J. G., Crystal, P., Monica, B., Ramani, S., et al. (2004). Quantitation of group A rotavirus by real-time reverse-transcription-polymerase chain reaction. Journal of Medical Virology, 73, 118–122.
Kapikian, A. Z., & Chanock, R. M. (1996). Rotaviruses. In B. N. Fields, D. M. Knipe, & P. M. Howley (Eds.), Fields virology (3rd ed., pp. 1657–1708). Philadelphia, PA: Lippincott-Raven Publishers.
Katzenelson, E., Fattal, B., & Hostovesky, T. (1976). Organic flocculation: An efficient second-step concentration method for the detection of viruses in tap water. Applied and Environment Microbiology, 32, 838–839.
Kuzuya, M., Fujii, R., Hamano, M., Ohata, R., Ogura, H., & Yamada, M. (2001). Seroepidemiology of human group C rotavirus in Japan based on blocking enzyme linked immunosorbent assay. Clinical and Diagnostic Laboratory Immunology, 8, 161–165.
Kuzuya, M., Hamano, M., Nishijima, M., Fujii, R., Ogura, H., Tanaka, M., et al. (2005). An outbreak of acute gastroenteritis caused by human group C rotavirus in a welfare institution in Okayama prefecture. Japanese Journal of Infectious Diseases, 58, 255–257.
Laine, J., Huovinen, E., Virtanen, M. J., Snellman, M., Lumio, J., Ruutu, P., et al. (2011). An extensive gastroenteritis outbreak after drinking-water contamination by sewage effluent. Finland. Epidemiology and Infection, 139, 1105–1113.
Leclerc, H., Schwartzbrod, L., & Dei-Cas, E. (2002). Microbial agents associated with waterborne. Critical Reviews in Microbiology, 28, 371–409.
Logan, C., O’Leary, J. J., & O’Sullivan, N. (2006). Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. Journal of Clinical Microbiology, 44, 3189–3195.
Maunula, L. (2007). Waterborne norovirus outbreaks. Future Virology, 2, 101–112.
Meleg, E., Ba´nyai, K., Martella, V., Jiang, B., Kocsis, B., Kisfali, P., et al. (2008). Detection and quantification of group C rotaviruses in communal sewage. Applied and Environment Microbiology, 74, 3394–3399.
Nilsson, M., Svenungsson, B., Hedlund, K. O., Uhnoo, I., Lagergren, A., Akre, T., & Svensson, L. (2000). Incidence and genetic diversity of group C rotavirus among adults. Journal of Infectious Diseases, 182, 678–684.
Offit, P. A., & Clark, H. F. (2000). Rotavirus. In G. L. Mandel, J. E.439 Bennet, & R. Dolin (Eds.), Principles and Practice of Infectious Diseases (5th ed., pp. 1696–1703) NewYork: Churchill Livingstone.
Parashar, U. D., Burton, A., Lanata, C., Boschi-Pinto, C., Shibuya, K., Steele, D., et al. (2009). Global mortality associated with rotavirus disease among children in 2004. Journal of Infectious Diseases, 200, 9–15.
Phan, T. G., Nishimura, S., Okame, M., Nguyen, T. A., Khamrin, P., Okitsu, S., et al. (2004). Virus diversity and an outbreak of group C rotavirus among infants and children with diarrhoea in Maizuru city, Japan during 2002-2003. Journal of Medical Virology, 74, 173–179.
Ramani, S., & Kang, G. (2009). Viruses causing childhood diarrhea in the developing world. Current Opinion in Infectious Diseases, 22, 477–482.
Rodger, S. M., Bishop, R. F., & Holmes, I. H. (1982). Detection of a rotavirus-like agent associated with diarrhea in an infant. Journal of Clinical Microbiology, 16, 724–726.
Rose, J. B., Singh, S. N., Gerba, C. P., & Kelley, L. M. (1984). Comparison of microporous filters for concentration of viruses from waste water. Applied and Environment Microbiology, 47, 989–992.
Saif, L. J., & Jiang, B. (1994). Nongroup A rotaviruses of humans and animals. Current Topics in Microbiology and Immunology, 185, 339–371.
Sánchez-Fauquier, A., Roman, E., Colomina, J., Wilhelmi, I., Glass, R. I., & Jiang, B. (2003). First detection of group C rotavirus in children with acute diarrhea in Spain. Archives of Virology, 148, 399–404.
Santosham, M., Chandran, A., Fitzwater, S., Fischer-Walker, C., Baqui, A. H., & Black, R. (2010). Progress and barriers for the control of diarrhoeal disease. Lancet, 376, 63–67.
Schnagl, R. D., Boniface, K., Cardwell, P., McCarthy, D., Ondracek, C., Coulson, B., et al. (2004). Incidence of group C human rotavirus in central Australia and sequence variation of the VP7 and VP4 genes. Journal of Clinical Microbiology, 42, 2127–2133.
Smith, E. M., & Gerba, C. P. (1982). Development of a method for detection of human rotavirus in water and sewage. Journal of Applied and Environmental Microbiology, 43, 1440–1450.
Steele, A. D., & James, V. L. A. (1999). Seroepidemiology 463 of human group Crotavirus in South Africa. Journal of Clinical Microbiology, 37, 4142–4144.
Szucs, G., Kende, M., & Uj, M. (1987). Atypical human rotaviruses in Hungary. Annales de l’Institut Pasteur. Virology., 138, 391–395.
Velazquez, F. R., Matson, D. O., Calva, J. J., Guerrero, L., Morrow, A. L., Carter-Campbell, S., et al. (1996). Rotavirus infection in infants as a protection against subsequent infections. The New England Journal of Medicine, 335, 1022–1028.
Wilhelmi, I., Roman, E., & Sánchez-Fauquier, A. (2003). Viruses causing gastroenteritis. Clinical Microbiology and Infection, 9, 247–262.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Senousy, W.M., Ragab, A.M.ES. & Handak, E.M.A.E.H. Prevalence of Rotaviruses Groups A and C in Egyptian Children and Aquatic Environment. Food Environ Virol 7, 132–141 (2015). https://doi.org/10.1007/s12560-015-9184-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9184-6