Abstract
Enteric viruses are a cause of waterborne disease worldwide, and low numbers in drinking water can present a significant risk of infection. Because the numbers are often quite low, large volumes (100–1,000 L) of water are usually processed. The VIRADEL method using microporous filters is most commonly used today for this purpose. Negatively charged filters require the addition of multivalent salts and acidification of the water sample to effect virus adsorption, which can make large-volume sampling difficult. Positively charged filters require no preconditioning of samples, and are able to concentrate viruses from water over a greater pH range than electronegative filters. The most widely used electropositive filter is the Virosorb 1MDS; however, the Environmental Protection Agency has added the positively charged NanoCeram filters to their proposed Method 1615. Ultrafilters concentrate viruses based on size exclusion rather than electrokinetics, but are impractical for field sampling or processing of turbid water. Elution (recovery) of viruses from filters following concentration is performed with organic (e.g., beef extract) or inorganic solutions (e.g., sodium polyphosphates). Eluates are then reconcentrated to decrease the sample volume to enhance detection methods (e.g., cell culture infectivity assays and molecular detection techniques). While the majority of available filters have demonstrated high virus retention efficiencies, the methods to elute and reconcentrate viruses have met with varying degrees of success due to the biological variability of viruses present in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to assess the occurrence of viruses in water, it has long been recognized that the best methods will be those that are simple, rapid, inexpensive, and consistent (Hill et al. 1971; Wallis et al. 1979). While a number of techniques have been developed and refined, it has proven difficult to achieve the detection of all relevant virus types over the spectrum of water quality matrices that exist in nature and human-constructed facilities. The objective of this review is to discuss the primary concentration, elution, and secondary concentration methods that have been developed over the past several decades in an effort to enhance the detection of pathogenic enteric viruses in water by traditional cell culture and molecular techniques.
Early Concentration Techniques
A number of methods were developed during the 1960s and 1970s for the concentration of enteric viruses (Table 1). The majority of these were for recovering viruses from small volumes of water. Aqueous polymer two-phase separation employed liquid–liquid partitioning to stimulate movement of viruses from water samples to an organic solution [e.g., dextran and polyethylene glycol (PEG)]. Although a number of research groups including Shuval et al. (1967, 1969) reported promising results as indicated in a review by Hill et al. (1971), the method was troublesome due to the minimal sample volumes that could be processed (usually ~1 L) and the reported inhibition of some echovirus and Coxsackievirus serotypes (Grinrod and Cliver 1970). Hydroextraction is a method which requires placement of the water sample into a dialyzing bag. Its volume is subsequently reduced by a hydrophilic agent surrounding the bag (usually PEG) that extracts water from the sample. While Shuval et al. (1967) reported a recovery efficiency of 40–50 %, there were several problems associated with the procedure. In addition to viruses, toxic substances present in the water samples were also concentrated in the remaining aqueous volume which resulted in cell culture toxicity. PEG was also found to counter-dialyze and contaminate the water sample.
Poliovirus was concentrated from various water sources using soluble ultrafilters composed of sodium alginate (Gartner 1967; Nupen 1970). The filters were subsequently dissolved in sodium citrate to recover the virus, and then applied to cell culture infectivity assays for detection. These filters proved ideal for low turbidity waters, but samples required pretreatment by passage through a membrane filter for more turbid natural waters. This resulted in a loss of solid-associated viruses (Gartner 1967), while their use with sewage water resulted in clogging of the filter (Nupen 1970). Ultracentrifugation was also attempted (Cliver and Yeatman 1965), but the method had little practical value due to the elevated cost of equipment, the ability to process only small water volumes, and excessive processing times (Hill et al. 1971). Although aqueous polymer two-phase separation, hydroextraction, soluble ultrafiltration, and ultracentrifugation worked well for bench-scale water quality experiments, they were not feasible for processing large volumes of water (Grabow 1968; Rao and Labzoffsky 1969).
Grab sample collection was one of the earliest approaches to evaluating viral presence in water, but proved most ideal for sewage containing high viral loads (Kelly 1953; Melnick et al. 1954; Gravelle and Chin 1961). The method was deemed only partially quantitative and impractical, however, for water sources containing few viruses (e.g., natural surface waters and tap water) (Grabow 1968). For processing larger water volumes, adsorbents such as polyelectrolytes, precipitable salts, and ferric oxides were evaluated. Co-polymer maleic anhydride-based electrolytes were investigated for their capacity to remove viruses from distilled water (Johnson et al. 1967), sewage, and streams receiving sewage effluents (Grinstein et al. 1970). Wallis et al. (1970) investigated the virus retention capacity of “sandwiched” insoluble polyelectrolyte layers (crosslinked isobutylene maleic anhydride copolymers; Monsanto Co., St. Louis, MO) from tap water. When preceded by an AP 20 fiberglass prefilter pad (Millipore, Billerica, MA), the polyelectrolyte layers adsorbed poliovirus 1 efficiently (≥90 %) over a wide pH range (5.0–8.4). A comprehensive review by Hill et al. (1971) describes additional studies employing polyelectrolytes, but indicates that their inefficient virus adsorption from turbid waters would result in increased equipment costs due to the need for sample prefiltration. Preformed flocs of precipitable salts including aluminum hydroxide [Al(OH)3], aluminum phosphate (AlPO4), and calcium phosphate (CaHPO4) effectively concentrated several enterovirus types including poliovirus 1 (Mahoney strain) and echovirus 7 (Wallis and Melnick 1967b). Nevertheless, adenovirus only adsorbed to Al(OH)3 and reovirus concentration was not achieved with any of the salt precipitates evaluated.
Gauze pads composed of cotton fibers were employed for the concentration of poliovirus from large volumes of water (5,000–8,000 L) originating from well and surface supplies during an epidemic in Paris, France (Coin 1967); however, the method proved to be primarily qualitative as it was difficult to determine the total volume of water that had passed through the material and the proportion of viruses that had been adsorbed relative to the actual numbers present in the surrounding waters (Coin 1967; Grabow 1968; Hill et al. 1971). In addition, the efficiency of cotton as a virus adsorbent was believed to be poor due to its large porosity which resulted in higher flow rates (Rao and Labzoffsky 1969). Later research by Liu et al. (1971) involved the design and construction of a flow-through gauze sampler for water-based field studies. While the sampling and recovery procedures were relatively simple and the device was inexpensive and effective for sampling large volumes of water, viruses suspended in tap water adsorbed poorly compared to viruses in seawater. The addition of sodium chloride (NaCl) salt to the tap water greatly increased the ability of poliovirus to adsorb to the cotton fibers.
Electronegative Filtration Media
The types of electronegative filtration media that have been used are summarized in Table 2.
The use of membrane filters to concentrate viruses began to increase as they had a greater potential to process large volumes of water (Anderson et al. 1967; Stevenson 1967). The ability of membrane filters to recover viruses from aqueous suspensions was first reported by Metcalf (1961), who found that cellulose nitrate HA membranes (Millipore) retained influenza viruses despite the pore size exceeding that of the viruses. It was later discovered that coating the cellulose nitrate membranes with proteinaceous or gelatin-based substances enhanced virus passage through the membrane as reactive sites were occupied by the coatings (Cliver 1965). Attempts to utilize the cellulose nitrate HA Millipore membrane (0.45-μm pore size) as an enteric virus concentrator were realized by the addition of low molarity concentrations of NaCl and magnesium chloride (MgCl2) to sample waters (Wallis and Melnick 1967a; Rao and Labzoffsky 1969). Adsorption of these salts to the cellulose nitrate membrane during passage facilitated the attachment of poliovirus 1 via salt-bridging, with lower concentrations of divalent ions required relative to greater amounts of monovalent ions (e.g., a lower amount of MgCl2 is required in comparison to NaCl). Wallis and Melnick (1967a) adjusted the suspension medium to pH 5 (similar to the isoelectric point of poliovirus 1, Mahoney strain), which also enhanced the adsorption capacity. Using McIlvaine’s Buffer (0.05 M Na2HPO4·7H2O, pH 7.0), Berg et al. (1971) concentrated poliovirus 1, reovirus 1, Coxsackievirus B3, and echovirus 7 from relatively clean raw waters with high efficiencies; however, the presence of organic materials ranging from moderate levels in clean surface waters to high levels in sewage, led to less efficient virus concentration. This was attributed to the preferential adsorption of the organic ‘membrane coating components’ that allowed for the viruses to pass through the filter and into the filtrate (Wallis and Melnick 1967c; Berg et al. 1971). Even tap waters have small quantities of dissolved organic materials (largely humic acids) that can clog primary adsorbent filters during the passage of large volumes (Farrah et al. 1976b). Placement of a clarifying filter (e.g., Millipore AP 25 prefilter pads) ahead in series with the cellulose nitrate Millipore membranes alleviated this obstacle, thereby enhancing the adsorption of viruses from 500 mL volumes of raw lake water (Rao and Labzoffsky 1969). The use of prefiltration to remove suspended organic solids, combined with the addition of trivalent aluminum salts [AlCl3, Al2(SO4)3] to tap water samples facilitated the adsorption of enteroviruses to cellulose nitrate membrane filters (Wallis et al. 1972a). Trivalent salts were favorable since much less was needed when compared to MgCl2. The use of these salts, however, required acidification of the samples before their addition due to the formation of insoluble aluminum hydroxide gels that form at pH values of 5 and higher.
The ability of both insoluble polyelectrolyte layers (PE 60) and membrane filters (Millipore HA) to process test waters at higher flow rates prompted the development of a field sampling device designed to filter large volumes of water (Wallis et al. 1972b) that would become the model for subsequent studies (Farrah et al. 1976a). Since the primary adsorbent filter was subject to clogging, a series of prefilters and resins were used to reduce turbidity and remove organic matter from the water. Processing 400 gallons (~1,500 L) of water resulted in a buildup of particulate matter (largely silts) on the clarifiers and subsequent clogging of the filters used for virus adsorption. Hill et al. (1972) also sought to determine the efficacy of membrane filtration (Millitube MF cartridge filter) in the concentration of low levels of poliovirus 1 from 100-gallon (378 L) volumes of tap water. No virus was detected in the filtrate (effluent) when the water samples were preconditioned with Ca2+ or Mg2+ cations (500 and 1,200 μg/mL, respectively). It was later demonstrated that the mere acidification of 100-gallon tap water samples to pH 4.0 via a simple injector eliminated the need for salts to facilitate virus adsorption to the filter surface (Sobsey et al. 1973).
This treatment was more effective for an epoxy-fiberglass filter (Cox) in comparison to the HA cellulose nitrate filter (Millipore). In addition, the placement of a K27 wound fiberglass depth filter (Carborundum Co., Lebanin, IN) in series with the Cox filter eliminated the need for a clarifying device. An all-inclusive instrument served each of the necessary functions for conditioning the waters before sampling including a fluid proportioner for pH adjustment and maintenance of the ionic strength by the addition of salts to the sample water (Hill et al. 1974). Fitted with a concentrator of choice (e.g., Cox epoxy fiberglass, Millipore cellulose nitrate membrane, or K27 fiberglass depth filter), the device facilitated the concentration of Coxsackieviruses A9 and B1, reovirus 1, and poliovirus 1 with minimal loss, as demonstrated by periodic assessment of effluent samples. This pump system, used in conjunction with the Balston epoxy fiberglass filter, was capable of concentrating viruses from 100-gallon (378 L) volumes of potable water (Jakubowski et al. 1974).
Cellulose nitrate membranes (Millipore), the epoxy-fiberglass Cox filter, the K27 fiberglass depth filter + Cox filter in series, and the Balston epoxy-fiberglass devices were compared by a number of parameters including size, cost, and their general advantages and disadvantages (Jakubowski et al. 1975). Combined with their housings, the Millipore and Cox filters were the bulkiest and exhibited the greatest initial costs; the Balston filter was the smallest and least expensive of the four. While the Millipore membrane filter had been the most researched to that point, it had proven very fragile, and was subject to clogging during the experiments. The Cox filter was more durable, but bulky and subject to leaking. The series filter device (K27 + Cox) was capable of removing interfering substances, yet exhibited the lowest flow rate. Despite exhibiting the highest flow rate, the Balston filter’s cadmium-coated center posts were subject to corrosion under the acidic conditions required for virus adsorption with aluminum salts; nevertheless, it proved effective in the simultaneous concentration of four enterovirus types from varying water matrices (Guttman-Bass and Nasser 1984), and the concurrent adsorption of both enteroviruses and Salmonella from surface water (Rolland and Block 1980).
Employing the Wallis-Melnick virus concentrator model (Wallis et al. 1972b) with additional modifications (Sobsey et al. 1973), Farrah et al. (1976a) sought to investigate potential replacements for the epoxy-fiberglass–asbestos Cox filter as the flow rates tended to decrease rapidly due to clogging as larger water volumes were sampled. The larger surface area of the pleated glass fiber melamine-impregnated epoxy Filterite filter (Filterite Corp., Timonium, MD) allowed for processing of seven times the volume of tap water relative to the Acropor (Ann Arbor, MI), Millipore, and Cox filters. The Filterite device also exhibited higher flow rates (5–40 L/min), particularly when preceded by a K27 clarifying prefilter. Similar to other electronegative filters, the adsorption of viruses to the Filterite filter was enhanced in the presence of trivalent Al3+ salts. The filter could also be reused after treatment with 0.1 N NaOH to remove the residual organic material and inactivate residual viruses. In addition to tap water, the Filterite filter could effectively retain polioviruses present in turbid estuarine water at an average flow rate of six gallons per minute (Payment et al. 1976), with virus retention efficiencies ranging from ~75 % (no sample preconditioning) to 100 % (sample acidification to pH 3.5 + 0.05 M AlCl3). Further testing of a pleated Filterite filter with in-line acid/salt injectors confirmed its ability to rapidly process large volumes of tap water, seawater, and secondary treated sewage, as well as to resist clogging relative to Balston tube filters and Millipore flat-disk membrane filters (Gerba et al. 1978).
The tendency of membrane filters to clog, coupled with the rising costs of pleated cartridge filters (e.g., Filterite filters), prompted the further evaluation of alternative electronegative virus adsorbent materials. In an effort to build a low-cost concentrator, Sarrette et al. (1977) employed negatively charged glass powder (Sovirel Glass Co., France) for the adsorption of poliovirus 1 in a Pyrex glass column apparatus. Microfiberglass filter disks produced by several manufacturers were mostly found to adsorb poliovirus 1 efficiently, although prefiltration was required for at least one model (Payment and Trudel 1979). Payment and Trudel (1988) later tested wound fiberglass depth filters (Diamond filter tubes; Filterite Corp., Timonium, MD) in an effort to find a low cost alternative to the pleated Filterite filter. Two 1-μm wound fiberglass filters placed in series adsorbed poliovirus 1 with the same efficiency (>99 %) as a 3-μm wound fiberglass filter placed first in series with a 0.2-μm pleated Filterite filter.
Although the pleated Filterite filter represented significant progress due to its ability to process large water volumes rapidly, new electropositive filters were becoming increasingly available for enteric virus concentration. During much of this time, most studies used Filterite filters as a negatively charged model for comparison with the new positively charged concentrators, or to further elucidate the factors affecting virus adsorption to filter surfaces. These studies are discussed in the Section entitled “Electropositive Filtration Media” of this review. Nevertheless, the need remained for an inexpensive method using electronegative filters to concentrate both enteric viruses and bacteriophages from large volumes of water due to the elevated costs associated with positively charged filters.
Nonetheless, acidifying water samples (pH 3.5) to enhance virus adsorption reportedly inactivated coliphages (Goyal et al. 1980). It was later discovered that preincubating both laboratory and indigenous (originating from raw sewage) strains of bacteriophages in 0.1 M manganese chloride (MnCl2) minimized the inactivation upon exposure to acidified water samples (pH 3.5, final MnCl2 concentration = 0.0001 M), allowing for the adsorption of PRD1 and MS2 bacteriophages to Filterite filters at efficiencies of 96.3 % (±2.1 %) and ≥99.9 %, respectively (Scott et al. 2002).
The implementation of advanced molecular detection techniques has seen a renewed interest in the development of adsorption–elution methodologies incorporating low cost, negatively charged Millipore cellulose nitrate membrane filters (0.45 μm). In one study, Millipore membranes recovered 80 % of seeded poliovirus 1 from 100-L samples (pH 3.5, 0.5 mM AlCl3), compared to granular activated carbon columns (74 %) for the eventual detection by reverse-transcriptase polymerase chain reaction (RT-PCR) (Jothikumar et al. 1995). Myrmel et al. (1999) slightly modified this procedure to concentrate human caliciviruses on Millipore membranes. Preconditioning the water samples to pH 5 with the addition of MgCl2 resulted in the strongest PCR amplification signal, demonstrating the importance of primary concentration parameters for optimal virus detection using molecular methods. Several enterovirus concentration procedures were evaluated using Millipore nitrocellulose membranes, a second electronegative filter (NM04701 020SP; CUNO, Meriden, CT), and the 1MDS Virosorb positively charged filter (Hsu et al. 2007). Detection by Most Probable Number-RT-PCR (MPN-RT-PCR) and real-time RT-PCR revealed that the most effective concentration methods employed the nitrocellulose membrane (Millipore) or the electronegative NM04701 020SP membrane (CUNO) coupled with an acid rinse (0.5 mM H2SO4) to elute the viruses.
Electropositive Filtration Media
The types of electropositive filtration media that have been used are summarized in Table 3.
Although electronegative filters had originally proven effective for the removal of viruses from water, their use required either acidification of or the addition of polyvalent salts to the water before concentration, which was difficult for large volume processing. Electrophoretic studies by Kessick and Wagner (1978) demonstrated that various types of adsorptive media were negatively charged in the pH range of 2–7, and that most exhibited very low isoelectric point values (average pI = 2). The negative charge of most viruses at neutral pH required the addition of polyvalent salts and/or HCl to precondition the water samples, thus imparting a positive charge to the filter to enhance the concentration of viruses. Field sampling equipment required expensive injector devices to enhance viral adsorption, although Payment and Trudel (1988) did develop a relatively low-cost device for this purpose. The conditioning of water could be tedious and unreliable due to the need to predict salt and/or acid requirements when dealing with the spectra of water quality present in both natural and treated waters. Positively charged filter media thus presented an alternative to complications associated with the use of highly electronegative virus adsorbents.
In Situ Charge Modification of Electronegative Adsorbent Materials
Several research groups led efforts to enhance the adsorption capacity of negatively charged surfaces to alleviate the need for pH or ionic strength adjustments of the test waters. A study by Zerda et al. (1985) demonstrated the ability of silica particles charged modified with primary amines, quaternary amines, and carboxyl groups to adsorb various enteroviruses and bacteriophages. The adsorption was found to correlate strongly with the pH of the dispersion medium (as it determines the ionization states of the adsorbents and the viruses). As reported in previous literature (Zerda et al. 1985), the isoelectric point (pI) of the viruses was also a significant factor in their attraction to the charge-modified silica surfaces. Viruses with a low pI (e.g., MS2 bacteriophage with a pI of 3.9) adsorbed to all three modified surface types at pH 4, which is near its pI. Increasing the pH of the medium, however, increased the negative net surface charge of MS2 and enhanced its adsorption to the positively charged primary amine and quaternary amine surfaces. Poliovirus 1 (Brunhilde strain, pI = 7.1) was also evaluated. At pH 4, its net surface charge was positive as the pH of the surrounding media was well below the virus isoelectric point. Thus, the virus preferentially adsorbed to the negatively charged carboxyl-modified silica surface. As the medium pH surpassed the isoelectric point for poliovirus 1, the virus acquired a negative net surface charge and adsorbed more readily to the positive charge-modified silica surfaces.
Poliovirus 1 was effectively concentrated from tap water using positively charged asbestos–cellulose filters (Seitz-grade S, Milldale, CT) and cellulose–diatomaceous earth charge-modified resin filter disks (50S and 60S, Zeta Plus; CUNO, Meriden, CT) with no requirement for salt addition (Sobsey and Jones 1979). Viruses adsorbed most efficiently in the pH ranges between 5.5 and 7.5 for the 50S filter and between 3.5 and 6.0 for the 60S filter, while the Seitz S filter adsorbed nearly 100 % of the virus from pH 3.5 to 9.0. The Filterite filter was also evaluated, adsorbing 93 % of the virus at pH 3.5, but an increase in pH of the water sample to 5.5 resulted in a substantial reduction in the filter’s ability to adsorb viruses in the absence of excess H+ and multivalent salts. The results of the Sobsey and Jones study (1979) supported the idea that electrostatic forces were instrumental in virus–filter interactions due to the correlations between zeta potential (i.e., electrokinetic potential) measured for the electronegative and positively charged adsorptive materials, and the retention efficiencies measured for each filter.
Previously, insoluble metallic salts including aluminum and magnesium had been observed to adsorb a number of virus types (Wallis and Melnick 1967c; Vilagines et al. 1982). Farrah and Preston (1985) were able to form in situ precipitated flocs of ferric and aluminum hydroxides within and on cellulose filters. Once dried, the modified filters were able to efficiently adsorb poliovirus 1 and MS2 coliphage relative to untreated filters at pH values of 5 and 7; adsorption was ineffective at pH 9 on both surface types. A subsequent study employing the same in situ precipitation method evaluated several Filterite electronegative systems and a broader spectrum of metallic salts (Toranzos et al. 1986). Flat disk, depth, and pleated microporous filters that were charge-modified were able to efficiently adsorb considerably greater numbers of enteroviruses and bacteriophages from 20-L dechlorinated tap water volumes than the non-modified control filters. The most effective combination of salts tested was ferric chloride with either aluminum or MgCl2; however, the percentage of virus adsorbed varied according to the filter type and the pH of the tap water. Epoxy fiberglass filters (Filterite) and G25 fiberglass prefilters (Fisher Scientific, Waltham, MA) soaked in an aqueous solution of cationic polymer (e.g., polyethyleneimine) and then air-dried were more effective at adsorbing viruses from water than the fiberglass surfaces alone (Preston et al. 1988), but the stability of these charge-modified filters was less than 2 weeks, making them less ideal for long-term storage before use.
A low-cost cation-coated filter method was developed for the concentration of noroviruses from dechlorinated tap water (Haramoto et al. 2004). Several electronegative filter types including cellulose nitrate HA filters (47 mm, Millipore) precoated with Al3+ ions adsorbed poliovirus 1 more efficiently from water than filters that did not receive the coating. Application of the Al3+ coating to a larger HA membrane (293 mm) allowed for the processing of large volumes of tap water (100–532 L). Noroviruses (genogroups I and II) were detected by TaqMan PCR in 4.1 and 7.1 % of the 98 samples collected, respectively. The surface area (518 cm2) of the 293-mm HA filters was much higher than that of the typically used 47-mm filters (9.6 cm2), decreasing the likelihood of clogging due to adsorption of dissolved organic materials in the water samples. The Al3+ cation method was also used to concentrate noroviruses, enteroviruses, adenoviruses, and torque teno viruses from river water receiving wastewater effluents (Haramoto et al. 2005). Nevertheless, the volume of the samples collected was much smaller (0.5 L) than for the tap water study (Haramoto et al. 2004) as such contaminated surface waters are characterized by higher viral loads. The processing of smaller volumes of surface water minimizes the potential for adsorption interference by dissolved organic matter. While the Al3+ cation-coated filter method is promising and inexpensive, it would likely necessitate a prefiltration step to process large volumes of natural surface waters typified by low numbers of viruses and high levels of organic material.
Glass Wool
Oiled sodocalcic glass wool is an adsorbent material capable of concentrating viruses from water due to its net positive charge and hydrophobicity (Vilagines et al. 1993). Large volumes of water can be processed without preconditioning (e.g., addition of multivalent salts and H+) with exceptions (Albinana-Gimenez et al. 2009a, b), and the glass wool is considerably less expensive than alternative electropositive filter media (e.g., Virosorb 1MDS). As glass wool filters are generally prepared in-house (usually by packing into stainless steel holders), they can be used in varying mass quantities and compacted to a desired bulk density. While many adsorption–elution studies have reported the virus retention efficiencies of various types of filtration media, those using glass wool have largely provided the elution (recovery) and secondary concentration efficiencies only. The recovery of poliovirus 1 (75 %) following concentration with glass wool (Vilagines et al. 1993) compared to the 25.5 % achieved by Menut et al. (1993) was attributed to the difference in the amount of glass wool used (50 g versus 5 g, respectively). A multi-laboratory collaborative study assessing the reproducibility of using glass wool to recover poliovirus 1 from drinking water and seawater reported elution efficiencies of 72 and 75 %, respectively (Vilagines et al. 1997). The adsorption efficiencies were not reported for these earlier studies employing glass wool, nor have they explicitly been stated in subsequent publications. In a study in our laboratory, the adsorption efficiencies for MS2 coliphage and poliovirus 1 were 56 % (±43 %) and 73 % (±32 %), respectively (Ikner and Bright, unpublished data), with high standard deviations. It therefore seems that virus adsorption to glass wool is quite variable.
Glass wool has largely been employed as part of an inexpensive and effective adsorption–elution methodology for monitoring the presence of viruses in several water matrices. Enterovirus detection via RT-PCR following adsorption to electronegative glass powder and glass wool revealed that the latter adsorbed greater amounts of organic material present in treated wastewater, resulting in subsequent assay inhibition not observed with the glass powder samples following secondary concentration (Gantzer et al. 1997). The inhibition of molecular techniques following the enhanced adsorption of organic matter (particularly humic acids) to positively charged media has been reported previously (Abbaszadegan et al. 1993; Kopecka et al. 1993). Seasonal monitoring of group A rotaviruses and enteroviruses in raw wastewater using glass wool for concentration saw the detection of viral RNA in 11 % of the samples each year of the study using RT-PCR (van Zyl et al. 2004) and 42.5 % using nested-PCR (Ehlers et al. 2005).
Gassilloud et al. (2003) compared the concentration of feline calicivirus F9 (FCV F9) and poliovirus 1 from tap water using an electropositive membrane (Zetapore NM047-01-045 SP) and glass wool, followed by both quantitative PCR (qPCR) and traditional cell culture infectivity assays for detection (Gassilloud et al. 2003). Membrane filtration facilitated a much greater recovery of infectious FCV F9 (75 %) than the glass wool method (0.5 %), with only a 5.3 % recovery of the genome, indicating that FCV F9-glass wool interactions were unfavorable. Group A rotaviruses were successfully detected using nested PCR in 6.5 % of the drinking water samples filtered through glass wool (van Zyl et al. 2004) and enteroviruses were found in 18.7 % of the finished water samples using nested-PCR for their detection (Ehlers et al. 2005). Several serotypes of adenoviruses (including 2, 40, and 41) were also detected in 5.3 % of the drinking water samples using conventional nested PCR followed by real-time PCR (van Heerden et al. 2005). Lambertini et al. (2008) reported a range of mean recoveries of several enteric viruses listed on the U.S. Environmental Protection Agency’s (USEPA) Contaminant Candidate List (CCL) from glass wool seeded with tap and/or well water samples (10 to >1,500 L) using qPCR for all of the viruses, and integrated cell culture PCR (ICC-PCR) to determine the presence of infectious poliovirus.
Surface waters have also been monitored using sodocalcic glass wool for group A rotaviruses (van Zyl et al. 2004), enteroviruses (Ehlers et al. 2005), and adenoviruses (van Heerden et al. 2005). More recently, Albinana-Gimenez et al. (2009a, b) found that preconditioning of tap water samples to pH 3.5 resulted in better recoveries of adenoviruses and JC polyomaviruses from glass wool filters, but did not apply this technique to 50-L tap and river water samples as it was deemed impractical. Adenoviruses and JC polyomaviruses were efficiently concentrated from river water samples using glass wool (10–50 L) for detection by real-time PCR. Glass wool therefore presents a viable and inexpensive option for the concentration and recovery of several enteric virus types from a broad spectrum of water matrices. It is also compatible for use with molecular detection techniques.
Virosorb 1MDS
The Virosorb 1MDS filter (CUNO, Meriden, CT) has been extensively researched for over 30 years, and recommended as an electropositive method for virus concentration by multiple agencies and organizations (Fout et al. 1996; American Public Health Association 2005). The electropositive surface-modified fiberglass–cellulose Virosorb 1MDS pleated cartridge filter (0.2-μm pore size) was evaluated relative to the Zeta Plus 50S filter disks (47 mm, CUNO) and the Filterite filter in small (3.8 L) and large volume experiments (1,000 L) (Sobsey and Glass 1980). The 50S Zeta Plus filter and the Filterite filter adsorbed poliovirus 1 equally well in the small volume experiments, supporting previous findings that water samples processed without preconditioning with the electropositive Zeta Plus 50S disk yielded similarly high virus adsorption efficiencies relative to Filterite. Adsorption efficiencies were also compared for the 1 MDS disk filter (single sheet thickness and double sheet thickness), Zeta-Plus 50S, and Filterite as a function of tap water pH. The Zeta-Plus filter exhibited the greatest virus adsorption capacity relative to the 1MDS (both single and double sheet), although all three media adsorbed ≥80 % of poliovirus between pH 3.5 and 7.5, with the efficiency declining as pH increased beyond this range.
The trend of decreasing poliovirus 1 adsorption in more alkaline tap water was later observed by Melnick et al. (1984), who recommended a pH adjustment to neutral for samples before processing with Virosorb 1MDS filters. This was explained in part by the electrophoretic mobility of the filter materials (Sobsey and Glass 1980). Virosorb 1MDS is slightly less electropositive than the Zeta-Plus 50S; both filters become more negatively charged as the water pH increases. The adsorptive capacity of Filterite becomes markedly reduced above pH 3.5, which has previously been reported (Sobsey and Jones 1979). Sobsey et al. (1981) reconfirmed the capability of the electropositive 1MDS Virosorb filter to adsorb poliovirus 1 efficiently from both large (1,000 L) and small (1.3 L) water volumes at ambient tap water pH levels (7.5) without the addition of salts or acidification of the sample relative to the Filterite filter at pH 3.5 with the addition of MgCl2. However, the small volume experiments revealed that echovirus 1 and reovirus 3 were more efficiently adsorbed by the Filterite filter under acidic conditions with added MgCl2 (99 and 100 %, respectively), with reovirus 3 also demonstrating 100 % adsorption without salt addition. Both filter types were able to process volumes of tap water at high flow rates (90 L/min), and accommodate storage at 4 °C without substantial loss of viruses. Nonetheless, a marked difference was observed in their capacity to filter secondary sewage before clogging occurred (Rose et al. 1984). Filterite (nominal pore size of 3.0 ± 0.45 μm) was capable of processing 19 L of secondary sewage compared to 1MDS (nominal pore size of 0.2 μm), which could only filter 11 L unless preceded by a clarifying filter (15 L).
Contrary to theoretical expectations that the electropositive filter would adsorb viruses more readily from clean water, the positively charged 1MDS filter adsorbed enteroviruses poorly from the surface waters evaluated (Hsu et al. 2007). However, this may have been due to the preconditioning of the water samples conducted in this study before filtration (pH 3.5, 50 mM MgCl2), which is normally not performed before processing samples using electropositive filters. Acidification and the addition of divalent salts would impart a positive electrical double layer around the negatively charged virus particles, leading to electrostatic repulsion with the positively charged filter surface.
NanoCeram Virus Sampler
Although Virosorb 1MDS has consistently demonstrated efficient virus adsorption from a range of water quality types for both small and large volumes, it is costly (~$250 USD). It is also relatively ineffective for the concentration of viruses from seawater, most likely due to the higher salt concentrations and pH levels relative to freshwater. The electropositive NanoCeram Virus Sampler (Argonide, Sanford, FL) has recently become available as a low cost alternative (~$45–$50 per unit). Similar to both the Filterite and 1MDS filters, NanoCeram is a pleated cartridge filter. It is composed of a non-woven matrix of microglass fibers (0.6 μm in length) coated with boehmite-derived nanoalumina fibers (~2 nm in diameter by ~250 nm in length). The nanoalumina (AlOOH) coating confers an extensive surface area to the NanoCeram filter (~500 m2/g), resulting in an effective pore size of ~2 μm.
Karim et al. (2009) reported a NanoCeram filter retention efficiency of poliovirus 1 (105 PFU) of 84 % (±9 %) from dechlorinated tap water (100 L) at a flow rate of 5.6 L/min. This adsorption efficiency is comparable to previously published reports for poliovirus 1 adsorption from large volumes of tap water using the 1MDS filter (Sobsey and Glass 1980; Sobsey et al. 1981), although NanoCeram (12.7 cm) is roughly half the size of a 1MDS filter (25.4 cm). Karim et al. (2009) also found that the NanoCeram filter capably adsorbed poliovirus 1 over the pH range of 6.0–9.5, relative to the 1MDS filter which demonstrates a substantial decrease in retention capacity above pH 7.5 (Sobsey and Glass 1980). This is likely attributed to the high isoelectric point of nanoalumina (~9.0), which would maintain an electropositive charge in aqueous systems characterized by slight to moderate alkalinity.
Ikner et al. (2011) reported a >99.8 % filter retention efficiency for MS2 coliphage, poliovirus 1, echovirus 1, Coxsackievirus B5, and adenovirus 2 from 20 L of dechlorinated tap water using a flow rate of 2.5 L/min. At a higher volume (100 L) and flow rate (5.7 L/min), the efficiency was slightly reduced, with retention of 96 % (±2 %) of poliovirus 1. In a study by Bennett et al. (2010), poliovirus 1 was also efficiently captured by the NanoCeram filter at neutral pH with no added salts. The MS2 coliphage adsorption efficiency from de-ionized water using NanoCeram (88 ± 5 %) was comparable to that with 1MDS under neutral pH conditions with no added salts (85 ± 8 %); however, both electropositive filters demonstrated lower adsorption efficiencies compared to the electronegative nitrocellulose Opticap filter, which efficiently captured MS2 coliphage (>99 %) from artificial seawater (0.1 M MgCl2). Coliphage Q β and adenovirus 41 were also evaluated for adsorption to NanoCeram from several water matrices. While both virus types were efficiently adsorbed to the NanoCeram from both dechlorinated finished water and natural seawater (>97 %) with a flow rate of 25 L/min, a decrease in retention efficiencies was measured with source water (~80 %). This may have been due to the turbidity (3.1 NTU) and dissolved organic content (4.87 mg/L) of the source water, which were higher than for the finished water (0.07 NTU, 2.34 mg/L) and seawater (2.4 NTU). There may have been preferential adsorption of the organic material to the filter, or enhanced association of the viruses with the organic solids.
NanoCeram filters have also been demonstrated to be effective for the recovery of noroviruses. In a study by Lee et al. (2011b), an eluting solution containing 1.5 % beef extract and 0.05 M glycine was able to recover greater numbers of murine norovirus (38.9 vs. 18.3 %) and human norovirus (61.1 vs. 26.5 %) from 1MDS than from NanoCeram disk (142 mm) filters, respectively. However, when 0.01 % tween 80 was added to the solution, the elution efficiencies increased for both murine norovirus (54.4 % from 1MDS vs. 23.4 % from NanoCeram) and human norovirus (67.5 % from 1MDS vs. 85.7 % from NanoCeram). In a subsequent study conducted in Korea using NanoCeram disk filters and beef extract/glycine elution without tween 80, human norovirus was detected via RT-PCR from 46 of 109 (43.4 %) groundwater samples (Lee et al. 2011a).
Based on the results of these numerous promising studies, the USEPA has recently included the NanoCeram filters as an alternative to 1MDS filters in their proposed Method 1615 concerning the detection of noroviruses and enteroviruses from surface waters and groundwaters (U.S. Environmental Protection Agency 2010).
Ultrafiltration (Molecular Filtration)
Ultrafiltration is an alternative to the use of adsorption–elution methods since it is based on size exclusion rather than electrostatic interactions between electronegative viruses and negatively or positively charged filter surfaces. It therefore does not require preconditioning of the water. Nevertheless, ultrafiltration requires more costly equipment, and is generally not feasible for field sampling relative to the virus adsorption and elution techniques. Ultrafiltration can also require additional operator training.
Although the concentration of viruses during ultrafiltration does not occur via electrostatic interactions, viruses may still become adsorbed to the ultrafilter membranes via van der Waals interactive forces and/or hydrophobic bonding. Organic solutions (e.g., calf serum, glycine, and beef extract) and sodium polyphosphates have been successfully employed as pretreatment or blocking solutions to prevent such viral adsorption to ultrafilter membranes (Berman et al. 1980; Hill et al. 2005). Alternately, prefiltration of samples using a 5-μm filter alleviated the need for preblocking, and resulted in high recoveries of poliovirus 3 (99 %) and echovirus 11 (100 %) by ultrafiltration (Garin et al. 1993).
Because of such viral adsorption, the recovery of viruses from ultrafilter membranes usually cannot occur by simply flushing the retentate volume remaining in the apparatus following concentration. Solutions capable of interfering with virus–membrane interactions have been employed in a reverse flow configuration (backflushing) to enhance the recovery of viruses. Jansons and Bucens (1986) found that the addition of 10 % beef extract to the retentate followed by backflushing gave the highest recovery of poliovirus 1 from groundwater processed through Amicon DC30EM ultrafilters (Amicon Corp., Danvers, MA). Several other solutions have been utilized to aid in virus recovery from ultrafilters including varying concentrations of glycine (Berman et al. 1980; Winona et al. 2001; Olszewski et al. 2005), 3 % beef extract (Garin et al. 1993), alternating solutions of 0.1 HNO3 and 0.1 M NaOH (Soule et al. 2000), and sodium polyphosphates (Hill et al. 2005, 2007, 2009; Polaczyk et al. 2008).
There are several ultrafiltration methodologies currently in use that differ principally by the type of flow currents generated within the apparatus to effectively facilitate the separation of particles by their molecular weights. These include dead-end flow and various types of cross flow (e.g., tangential flow, vortex flow) ultrafiltration. There are also several types of filter geometries that are used for ultrafiltration such as spiral wound, tubular, and hollow fiber membranes; plate-and-frame (flat sheet) systems are also infrequently used (Wagner 2001).
Filter Geometries
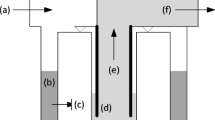
Spiral wound membranes have layers of filter material wound around a central permeate collection tube. They are the least expensive type of ultrafilter available and thus are used widely in the water industry (Wagner 2001). Tubular membranes have a wide central channel where the water sample is fed. The concentrated sample is collected on the far side. The filter permeate (waste) is collected on the outside of the tubular ultrafilter in the filter housing. Plate-and-frame (flat sheet) systems all use flat sheet membranes; however, the configuration of these membranes and the plates varies widely between brands (Wagner 2001). With hollow fiber ultrafiltration, the water sample is fed through the fibers of the membrane and the filter permeate is collected in the filter housing. The flow can be from inside to outside or vice versa. The viruses are concentrated within the hollow fibers of the membrane (retentate).
The earliest ultrafiltration methods operated via reverse osmosis and employed flat, asymmetrical cellulose acetate membranes with a specified molecular weight cutoff (MWCO) that were subject to random incidences of viral penetration (Sweet et al. 1971; Sorber et al. 1972). A significant loss of poliovirus 1 was observed following the passage of samples through the interior of hollow fiber cellulose acetate membranes lacking a non-permeable skin in contact with the feed solution (Belfort et al. 1975). Hollow fiber ultrafilters composed of asymmetric polysulfone fibers were later found to be superior to cellulose acetate membranes as they were less vulnerable to pH and temperature variations, bacterial degradation, and the loss of viruses due to the dense inner skin lining that was in direct contact with the sample feeds (Belfort et al. 1976). Hollow fiber ultrafilters have since been principally composed of polysulfone or polyacrynitrile materials, although these fibers generally require pretreatment to prevent the substantial loss of viruses due to adsorption.
Tangential Flow Ultrafiltration
Tangential flow filtration is a type of cross flow process during which the water sample feed is rapidly directed tangentially along the surface of the ultrafilter membranes. The rapid flow across the membranes creates a pressure differential, forcing any particles smaller than the MWCO along with some feed solution through the membrane and into a filtrate (effluent) holding container. The swift crossflow also prevents the build-up of foulants and clogging of the membranes. Tangential flow is thus used commonly with lower quality waters such as wastewater. The proper selection of a MWCO should ensure a minimal loss of virus into the filtrate; several are available, and range from 5,000 to 100,000 MWCO [5–100 kiloDaltons (kDa)]. Viruses and other particles that are larger than the MWCO cannot pass through the membranes and are retained in the feed solution, which is recirculated through the device until a desired concentrate volume is achieved.
Two-liter tap water samples seeded with poliovirus 1, hepatitis A virus, and a bovine strain of rotavirus were effectively concentrated in a primary step using a tangential flow Minitan system (Millipore), which uses four stacked polysulfonate membranes (Soule et al. 2000). Following secondary ultrafiltration, 5.6 % of the tap water samples were positive for enteric viruses using RT-PCR, including samples collected at the homes of children ill with rotaviral gastroenteritis. Winona et al. (2001) compared hollow fiber dead-end ultrafiltration and tangential flow concentration methods using poliovirus 2 and phages T1 and PP7 seeded in 2-L volumes of tap, ground, and surface waters. While both systems performed similarly well, different elution strategies were required. Optimal recoveries of T1 phage (87 ± 3 %), PP7 phage (88 ± 23 %), and poliovirus 2 (90 ± 10 %) were obtained from groundwater when the hollow fibers were preblocked with 5 % fetal bovine serum (FBS) and then eluted with 0.05 M glycine (pH 7 or 9). Processing of groundwater using the tangential flow system (5 % FBS block, 0.05 M glycine with recirculation) also produced efficient virus recoveries of 61 % (±19 %), 72 % (±20 %), and 43 % (±10 %) for T1 phage, PP7 phage, and poliovirus 2, respectively. Olszewski et al. (2005) scaled up the hollow fiber and tangential flow methods developed by Winona et al. (2001) to conduct large volume (100 L) experiments with the same viruses. Again, the hollow fiber dead-end system provided greater recoveries of all three virus types in a more consistent manner than the tangential flow arrangement. The costs associated with the hollow fiber ultrafilters were lower than the tangential flow apparatus by several thousands of dollars. The hollow fiber system also demonstrated a greater potential for portability to the field than the bulky, stainless steel housings required for the tangential flow ultrafiltration.
The hollow fiber ultrafiltration system was further evaluated using small and large volume samples from storm and drainage ditches spiked with phage PP7 as an internal standard (Rajal et al. 2007). Using real-time TaqMan PCR and plaque assay methods, phage PP7 detection was greatly enhanced by the recirculation of a 0.05 M glycine-eluting solution; however, the authors found that preblocking of the filters with calf serum as performed in previous studies reduced the virus recovery efficiency. The problem was alleviated by replacing the plastic feed container with a stainless steel vessel containing mixing impellers.
Dead-End Flow Ultrafiltration
Although ultrafiltration is an effective concentration method that generally yields high recoveries of diverse virus types, it has not transferred as readily to the field similar to the aforementioned methods employing charged filters. Tangential flow and vortex flow systems are largely laboratory-based procedures using immobile equipment and instruments that would be cumbersome and impractical to transport quickly to the field. These issues can potentially be overcome by the use of dead-end ultrafiltration. In dead-end ultrafiltration processes, the sample is fed through the ultrafilter once. An eluting aid is added to the retentate as with tangential flow, but it is not recirculated through the system. The set-up is less awkward to transport, and easier to construct onsite. While most published studies have reported the use of the dead-end configuration to study the concentration of bacteria, Smith and Hill (2009) evaluated several disposable ultrafilters in this configuration to concentrate multiple microbial classes from water. Using the REXEED 35S ultrafilter (Asahi Kasei Corp., Tokyo, Japan), the recoveries of MS2 coliphage from 100 L volumes of tap water and tap water amended with surface water measured 57 % (±7.7 %) and 73 % (±13 %), respectively. This method was found to be more efficient with less turbid waters, however (Smith and Hill 2009).
Ultracentrifugation
Centrifugation with forces around 1,000–3,000×g for 10–15 min is sufficient to pellet whole cells and bacteria and high speed centrifugation (≥5,000×g for long time periods) is sufficient to pellet larger organelles such as mitochondria; nevertheless, ultracentrifugation (≥100,000×g) is required when attempting to pellet most macromolecules and viruses (Ammersbach and Bienzle 2011). Such pellets may subsequently be suspended in a much smaller volume to concentrate viruses. Ultracentrifugation may also be used in conjunction with a density gradient to purify viruses (Ammersbach and Bienzle 2011). In isopycnic ultracentrifugation (e.g., sucrose, cesium chloride, or glycerol gradient), the particles in the sample are separated based on their size, the particle density relative to the solution, the rotor clearance factor, the g-force, and sometimes the particle shape or viscosity of the solution. Particles migrate through the gradient to the position where the gradient density matches that of the particle. In rate zonal ultracentrifugation (e.g., iodixanol gradient), particles are separated by their size and mass and not just their density (Ammersbach and Bienzle 2011). Many density gradients are hyperosmotic and cause increases in viral densities. They can also be toxic or corrosive and therefore purified samples must be dialyzed before cell culture, electrophoresis, or high-performance liquid chromatography (HPLC) (Ammersbach and Bienzle 2011).
Ultracentrifugation has been used for decades to concentrate viruses (Cliver and Yeatman 1965; Hill et al. 1971). This method exhibits a similar and sometimes greater recovery of viruses from environmental samples than other concentration methods. For instance, Schultz et al. (2011) found that ultracentrifugation (165,000×g for 4 h at 4 °C) worked better than ultrafiltration as a secondary concentration step (following primary concentration using a positively charged filter) for recovering feline calicivirus and hepatitis A virus from bottled water. In a study by Fumian et al. (2010), ultracentrifugation (100,000×g for 1 h at 4 °C) had a mean recovery rate of 47 % (range of 34–60 %) of rotavirus A from wastewater samples in comparison to concentration using a negatively charged membrane followed by secondary concentration with a centrifugal ultrafilter (mean recovery rate of 3.5 %, range of 1.5–5.5 %). Prata et al. (2012) found average overall virus recovery efficiencies of 69 % for wastewater and 76 % for recreational water samples in comparison to only 38 and 22 %, respectively, using an organic flocculation method.
Ultracentrifugation has several advantages over various other virus concentration methods. It is has a low cost for materials per sample since containers may be reused. Also, the samples can be processed without adjustments to pH and there are no elution (Prata et al. 2012) or secondary concentration steps required (Fumian et al. 2010). Ultracentrifugation requires less time than organic flocculation methods and does not introduce any PCR inhibitory substances into the sample (e.g., beef extract) (Prata et al. 2012).
There are, however, several significant drawbacks to the use of ultracentrifugation to concentrate viruses. First of all, the initial cost to obtain an ultracentrifuge may discourage the use of such a method and may be cost-prohibitive for many laboratories (particularly if included in the calculations of per sample costs) (Fumian et al. 2010; Schultz et al. 2011). Additionally, only small volumes (~10 mL–1 L) may be reasonably processed (Fumian et al. 2010; Prata et al. 2012). Therefore, this method is only useful for highly impacted waters such as wastewater. These waters usually require a clarification step (e.g., high speed centrifugation of 3,000–12,000×g for 10–15 min) (Nordgren et al. 2009; Fumian et al. 2010) or a prefilter step (e.g., filtration through a filter with a pore size of 0.2–0.45 μm) (Fumian et al. 2010; Ammersbach and Bienzle 2011; Schultz et al. 2011; Prata et al. 2012) to remove most of the larger debris and cells from the sample before the ultracentrifugation step(s). Such prefiltration can cause the loss of viruses in the sample due to membrane clogging or adsorption (Prata et al. 2012). Although ultracentrifugation is not useful for large volumes of water, the samples can be greatly concentrated down to approximately 100 μL to a few milliliters (Prata et al. 2012).
Virus Elution (Recovery)
As previously described, a number of highly efficient methods have been developed to concentrate viruses from water. The VIRus ADsorption and ELution (VIRADEL) method is the most common and is based on electrostatic interactions between electronegative viruses and either negatively or positively charged filters in aqueous systems. Virus adsorption to filters is governed by several energy contributions including electrostatic interactions, repulsive double layer van der Waals attractive forces, and hydrophobic interactions, among others (Gerba 1984). Therefore, the elution of viruses from filter surfaces has been most successful with solutions capable of disrupting these interactions. A number of organic and inorganic solutions at varying pH levels have been assessed for this purpose (Tables 4, 5).
Alternatively, the process of ultrafiltration separates viruses from other particles in a water sample feed according to differences in their molecular weights. As these methods are mechanistically different, the methods for the recovery of viruses following these concentration steps also differ; however, virus adsorption to ultrafilter membranes has frequently been implicated in lower than expected virus recoveries. Several solutions have been successfully employed as additives to the retentate volume in ultrafilters to enhance the elution and/or backflushing efficiency. Despite the high recovery levels that have been reported by various research groups, the elution efficiency of viruses from both charged filters and ultrafilters remains highly variable.
Organic Solutions
Glycine
Glycine has been in use for several decades to recover viruses following primary concentration. An amphoteric amino acid, glycine exhibits both acidic and basic properties imparted by its α-carboxyl (pKa1 = 2.34) and α-ammonium (pKa2 = 9.60) functional groups (McMurry 2004). The acid dissociation constant of the α-ammonium functional group combined with the isoelectric point of glycine (pI = 5.97) indicates that glycine exhibits both a high buffering capacity and a strong net negative charge under alkaline conditions (McMurry 2004). Glycine was initially employed as an eluting solution because it was free of the ‘membrane coating components’ characteristic of proteinaceous eluting solutions (e.g., beef extract and calf serum) that could clog smaller filters used for secondary concentration (Wallis et al. 1972a). The recommended solution pH was extremely alkaline (pH = 11.5), and varying levels of virus inactivation were reported (Table 4). Upon exposure to a high pH solution, a negative charge would be imparted to the filter surface, creating unfavorable conditions for viruses to remain adsorbed as they also carry a net negative charge. Metcalf et al. (1974) stated that short-term exposures (up to 10 min) before pH adjustment to neutral did not inactivate poliovirus, with a 5 % loss observed after 20 min. In contrast, a 50 % decrease in poliovirus titer was reported following a 3-min exposure period to pH 11.5 glycine–NaOH buffer (Jakubowski et al. 1975). Nevertheless, a number of studies showed that increasing the alkalinity of glycine resulted in greater enterovirus and bacteriophage recovery efficiencies from several types of negatively charged filters (Wallis et al. 1972a; Jakubowski et al. 1975; Farrah et al. 1976a; Sarrette et al. 1977; Goyal et al. 1980) and electropositive filters composed of different materials (Sobsey and Jones 1979; Logan et al. 1980). This trend was not observed, however, for other virus types including adenoviruses and reoviruses which exhibited inactivation above pH 10.0 (Sobsey et al. 1980). Varying concentrations of glycine continued to be used, however, as elution aids in ultrafiltration systems at pH values of 7.0 and 9.0 (Winona et al. 2001; Olszewski et al. 2005; Rajal et al. 2007).
As mentioned previously, one of the principle issues with the VIRADEL method is the broad range of virus elution efficiencies that are obtained. Wallis et al. (1972a) achieved ≥90 % elution using 0.05 M glycine (pH 11.5) of poliovirus 1 (LSc2ab strain) from negatively charged Millipore HA nitrocellulose membranes adsorbed from tap water, relative to the much lower efficiencies obtained by Jakubowski et al. (1975) of 38.5 %. Both groups filtered tap water with similar levels of total dissolved solids (TDS); however, the membrane diameters were not the same (27 mm and 293 mm, respectively) and the sample volumes were substantially different; Wallis et al. (1972a) filtered 10-mL samples relative to the 380 L used in the later study. The decrease in the elution efficiency was most likely attributable to the increase in the sample volume, a trend that has subsequently been observed (Singh and Gerba 1983).
The elution efficiency of polioviruses from electronegative Filterite filters (fiberglass melamine-impregnated paper with epoxy) using alkaline glycine solutions has also proven variable, but less so than with the nitrocellulose Millipore filters. Most studies have reported mean elution efficiencies for poliovirus 1 ranging from 60 to 70 %, despite varying sample volumes and sample water sources (Farrah et al. 1976a; Farrah and Bitton 1978; Sobsey and Jones 1979; Sobsey and Glass 1980). Farrah et al. (1977) reported an elution range of 31–97 % using 0.05 M glycine (pH 10.5), with an average of 69 %. Coliphages were also efficiently recovered with glycine at pH levels of 11.0 (64.7 %) and 11.5 (53.9 %) following the concentration of secondary sewage using a Filterite filter (Goyal et al. 1980).
The elution of viruses from positively charged filter media using glycine gave similar recoveries compared to those observed with the Filterite filters. Mean poliovirus elution efficiencies of 67 and 60 % were reported from cellulose–diatomaceous earth “charge modified” resin filters (Zeta Plus 50S, 0.75-μm pore size) in separate studies (Sobsey and Jones 1979; Sobsey and Glass 1980); a decrease in porosity (Zeta Plus 60S, 0.45-μm pore size) resulted in a lower virus recovery of 42 % (Sobsey and Jones 1979). A similar recovery from the Zeta Plus 60S filter using 0.05 M glycine (pH 10.0) was observed for T2 phages of 41.7 % (Logan et al. 1980). The elution efficiency was only 40 % for MS2 coliphage seeded into secondary sewage and adsorbed to Zeta Plus 50S filters, which was likely due to virus association with suspended solids (Goyal et al. 1980). Virosorb 1MDS filters (surface-modified fiberglass and cellulose mixtures) in both single-sheet and double-sheet configurations demonstrated 51 and 62 % recoveries of adsorbed polioviruses, respectively, when eluted with glycine at pH 11.0 (Sobsey and Glass 1980).
Although the elution of viruses using glycine solutions appeared to be fairly consistent among different filter types, it was more effective at extreme pH values that can cause substantial viral inactivation. This can be particularly problematic for waters containing low numbers of virus. This prompted ongoing research into alternative virus recovery solutions.
Beef Extract
The use of beef extract to facilitate virus elution following primary concentration has been in practice for over four decades (Table 4). Early studies reported variable recoveries of several enteroviruses with beef extract under different experimental conditions. For instance, Rao and Labzoffsky (1969) eluted 53–123 % of polioviruses adsorbed to Millipore HA filters from raw waters using 3 % beef extract. Sonication, in conjunction with beef extract elution performed following varying hold times, later recovered 100 % of poliovirus 1, Coxsackievirus B3, and reovirus 1, and 90 % of echovirus 7 adsorbed to Millipore HA filters (Berg et al. 1971). It was also demonstrated early on that increasing the alkalinity of beef extract from pH 6 to 8, as well as increasing the contact hold time between the eluting solution and the filter to at least 30 min, resulted in greater virus recoveries (Rao et al. 1972). However, the use of beef extract fell out of favor for several years as it was composed of proteins that would preferentially adsorb to reactive filter sites during adsorption–elution secondary concentration methods (Wallis et al. 1972a).
Although glycine (0.05 M) was used for a number of years to elute viruses from filters, its optimum efficacy at extreme alkaline pH values had the potential to cause virus inactivation beyond short exposure times. In contrast, beef extract solutions of varying concentrations are capable of eluting numerous virus types at moderate alkaline pH conditions (pH 9.0–9.5) from both negatively and positively charged filter adsorbents following the concentration of viruses from water quality matrices ranging from dechlorinated tap water to primary sewage. Studies seeking to evaluate several eluting solutions for their ability to recover enteroviruses adsorbed to electronegative Filterite filters from 40 to 50 mL volumes of dechlorinated tap water revealed that 3 % beef extract (pH 9.0) eluted from 75 % (Farrah and Bitton 1978) to 92 % (Farrah and Bitton 1979) of poliovirus 1; echovirus 1 and Coxsackievirus B3 were also efficiently recovered at levels of 84 and 85 %, respectively (Farrah and Bitton 1979). Farrah and Bitton (1979) further demonstrated the ability of 3 % beef extract (pH 9.0) to consistently recover poliovirus 1, echovirus 1, and Coxsackievirus B3 from several electronegative membrane filters (Acropor–acrylic copolymer, Millipore, Filterite, and Zeta Plus C-10-cellulose diatomaceous earth) at efficiencies of ≥84 %, with the exception of poliovirus 1 from Zeta Plus C-10 filters (76 %) (Farrah and Bitton 1979). The simultaneous concentration of Coxsackieviruses A9 and B1, echovirus 7, and poliovirus 1 from tap water (5 L) using Balston filters (epoxy-fiberglass), followed by elution with 3 % beef extract (pH 9.0) gave an average recovery of 78 %, with poliovirus 1 eluted the most efficiently (87 %) and echovirus 7 the least efficiently (72 %) (Guttman-Bass and Nasser 1984). A mixture of these four viruses was then seeded into tap water, wastewater, seawater, and two different surface water samples, all varying according to pH, chemical oxygen demand (COD), and conductivity. The samples were filtered and recovered in the eluates at efficiencies ranging from 73 % (±7 %) for wastewater to 107 % (±20 %) for seawater.
Viral elution using beef extract has proven more inconsistent among the variety of positively charged filters and for different virus types. A solution of 0.3 % beef extract with 0.05 M glycine (pH 9.5) recovered poliovirus 1 more efficiently (66 %) from 1 MDS filter sheets than 0.05 M glycine (pH 11.0) alone (54 %) in a small volume study (3.8 L) (Sobsey and Glass 1980), whereas the same beef extract solution recovered 86 and 62 % of poliovirus 1 adsorbed to 1MDS and Filterite filters from 1,000-L volumes of tap water, respectively (Sobsey et al. 1981). The trend of more efficient poliovirus 1 elution relative to those observed for echovirus 1 and reovirus 3 using a 0.3 % beef extract with 0.05 M glycine solution (pH 9.5) from Filterite and 1MDS filters (Sobsey et al. 1981) was also observed in a later study employing the same filters to assess the recovery of the aforementioned viruses from raw surface and finished waters from a drinking water-treatment plant (Sobsey and Glass 1984).
Beef extract has been mandated for use by the USEPA to facilitate virus recovery from filters following sample collection (Fout et al. 1996). Dahling (2002) performed several modifications of the E.P.A.’s Information Collection Rule elution procedure to determine the most effective strategy for use with the 1MDS filter, finding that two separate filter washings (total volume = 3,200 mL) using 1.5 % beef extract with an overnight hold at room temperature yielded the highest virus recovery. However, an overnight hold is not practical for rapid virus detection, and smaller eluate volumes are preferable to better achieve virus concentration. A comparison of 1.5 and 3.0 % beef extract solutions for the recovery of poliovirus 1 from both 1MDS and MK filters (molded waste fibers coated with melamine resin) revealed that recovery was best achieved with the 3.0 % solution (Ma et al. 1994).
Karim et al. (2009) later evaluated several elution strategies to recover poliovirus 1 adsorbed to NanoCeram filters (nanoalumina fibers) from 100 L of deionized tap water using 1.5 % beef extract with 0.05 M glycine (pH 9.0). Two washings with hold times of 1 and 15 min, respectively, resulted in a mean recovery of 77 % following secondary concentration of the eluates. The same elution strategy (without secondary concentration) was later employed to assess the recovery of MS2 coliphage and poliovirus 1 adsorbed to the ViroCap capsule (containing NanoCeram adsorbent material) from 20-L volumes of deionized water and artificial seawater (Bennett et al. 2010). MS2 coliphage seeded in both water types was more readily recovered from the ViroCap device. Higher recoveries were measured for polioviruses adsorbed from deionized water using the 1MDS filter, and the elution was more efficient from the ViroCap following concentration from artificial seawater. The NanoCeram filter was further evaluated using 3.0 % beef extract (pH 9.5) and the soy-based OptimaRE (pH 7.0) eluting solutions recirculated with a peristaltic pump to recover adenovirus 40 adsorbed from 40-L volumes of seawater (Gibbons et al. 2010). The elution efficiencies were poor (<1 %) when using OptimaRE, and the 3 % beef extract solution recovered only 4.5 % of the adenoviruses (real-time PCR units). In contrast, noroviruses were eluted effectively using 3 % beef extract alone (111 ± 28 %) and amended with 0.1 % Tween 80 (119 ± 26 %). Further, Gibbons et al. (2010) compared the elution efficiencies of adenovirus 40 and Qβ male-specific coliphages (norovirus surrogate) from 40-L volumes of seawater, source water, and finished water, reconfirming the low recovery of adenovirus (2.5, 2.4, and 1.4 %, respectively), and the high recovery observed for noroviruses from seawater with Qβ recoveries measuring 96, 34, and 35 %, respectively.
Beef extract has proven to be an effective eluting solution for enteric viruses and coliphages adsorbed to charged filter surfaces and ultrafilters from a spectrum of water quality matrices. Nevertheless, the molecular detection methods (e.g., PCR) currently used are sensitive to the presence of organic inhibitors (e.g., humic and fulvic acids) naturally present in waste, surface, and tap waters (Farrah et al. 1976b; Sobsey and Glass 1984; Sobsey and Hickey 1985). When employed to elute viruses from filters, the heterogeneous organic composition of beef extract further contributes to the levels of these inhibitors. The methods subsequently developed to reconcentrate the beef extract eluates (e.g., organic flocculation which is discussed later) simultaneously collect these inhibitory organic materials, which must be removed before molecular analysis (Abbaszadegan et al. 1993; Schwab et al. 1993). The elimination of molecular inhibitors necessitates further steps for virus concentration which may result in additional losses, prompting ongoing research into alternative eluting solutions that do not present such problems.
Inorganic Solutions
Acids/Bases
A technique recently developed by Katayama et al. (2002) utilizing electronegative nitrocellulose membranes (Millpore HA) addresses both elution and the removal of organic inhibitors. Following the primary concentration of drinking water or seawater samples seeded with poliovirus, the filters were initially rinsed with 0.5 mM H2SO4 to remove the Mg2+ cations and organic materials which are both capable of inhibiting PCR. The elution of viruses was then achieved by a second washing with 1.0 mM NaOH (pH 10.5–10.8), followed by immediate neutralization using 50 mM H2SO4 and 100× Tris–EDTA buffer (pH 8.0) (Table 5). The method eluted 95 % of poliovirus from pure water and from 82 to 95 % from seawater, compared to the standard method (1MDS filter, beef extract pH 9.5), which recovered only 50 % of viruses from pure water and 6 % from seawater for detection by reverse-transcriptase PCR. While this method was effective, the preconditioning requirement for freshwaters to achieve virus concentration makes it more useful for small-volume processing of waters characterized by high viral loads (e.g., surface waters receiving wastewater effluents). It has since been utilized to confirm the offshore transport of human enteric viruses from groundwater to coral reefs (Futch et al. 2010), and to assess the prevalence of human noroviruses and torque teno viruses in a variety of water sources in Brazil (Diniz-Mendes et al. 2008; Victoria et al. 2009).
The acid–base elution strategy was also used with an Al3+-coated nitrocellulose filter method and TaqMan PCR to detect from 88 to 109 % of polioviruses adsorbed from varying volumes of MilliQ water, and 82–99 % of viruses adsorbed from 500-mL and 1-L volumes of tap water, respectively (Haramoto et al. 2004). Compared to other VIRADEL methods, the cation-coated filter method (Al3+) followed by acid–base elution consistently recovered higher levels of the seeded poliovirus and human noroviruses from MilliQ, tap, and river water as detected by real-time PCR (Haramoto et al. 2009), and enteroviruses from raw water samples analyzed by most probable number-RT-PCR (MPN-RT-PCR) and real-time PCR (Hsu et al. 2007). The method is inexpensive and effective for use with both traditional cell culture and molecular assays, but more research is needed to further evaluate its efficiency for recovering low numbers of viruses adsorbed from large water volumes.
Researchers have also conducted studies using an acid (H2SO4) or a base (NaOH) individually to recover poliovirus 1 from various water matrices using 1MDS and Zetapor positively charged filters. Katayama et al. (2002) recovered 62 % of the poliovirus used to seed MilliQ water using a 1MDS filter eluted with 1.0 M NaOH. In contrast, Haramoto et al. (2004) were only able to recover 6 % of poliovirus from MilliQ water using Zetapor filters and 1.0 M NaOH. In unpublished studies by Eiji Haramoto (personal communication), H2SO4 was not effective for the elution of poliovirus from 1MDS or Zetapor filters from tap water, raw sewage, or secondary treated sewage. The recoveries were 2.9 % from raw sewage and 2.8 % from treated sewage using 1MDS filters and 16, <0.3, and <2.9 % using Zetapor filters from tap water, sewage, and treated sewage, respectively. In a separate study using 1MDS filters to concentrate poliovirus seeded into 40 L volumes, a low pH (using H2SO4; pH 1.9–3.0) and high pH (using NaOH; pH 10.5–12.1) elution step was effective in eluting poliovirus from MilliQ water (96 ± 21 and 66 ± 16 % elution efficiency, respectively) and tap water (65 ± 16 and 51 ± 11 %, respectively), but were not as effective from river water (8 ± 2 and 33 ± 12 %, respectively) and seawater (9 ± 2 and 6 ± 2 %, respectively). Thus, the water matrix appears to have a significant effect on the efficacy of acid/base elution from 1MDS filters. The information regarding the elution of viruses from positively charged filters using acids or bases is nevertheless limited and potentially merits further exploration.
Sodium Polyphosphates
Sodium polyphosphates (Na-PP) are strongly anionic, long-chain polymer dispersants that have been used to enhance the simultaneous recovery of viruses and other microbial classes from ultrafilter membranes. When 0.01 % (w/v) Na-PP was added to 10 L of bulk tap water volumes before sample processing in a study by Hill et al. (2005), followed by retentate collection and elution of the membranes with 0.01 % Tween-80, 100 % of infectious MS2 coliphages were detected by plaque assay (USEPA Method 1602); backflushing did enhance the recovery, but this was not statistically significant. Backflushing requires more technical expertise to perform with ultrafilters (Polaczyk et al. 2008). A subsequent study by Polaczyk et al. (2008) further evaluated this method with lower numbers of virus (MS2 coliphage and echovirus 1) seeded into 100-L tap water volumes amended with 0.01 % Na-PP. This study placed a heightened focus on the elution step (using 0.1 % Tween 80, 0.01 % Na-PP, and 0.001 % Antifoam A) rather than on backflushing. A recovery of 53 % was found for the elution step, whereas 84 % of MS2 was recovered using backflushing. In contrast, 49 % of echovirus 1 was recovered using elution relative to only 37 % recovered using a backflushing step (detected by real-time PCR). This more simple elution strategy (using 0.01 % Tween 80, 0.01 % Na-PP, and 0.001 % Antifoam A) was subsequently able to recover averages of 120 % (±22%) and 86 % (±13 %) of MS2 and φX174, respectively, seeded into 100-L tap water samples collected from eight U.S. cities (Hill et al. 2007).
Na-PP has rarely been used as an eluent in applications other than for ultrafilters. However, Ikner et al. (2011) recently utilized a phosphate buffer containing 1.0 % Na-PP and 0.05 M glycine as an effective eluting solution to recover viruses from NanoCeram positively charged cartridge filters. The authors were able to recover 57 % (±3 %) of MS2 coliphage, 69 % (±8 %) of poliovirus 1, 134 % (±27 %) of echovirus 1, 72 % (±13 %) of Coxsackievirus B5, and 39 % (±13 %) of adenovirus 2 from 20 L of seeded tap water. Nevertheless, the Na-PP appeared to inhibit subsequent PCR detection, though this inhibition was removed during the secondary concentration step using an ultrafiltration device.
Chaotropic Agents
In addition to the repulsive double layer, van der Waals attractive forces (DLVO forces) that govern the adsorption of viruses to filters, hydrophobic interactions have also been suggested to play a significant role (Farrah et al. 1981; Gerba 1984). Chaotropic agents disrupt the structure of water to allow for the accommodation of hydrophobic functional groups. These include salts, organic acids, and un-ionized detergents (e.g., Tween-80). In contrast, antichaotropic salts (e.g., Na2HPO4) enhance the structure of water, resulting in an environment that is less energetically favorable for hydrophobic compounds, thereby promoting their sequestration (Gerba 1984). Farrah et al. (1981) investigated several chaotropic and antichaotropic agents for their ability to elute poliovirus 1 from nitrocellulose membranes (Millipore) and electropositive Zeta Plus C30 filters (diatomaceous earth–anion exchange resin; CUNO, Meriden, CT). Their findings suggested that virus–filter interactions were affected by the solution pH. Neither chaotropic nor antichaotropic agents were able to elute viruses at pH 4, as both the filter and the viruses are oppositely charged and electrostatic forces predominate. At pH 9.5, the filter and viruses are both negatively charged; therefore, virus–filter interactions are largely hydrophobic. These results demonstrated that the chaotropic agents tested (e.g., trichloroacetic acid and Tween-80) promoted high elution efficiencies from both filter types at pH 9.5 in which hydrophobic interactions predominate. The most commonly used chaotropic agent is Tween-80, which has been employed as an eluting solution additive in several studies to lessen the hydrophobic interactions between viruses and filters to promote elution (Rajal et al. 2007; Holowecky et al. 2009; Smith and Hill 2009).
Secondary Concentration Methods
Secondary concentration of primary viral eluates (Table 6) is generally performed to reduce the volume that will be assayed using traditional cell culture and/or molecular assay techniques (e.g., PCR). Ideally, the method should be rapid, efficient (no loss of viruses), simple, inexpensive, and able to achieve a desired concentrate volume appropriate for subsequent virus detection methods.
Aqueous Polymer Two-Phase Separation
Early secondary concentration methods included those originally developed for the primary concentration of viruses from water. Hill et al. (1972) used the previously described aqueous polymer two-phase separation method to reconcentrate low numbers of polioviruses seeded into 100-gallon (378 L) volumes of estuarine water, achieving a final concentrate volume of approximately 24 mL and a total virus recovery efficiency ranging from 42 to 97 %. However, the use of aqueous polymer two-phase separation for secondary concentration was short lived due to the tediousness and time-consuming nature of the method as an overnight period was required.
Aluminum Hydroxide Precipitation–hydroextraction
A two-step procedure employing aluminum–hydroxide precipitation followed by hydroextraction was also developed for the secondary concentration of glycine eluates (Farrah et al. 1977) as the adsorption of viruses to aluminum hydroxide flocs had been previously demonstrated (Wallis and Melnick 1967d). Following neutralization of the eluates (1,600 mL), aluminum chloride was added to a final concentration of 0.003 M, and then neutralized again with 1 M sodium carbonate to produce flocs. The flocs obtained both by settling and centrifugation were resuspended three times in equal volumes of fetal calf serum (2 %) and glycine (0.05 M, pH 11.5) and subsequently centrifuged to extract the viruses adsorbed to the flocs. PEG-hydroextraction of the floc eluates in dialysis bags (4 °C) further reduced the concentrate volume to 20 mL, and the concentrates were dialyzed once more against phosphate-buffered saline. Twelve 400-L samples of estuarine waters receiving sewage effluent underwent secondary aluminum hydroxide precipitation–hydroextraction, and revealed mostly the presence of indigenous polioviruses (47 %) and echoviruses types 1 (23 %) and 5 (30 %). A subsequent study by Gerba et al. (1978) also employed the aluminum hydroxide precipitation–hydroextraction procedure, recovering 52 % of seeded polioviruses from 472 to 1,000 L of tap water, 53 % from 378 L of seawater, and 50 % from 19 to 190 L of secondary sewage. However, a round-robin investigation of poliovirus recovery methods later revealed that the aluminum hydroxide precipitation followed by hydroextraction demonstrated a wide range of recovery efficiencies for both low input experiments (0–67 %) and high input experiments (0–18 %) (Melnick et al. 1984). This variation, in addition to the advent of organic flocculation for the secondary concentration of beef extract eluates, and the time-consuming nature of hydroextraction further demonstrated the impracticality of the method for the rapid and efficient detection of viruses from water.
Adsorption–Elution
Viral eluates can be further concentrated by performing an additional adsorption–elution step using microporous filters. Wallis et al. (1970) adjusted 0.05 M borate eluates (pH 9, 200 mL) containing poliovirus to pH 8, then filtered the solution first through a Tween-80 treated AP 20 clarifying pad (90 mm) followed by passage through an untreated 0.45-μm cellulose ester membrane filter (Millipore) for adsorption. A 10-mL volume of 2 % casein–Tris buffer (pH 7.5) was recirculated twice through the Millipore filter to recover the adsorbed polioviruses, achieving a 75 % overall recovery and a 1,000-fold volume reduction relative to the initial sample volume of 10 L. The method was then applied to concentrate polioviruses filtered from 300 gallons (~1,100 L) of swimming pool water on insoluble polyelectrolyte filters and eluted as previously described; however, only 41 % of polioviruses were recovered on average. Wallis et al. (1972a) later determined that glycine-based eluates (0.05 M, pH 11.5) could be reconcentrated via adsorption–elution following acidification to pH 4.0–4.5, and the addition of AlCl3 to a final concentration of 0.0005 M. The passage of eluates through cellulose membranes facilitated virus adsorption, and elution was carried out (after washing of the membranes with saline to remove excess Al3+) using a small volume of glycine (0.05 M, pH 11.5) that further concentrated the viruses. Performing this process several times over was purported to recover 100 % of the adsorbed viruses; however, the viruses were not monodispersed before experimentation. The secondary concentration method was applied following the processing of 500 gallon (1,890 L) volumes of tap water through 293-mm cellulose membranes (Millipore) with an initial recovery with 0.05 M glycine (pH 11.5). The total method efficiencies ranged from 88 to 94 % of the original input viruses recovered.
During primary concentration, the suspended organic solids naturally present in water can reduce the ability of filters to effectively adsorb viruses due to preferential adsorption. As elution occurs, they are simultaneously recovered with the viruses; therefore, adsorption–elution reconcentration methods are also vulnerable to the presence of these materials. Sobsey et al. (1973) deduced that these virus-interfering components are largely anionic organic compounds, and that the addition of AlCl3 (final concentration of 0.005 M) to eluates before the reconcentration step alleviated the problem to allow for enhanced virus adsorption. The processing of 100-gallon (378 L) tap water samples seeded with poliovirus through K27-Cox filters and subsequent elution with pH 11.5 glycine buffer produced 1-L eluates. The eluates were adjusted to pH 3.5, amended to a final concentration of 0.0005 M AlCl3, and then filtered through a series of Cox filters (47 mm diameter, 5.0 and 0.45-μm pore size) which were subsequently washed with pH 3.5 saline to remove the excess Al3+ ions. The adsorbed viruses were then eluted with 7 mL of pH 11.5 glycine buffer. After pH adjustment to neutral and the addition of NaCl to maintain isotonicity, a final concentrate volume of 10 mL was obtained, achieving a 40,000-fold concentration relative to the original 378-L tap water sample. The overall virus recovery efficiencies ranged from 48 to 95 %, with low virus input experiments generating total recoveries ≥92 %, and high input trials recovering fewer viruses (≤63 %) (Sobsey et al. 1973).
This method developed by Sobsey et al. (1973) was slightly modified in an effort to reconcentrate low numbers of poliovirus from tap water (12–22 PFU/1,900 L) using cellulose membrane filters (293 mm, Millipore) and borosilicate glass microfiber–epoxy resin tube filters (Balston) (Jakubowski et al. 1975). To achieve secondary adsorption, a 1-μm porosity filter was added to the Cox series (47 mm diameter, 5.0 and 0.45-μm porosity), and two 7-mL portions of glycine buffer (pH 11.5) were used for the recovery to achieve a final concentrate volume of 20 mL. The method efficiencies ranged from 25 to 50 and 25 to 80 % when the Millipore membranes and Balston tubes were used for the primary concentration, respectively (Jakubowski et al. 1975). The use of adsorption–elution for secondary concentration sufficiently aided in the detection of low multiplicities of polioviruses in large water volumes, which was confirmed during the early development of a highly sensitive and consistent standard method to detect viruses in 1,900-L sample volumes (Hill et al. 1976).
As positively charged filters were increasingly used for the primary concentration of viruses from water, they were also investigated for their ability to reconcentrate viral eluates. Zeta Plus 50S filters were compared to Cox filters for the secondary concentration of poliovirus (388 PFU average) seeded in 378 L of tap water. Alkaline glycine eluates (1 L, pH 11.5) were adjusted to pH 6, passed through Zeta Plus 50S filters, and eluted with two 7.5-mL volumes of glycine (pH 10.0). Secondary recovery efficiencies averaged 22.5 % with the Zeta Plus filters, in comparison to 4 % for the electronegative Cox filter when used according to the Standard Methods, and 2 % for the Cox filter when pH adjustments used for the Zeta Plus filter were applied (Sobsey and Jones 1979). The preconditioning of eluates by the addition of AlCl3 and acidification was not necessary to obtain high recoveries when using electropositive filters for secondary concentration, simplifying the method considerably. Shields et al. (1985) developed a modified concentration method for enteroviruses that used an electronegative filter (Filterite) for primary filtration and trichloroacetic acid to elute, in addition to a positively charged 47-mm Seitz S filter for secondary reconcentration. The two eluting solutions tested during reconcentration were casitone (3 %, pH 9) and fetal calf serum (pH 9.0), which recovered collective averages of 54 and 43 % of four enterovirus types, respectively.
Organic Flocculation
While secondary adsorption–elution methods were able to concentrate viruses sufficiently for their detection by traditional cell culture assays, the increased use of beef extract solutions to elute viruses necessitated the development of alternative reconcentration methods. Beef extract had been demonstrated to coat membranes and occupy reactive sites, allowing viruses to pass through and into the filtrate (Cliver 1965). Katzenelson et al. (1976) showed that a combination of chemical and physical interactions could facilitate the secondary concentration of poliovirus 1 in 450-mL beef extract eluates. Acidifying 3 % beef extract to pH 3.5 increased the H+ ion concentration substantially, thereby decreasing the length of the repulsive double layer surrounding the particles in solution and encouraging coagulation. A subsequent 30-min period of slow stirring then stimulated physical particle–particle interactions and the formation of virus–protein flocs, which could then be centrifuged (3,000 × g, 10 min) and the pellet redissolved in 7.5 mL of 0.15 M Na2HPO4 by pipetting. The addition of several antibiotics, and the final pH adjustment to neutral gave a final concentrate volume of ~15 mL. This process, termed organic flocculation, achieved recoveries ranging from 60 to 91 %, compared to 30–47 % recovery with the glycine buffer reconcentration method developed by Hill et al. (1976). It also had a greater capacity to reconcentrate viruses from waters characterized by higher levels of organic matter than the secondary adsorption–elution technique, which generally exhibited low recoveries in such waters.
Nevertheless, organic flocculation does not concentrate all viruses equally well. A comparison of the organic flocculation efficiencies for a simian strain of adenovirus (SV-11), mouse parvovirus, and reovirus 3 resulted in recoveries of 68, 42, and 22 %, respectively (Sobsey et al. 1980). This may have been caused by viral inactivation at low pH, or inconsistent adsorption to flocs due to the varying physico-chemical surface properties of the viruses tested. Inconsistencies in organic flocculation recoveries were later validated among the three types of enteroviruses, including variations evident at the strain level (Morris and Waite 1980). A series of experiments, during which 0.3 % beef extract with 0.05 M glycine mock eluates from both Filterite and 1MDS filters were seeded with echovirus 1 and reovirus 3 showed that organic flocculation was a less efficient secondary concentration method for these viruses (Sobsey et al. 1981). The recovery of echovirus 1 was 74 % from the 1MDS filter mock eluates in contrast to 20 % measured for Filterite eluates. Recoveries of Reovirus 1 were exceptionally low at 6 % (1MDS) and <0.1 % (Filterite). The substantial loss of infectivity observed was likely due to the virus exposure to extreme acidity (pH 3.5) during organic flocculation, since viruses were added to the eluates just before the procedure, and very few viruses were detected in the supernatants and the flocs.
In order to correct for the differences in experimental conditions that might account for the range of efficiencies reported using the organic flocculation method, Guttman-Bass and Nasser (1984) developed a method for the simultaneous concentration of four representative enteroviruses from the four major groups: Coxsackievirus A9, Coxsackievirus B1, echovirus 7, and poliovirus 1. Pooled antibodies specific to any group of the three were added to samples before cell culture inoculation to ensure that only one viral strain was being quantified at a time. Organic flocculation recovered from 80 % (echovirus 7) to 99 % (Coxsackievirus B1) of the viruses seeded into tap water (5 L) and processed through the Balston filters. When the four viruses were simultaneously seeded into four different water matrices (tap water, surface water, seawater, and wastewater) and processed as described before, the organic flocculation recoveries ranged from 70 % (wastewater) to 98 % (lake water).
A study comparing the electronegative Filterite filter and the positively charged 1MDS filter for the concentration of high input poliovirus from 1,000 L of tap water further revealed the variation of virus recovery from organic flocs (Sobsey and Glass 1980). During the organic flocculation of two separate volumes of 1.5 % beef eluates (Filterite), 95 and 99 % of polioviruses adsorbed to the flocs (determined by assay of the supernatants) and 52 and 100 % of the viruses were recovered from the flocs after resuspension with Na2HPO4, respectively. The polioviruses eluted from the 1MDS filter demonstrated floc adsorption efficiencies of 99 % for both trials, with recoveries of 65 and 72 % measured from the flocs. Since it was shown that most of the polioviruses did adsorb efficiently to the flocs, the loss of virus may have been due to the partial removal of the pellet during aspiration of the supernatant. The overall method efficiencies relating the poliovirus titers in the final resuspended floc concentrates to the original titers added to the 1,000-L sample volumes measured 34 and 48 % for the Filterite and 1 MDS filters, respectively. On average, the 1MDS filter adsorbed more polioviruses, and elution was also higher from these filters relative to the Filterite concentrators. This most likely contributed to the greater overall recovery observed for the 1MDS method.
Organic flocculation has also been evaluated for virus reconcentration after sample processing using positively charged glass wool filters. The overall concentration efficiencies following inoculation of poliovirus 1 into 400- and 1,000-L tap water samples were 72 and 62 %, respectively (Vilagines et al. 1993). Poliovirus 1 was also recovered relatively efficiently from surface water (63 %) and treated wastewater (57 %) using organic flocculation. Five other viruses passed through glass wool filters in 100-L tap water volumes further demonstrated moderate secondary recovery efficiencies of 62 % (echovirus 11), 75 % (Coxsackievirus B2), 72 % (Coxsackievirus B4), 65 % (rotavirus SA-11), and 71 % (poliovirus 2).
The organic flocculation secondary concentration method developed by Katzenelson et al. (1976) underwent modifications to correct for the varying capacity of powdered beef extract lots to form heavy precipitates (Hurst et al. 1984). The addition of ferric chlorides to beef extract solutions that would not flocculate sufficiently was found to facilitate the formation of flocs that enhanced virus recovery (Payment et al. 1984). Dahling and Wright (1986) evaluated several potential materials that could enhance the recovery of viruses from beef extract eluates. The addition of Celite filter aid, T-21 silicate, and aluminum sulfate to several brands of powdered and paste beef extract solutions (3 %) resulted in comparable recoveries of poliovirus 1, echovirus 7, and Coxsackievirus A9. However, the Celite filter aid (diatomaceous silica) was deemed more effective relative to the other test materials as it resuspended more readily, was autoclavable, and formed more structured pellets upon centrifugation. Ma et al. (1994) compared organic flocculation (Katzenelson et al. 1976) to the modified method (Dahling and Wright 1986) employing a diatomaceous earth filter aid for the secondary recovery of polioviruses seeded in 378 L of tap water and passed through electropositive MK filters. The modified diatomaceous earth method was more effective when used with 1.5 % beef extract solutions, while the classic organic flocculation procedure recovered more viruses when used with 3 % beef extract. Most recently, the Celite method was utilized with modifications to concentrate polioviruses from 1.5 % beef extract with 0.05 M glycine eluates derived from NanoCeram filters (Karim et al. 2009). The combined recovery efficiencies following secondary concentration for poliovirus 1, Coxsackievirus B5, and echovirus 7 were 54, 27, and 32 %, respectively.
Modifications of the standard organic flocculation technique have also proven necessary to address viral inactivation, which can occur at the extreme acidic pH value of 3.5 recommended by the method. Shields and Farrah (1986) recovered >100 % of coliphages (MS2, φX174, and T3) using 2:1 solutions composed of 80 % (w/v) ammonium sulfate with 10 % beef extract to form flocs at pH 7, compared to a 1 % recovery average gained with organic flocculation. Poliovirus 1, echovirus 5, and Coxsackievirus B5 were also concentrated at considerably higher efficiencies using ammonium sulfate flocculation. While effective, the sizable quantity of beef extract required (10 %) could be rather costly. Further optimization of the method ascertained that a minimum concentration of 50 % (w/v) ammonium sulfate was required to achieve a 100 % recovery of poliovirus 1 when used with a 1 % beef extract solution (Payment and Trudel 1987). Beef extract eluates (1 %) amended with 50 % (w/v) ammonium sulfate also achieved high recoveries of poliovirus 1 (88 %), Coxsackievirus B4 (88 %), and Simian rotavirus SA-11 (97 %), of which the latter had previously exhibited 65 % recovery using the organic flocculation method (Vilagines et al. 1993).
As previously mentioned, humic substances naturally present in water are captured by filters during primary concentration and recovered with viruses during elution. When highly proteinaceous beef extract solutions are employed for virus elution and subsequently undergo secondary concentration, both viruses and proteins form part of the resultant flocs and are resuspended in the final concentrates. This has proven problematic for molecular detection techniques currently in use (e.g., PCR) to detect the presence of viruses in water as organic substances have been demonstrated to inhibit the enzymes necessary for genomic amplification (Schwab et al. 1993). This trend has been observed more frequently following primary concentration using electropositive filters (Abbaszadegan et al. 1993; Kopecka et al. 1993; Gantzer et al. 1997). Therefore, methods have been developed to remove these inhibitory substances from secondary concentrates. Abbaszadegan et al. (1993) evaluated spin chromatography using several types of resins to assess their capacity to remove organic inhibitors from secondary groundwater concentrates. It was found that a combination of Sephadex G-100 spun columns (Pharmacia, Stockholm, Sweden) and Chelex-100 chelating ion-exchange resins (Bio-Rad Laboratories, Hercules, CA) were most effective, allowing for the successful amplification of the 149-base pair target sequence (103 PFU poliovirus per reaction) in each of the ten sample concentrates tested.
As further evaluation of the spin chromatography method demonstrated only a 15 % recovery of 14C-labeled poliovirus, an alternative method to remove organic inhibitors was developed employing ultracentrifugation and solvent extraction techniques (Fout et al. 2003). The concentrates were generated by passing groundwater through 1MDS filters followed by elution using non-flocculating beef extract, and reconcentration by the Celite method developed by Dahling and Wright (1986). Ultracentrifugation of the concentrates through a sucrose pad achieved an 80 % recovery of the seeded polioviruses, but removal of the inhibitors was not observed for the concentrate samples from all of the test sites. Therefore, an organic solvent extraction technique was implemented to further remove the remaining inhibitors, followed by centrifugation, and finally secondary concentration using an ultrafilter (Microcon-100 concentrators precoated with 0.1 % bovine serum albumin). This led to a 74 % recovery of the seeded polioviruses. This method has since proven effective for the removal of organic inhibitors from 1MDS and NanoCeram filter concentrates originating from tap and river water samples (Karim et al. 2009).
Ultrafiltration
Ultrafilters have also been used for the secondary concentration of eluates. They are available in a variety of MWCO, and separate particles according to size. As eluates pass through the filter, either by centrifugation or vacuum filtration, solution components that are smaller than the specified size parameter pass through and into the filtrate for disposal. The captured viruses are then collected along with the small volume of retentate remaining in the ultrafilter, and thus are more concentrated than they were previously in the eluate. The processing times for eluates also tend to be relatively short, averaging 10–15 min with many of the newer ultrafilter systems. Although ultrafilters tend to be composed of inert materials (e.g., polysulfonates), the loss of viruses still can occur via physical adsorption (e.g., van der Waals attractive forces) or embedding into membranes.
The early use of ultrafilters for secondary concentration proved advantageous over other secondary concentration methods (e.g., adsorption–elution) as samples required neither pH adjustment nor the addition of multivalent cations. Ultrafilters (MWCO 100 kDa) successfully reconcentrated indigenous bacteriophages from 1.5-L eluates (1 % beef extract–0.05 M arginine) originating from small (65 L, Bio-Rad ultrafilters) and large volumes (100–200 L, Amicon hollow fiber ultrafilters) of surface water receiving wastewater effluents, achieving concentrate volumes ranging from 50–100 mL (Logan et al. 1980, 1981). Markedly different recoveries were measured for poliovirus 1 and hepatitis A virus from polysulfonate ultrafilters despite their similarity in structure (10 kDa MWCO; Minitan System, Millipore) (Divizia et al. 1989). The recovery of hepatitis A virus from 1-L seeded tap water volumes was 100 % using secondary ultrafiltration (four membranes, 10–12 psi, retentate:filtrate ratio of 6:7), poliovirus 1 recovery was exceedingly low (15 %). Pretreatment of the polysulfonate membranes with 3 % beef extract prevented absorption of the polioviruses into the membranes, increasing the recovery to 100 %, although processing times were increased significantly.
Ultrafiltration has become a more widely practiced method for the processing of eluates because viruses can be concentrated into very small volumes of solution, increasing the likelihood of detection by both cell culture and molecular techniques. Gilgen et al. (1997) applied ultrafiltration as the second step of a three-stage concentration methodology to detect multiple enteric virus types in river water samples using RT-PCR. One-liter samples were prefiltered using an AP 20 pad (Millipore), and then passed through an electropositive membrane filter (0.45 μm, Zetapore, Millipore). A small volume (3 mL) of 1 % beef extract with 0.05 M glycine eluent was used to recover the viruses, followed by reconcentration of two-thirds of the eluate volume down to 100 μL using a Centricon-100 microconcentrator (Amicon). Further concentration of the liberated viral RNA and RT-PCR to a 10 μL reaction volume yielded positive results in all six river water samples of enteroviruses, rotavirus, and small round structure virus genogroup I (i.e., norovirus GI), and in 33 % of the samples for small round structure virus genogroup II (i.e., norovirus GII). None of the samples tested positive for hepatitis A virus.
The VIRADEL method employing negatively charged HA nitrocellulose filters (Millipore) and acid–base rinse steps to remove inhibitors and elute viruses also incorporated secondary ultrafiltration to achieve small volumes. Seawater samples (2 L) collected from three separate locations and spiked with poliovirus 1 were reconcentrated to final volumes of ~2 mL using the Centriprep Concentrator 50 (Millipore); the overall method efficiencies ranged from 61 to 73 % as detected by RT-PCR (Katayama et al. 2002). Two-liter river water samples from a system of rivers and streams receiving untreated sewage were processed according to Katayama et al. (2002), obtaining final concentrate volumes of 2 mL with the Centriprep Concentrator 50 (Diniz-Mendes et al. 2008). Torque teno virus DNA was subsequently detected in 92 % of the water samples using real-time PCR. The presence of noroviruses (I and/or II) in water samples of varying qualities, including treated sewage and chlorinated drinking water, has also been established following the ultrafiltration of samples as previously described (Katayama et al. 2002) in combination with seminested PCR (Victoria et al. 2009).
Studies employing in situ Al3+-coated nitrocellulose filters for the primary concentration of viruses have also employed secondary ultrafiltration methods. Large volumes of tap water ranging from 141 to 505 L were filtered during a 13-month period, rinsed with 4 L of 0.5 mM H2SO4 to remove inhibitors, and eluted with 200 mL of 1.0 M NaOH (Haramoto et al. 2004). Reconcentration followed with the Centriprep YM-50 ultrafilters, resulting in final volumes of ~0.9 mL. Noroviruses (genogroups I and II) were detected using TaqMan PCR in 10.2 % of the 98 water samples tested. The Al3+-coated filter method with acid–base elution and ultrafiltration for the reconcentration step was further utilized to assess the presence of several virus types in an urban river system (Haramoto et al. 2005). Surface water samples (500 mL) collected from six sites were concentrated to ~0.7 mL. Real-time PCR detection revealed the presence of norovirus genogroups I and II in 44–53 % of the 64 collected samples, in addition to enteroviruses (9 %), adenoviruses (45 %), and torque teno viruses (5 %).
Recently, Ikner et al. (2011) utilized Centricon Plus-70 centrifugal ultrafilters (30 kDa MWCO; Millipore, Billerica, MA) in a secondary concentration step following sodium polyphosphate-based elution from positively charged NanoCeram cartridge filters. The ultrafilters recovered 75 % of MS2 coliphage, 61 % of Echovirus 1, 95 % of poliovirus 1, and 109 % of Coxsackievirus B5; however, only 33 % of adenovirus 2 was recovered. Nevertheless, the Centricon filters were also able to remove inhibitors to RT-PCR while reducing the volume from 420 to <20 mL.
Ultracentrifugation
Ultracentrifugation has also been used as a secondary concentration step. The advantages and disadvantages of this method are described in the section entitled “Ultracentrifugation” of this paper.
Conclusions
Although the methods to concentrate viruses from water have not changed substantially in several decades, they have undergone a number of modifications to increase efficiency. The use of electronegative filters to sample both large and small volumes of water has become fairly streamlined, but remains most ideal for the sampling of smaller volumes of water characterized by high viral loads as preconditioning remains a requirement. In addition, coliphages and some enteric viruses (e.g., reoviruses) are susceptible to inactivation at the low pH levels required to facilitate virus capture. The cation-coated filter method developed by Haramoto et al. (2004) does not require sample preconditioning, is simple to perform, and inexpensive. However, more large-volume studies need to be performed to further evaluate the efficacy of the method.
The electropositive 1MDS filter is the recommended concentrator for viruses from water (Fout et al. 1996), and has proven effective in the capture of etiologic agents implicated in several waterborne outbreaks. However, it continues to be quite costly (~$250 USD per filter). In addition, inhibition of molecular assays continues to be an issue with the secondary organic flocculation concentration step typically used with 1MDS eluates. NanoCeram filters are significantly less expensive and able to capture viruses from several different water types including seawater, which has proven difficult to accomplish with the 1MDS filter. Nevertheless, the NanoCeram filters require additional research to fully assess their capacity to retain a broader spectrum of viruses exhibiting different surface properties.
Ultrafiltration has been increasingly researched over the last decade, particularly for the concurrent concentration of multiple microbial classes. While it does remain a costly procedure, the development of field-based methods (e.g., dead-end ultrafiltration) shows a transition toward more practical use that requires neither extensive specialized training nor expensive equipment. Small centrifugal ultrafilters also show promise for use in secondary concentration steps following primary concentration by a variety of methods.
The ability to recover viruses following adsorption to filters remains highly variable. Organic and inorganic solutions, including surfactant additives have been extensively researched, but a solution capable of eluting all pathogenic viruses from the abundance of filter types available has yet to be developed. The biological variability of viruses, in terms of surface composition, net charge, and the tendency to form aggregates has contributed significantly to the differences measured in elution efficiency as markedly different recoveries have been observed even at the strain level. Glycine was used for many years to recover viruses, but demonstrated viral inactivation at its optimal alkaline eluting pH. Beef extract is capable of elution at more moderate alkaline pH values, but secondary concentration methods (e.g., organic flocculation) require extremely acidic conditions to work properly which also can lead to the inactivation of acid-sensitive viruses. In addition, the highly proteinaceous composition of beef extract may lead to the inhibition of molecular detection assays. The acid–base rinse procedure by Katayama et al. (2002) that removes inhibitors and elutes targeted viruses has the potential to alleviate these issues when used with electronegative nitrocellulose membrane filters. The use of sodium polyphosphates has demonstrated high recovery efficiencies of viruses and other microorganisms from ultrafilter systems. The application of these highly anionic polymers to elute viruses from electropositive filters has also demonstrated potential (Ikner et al. 2011).
Secondary concentration methods have proven vital to accurately discern the presence of viruses in water that may be present in very low numbers. As beef extract is the current recommended eluting solution, organic flocculation (including modifications) will continue to see widespread use. While the potential for organic inhibition of PCR methods remains an issue, inhibitor-removal techniques (Abbaszadegan et al. 1993; Fout et al. 2003) have provided the means for the continual use of beef extract (or modern beef extract substitutes) and organic flocculation. The utilization of ultrafilters for secondary concentration has been largely demonstrated with inorganic eluates. Ultrafiltration has the advantage of producing very small concentrate volumes relative to organic flocculation in short periods of time (10–15 min), but some models can be relatively expensive whereas organic flocculation merely requires pH adjustment and resuspension in a low molarity salt solution. Clearly, there are both advantages and unfavorable aspects with these reconcentration methods, but they have had demonstrated efficiency for use with a broad spectrum of water quality types.
As the methods for virus capture, recovery, and reconcentration have developed, understanding of the factors influencing each step has broadened significantly, often resulting in further modifications. However, each additional adjustment to the overall process train, even when necessary, still has the potential to result in further loss of viruses along the way, whether due to their inactivation or irreversible adsorption. While the increased sensitivity of molecular detection methods has partially alleviated these issues, their inability to differentiate between infectious and inactivated viruses indicates that concentration and recovery methods should strive to maintain the viability and structural integrity of these pathogens to more accurately assess the potential risks to human health.
References
Abbaszadegan, M., Huber, M. S., Gerba, C. P., & Pepper, I. L. (1993). Detection of enteroviruses in groundwater with the polymerase chain reaction. Applied and Environmental Microbiology, 59, 1318–1324.
Albinana-Gimenez, N., Clemente-Casares, P., Calgua, B., Huguet, J. M., Courtois, S., & Girones, R. (2009a). Comparison of methods for concentrating human adenoviruses, polyoma virus JC, and noroviruses in source waters and drinking waters using quantitative PCR. Journal of Virological Methods, 158, 104–109.
Albinana-Gimenez, N., Miagostovich, M. P., Calgua, B., Huguet, J. M., Matia, L., & Girones, R. (2009b). Analysis of adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water plants. Water Research, 43, 2011–2019.
American Public Health Association. (2005). Standard Methods for the Examination of Water and Wastewater (20th ed.). Washington, DC: American Public Health Association.
Ammersbach, M., & Bienzle, D. (2011). Methods for assessing feline immunodeficiency virus infection, infectivity and purification. Veterinary Immunology and Immunopathology, 143, 202–214.
Anderson, N. G., Cline, G. B., Harris, N. W., & Green, J. G. (1967). Isolation of viral particles from large fluid volumes. In G. Berg (Ed.), Transmission of viruses by the water route (pp. 75–88). London: Interscience.
Belfort, G., Rotem, Y., & Katzenelson, E. (1975). Virus concentration using hollow fiber membranes—I. Water Research, 9, 75–85.
Belfort, G., Rotem, Y., & Katzenelson, E. (1976). Virus concentration using hollow fiber membranes—II. Water Research, 10, 279–284.
Bennett, H. B., O’Dell, H. D., Norton, G., Shin, G., Hsu, F.-C., & Meschke, J. S. (2010). Evaluation of a novel electropositive filter for the concentration of viruses from diverse water matrices. Water Science and Technology, 61, 317–322.
Berg, G., Dahling, D. R., & Berman, D. (1971). Recovery of small quantities of viruses from clean waters in cellulose nitrate membrane filters. Applied Microbiology, 22, 608–614.
Berman, D., Rohr, M. E., & Safferman, R. S. (1980). Concentration of poliovirus in water by molecular filtration. Applied and Environmental Microbiology, 40, 426–428.
Cliver, D. O. (1965). Factors in the membrane filtration of enteroviruses. Applied Microbiology, 13, 417–425.
Cliver, D. O., & Yeatman, J. (1965). Ultracentrifugation in the concentration and detection of enteroviruses. Applied Microbiology, 13, 387–392.
Coin, L. (1967). The viruses in water. In G. Berg (Ed.), Transmission of Viruses by the Water Route (p. 367). London: Interscience.
Dahling, D. (2002). An improved filter elution and cell culture assay procedure for evaluating public groundwater systems for culturable enteroviruses. Water Environment Research, 74, 564–568.
Dahling, D. R., & Wright, B. A. (1986). Recovery of viruses from water by a modified flocculation procedure for second-step concentration. Applied and Environmental Microbiology, 51, 1326–1331.
Diniz-Mendes, L., de Paula, V. S., Luz, S. L. B., & Niel, C. (2008). High prevalence of human torque teno viruses in streams crossing the city of Manaus, Brazilian Amazon. Journal of Applied Microbiology, 105, 51–58.
Divizia, M., Santi, A. L., & Pana, A. (1989). Ultrafiltration: An efficient second step for hepatitis A virus and poliovirus concentration. Journal of Virological Methods, 23, 55–62.
Ehlers, M. M., Grabow, W. O. K., & Pavlov, D. N. (2005). Detection of enteroviruses in untreated and treated drinking water supplies in South Africa. Water Research, 39, 2253–2258.
Farrah, S. R., & Bitton, G. (1978). Elution of poliovirus adsorbed to membrane filters. Applied and Environmental Microbiology, 36, 982–984.
Farrah, S. R., & Bitton, G. (1979). Low molecular weight substitutes for beef extract as eluents for poliovirus adsorbed to membrane filters. Canadian Journal of Microbiology, 25, 1045–1051.
Farrah, S. R., Gerba, C. P., Goyal, S. M., Wallis, C., & Melnick, J. L. (1977). Regeneration of pleated filters used to concentrate enteroviruses from large volumes of tap water. Applied and Environmental Microbiology, 33, 308–311.
Farrah, S. R., Gerba, C. P., Wallis, C., & Melnick, J. L. (1976a). Concentration of viruses from large volumes of tap water using pleated membrane filters. Applied and Environmental Microbiology, 31, 221–226.
Farrah, S. R., Goyal, S. M., Gerba, C. P., Wallis, C., & Shaffer, P. T. B. (1976b). Characteristics of humic acid and organic compounds concentrated from tap water using the Aquella virus concentrator. Water Research, 10, 897–901.
Farrah, S. R., & Preston, D. R. (1985). Concentration of viruses from water by using cellulose filters modified by in situ precipitation of ferric and aluminum hydroxides. Applied and Environmental Microbiology, 50, 1502–1504.
Farrah, S. R., Shah, D. O., & Ingram, L. O. (1981). Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proceedings of the National Academy of Sciences of the United States of America, 75, 1229–1232.
Fout, G. S., Martinson, B. C., Moyer, M. W. N., & Dahling, D. R. (2003). A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater. Applied and Environmental Microbiology, 69, 3158–3164.
Fout, G. S., Schaefer III, F. W., Messer, J. W., Dahling, D. R., & Stetler, R. E. (1996). Information collection rule (ICR) microbial laboratory manual. EPA/600/R-95/178. Washington, DC: U.S. Environmental Protection Agency.
Fumian, T. M., Leite, J. P. G., Castello, A. A., Gaggero, A., de Caillou, M. S. L., & Miagostovich, M. P. (2010). Detection of rotavirus A in sewage samples using multiplex qPCR and an evaluation of the ultracentrifugation and adsorption-elution methods for virus concentration. Journal of Virological Methods, 170, 42–46.
Futch, J. C., Griffin, D. W., & Lipp, E. K. (2010). Human enteric viruses in groundwater indicate offshore transport of human sewage to coral reefs of the Upper Florida Keys. Environmental Microbiology, 12, 964–974.
Gajardo, R., Díez, J. M., Jofre, J., & Bosch, A. (1991). Adsorption-elution with negatively and positively-charged glass powder for the concentration of hepatitis A virus from water. Journal of Virological Methods, 31, 345–352.
Gantzer, C., Senouci, S., Maul, A., Levi, Y., & Schwartzbrod, L. (1997). Enterovirus genomes in wastewater: Concentration on glass wool and glass powder and detection by RT-PCR. Journal of Virological Methods, 65, 265–271.
Garin, D., Fuchs, F., Crance, J. M., Deloince, R., Aymard, M., & Bartoli, M. (1993). Validation of an ultrafiltration process to concentrate viruses from large volumes of water. Environmental Technology, 14, 397–400.
Gartner, H. (1967). Retention and recovery of polioviruses on a soluble ultrafilter. In G. Berg (Ed.), Transmission of viruses by the water route (pp. 121–127). London: Interscience.
Gassilloud, B., Duval, M., Schwartzbrod, L., & Gantzer, C. (2003). Recovery of feline calicivirus infectious particles and genome from water: Comparison of two concentration techniques. Water Science and Technology, 47, 97–101.
Gerba, C. (1984). Applied and theoretical aspects of virus adsorption to surfaces. Advances in Applied Microbiology, 30, 33–68.
Gerba, C. P., Farrah, S. R., Goyal, S. M., Wallis, C., & Melnick, J. L. (1978). Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Applied and Environmental Microbiology, 35, 540–548.
Gibbons, C. D., Rodriguez, R. A., Tallon, L., & Sobsey, M. D. (2010). Evaluation of positively charged alumina nanofibre cartridge filters for the primary concentration of noroviruses, adenoviruses, and male-specific coliphages from seawater. Journal of Applied Microbiology, 109, 635–641.
Gilgen, M., Germann, D., Luthy, J., & Hubner, P. (1997). Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. International Journal of Food Microbiology, 37, 189–199.
Goyal, S. M., Zerda, K. S., & Gerba, C. P. (1980). Concentrations of coliphage from large volumes of water and wastewater. Applied and Environmental Microbiology, 39, 85–91.
Grabow, W. O. K. (1968). The virology of waste water treatment. Water Research, 2, 675–701.
Gravelle, C. R., & Chin, T. D. Y. (1961). Enterovirus isolations from sewage: A comparison of three methods. Journal of Infectious Disease, 10, 205.
Grinrod, J., & Cliver, D. O. (1970). A polymer two phase system adapted for virus detection. Archive für Gesamte Virusforsch, 31, 365–372.
Grinstein, S., Melnick, J. L., & Wallis, C. (1970). Virus isolations from sewage and from a stream receiving effluents of sewage treatment plants. Bulletin of the world Health Organization, 42, 291–296.
Guttman-Bass, N., & Nasser, A. (1984). Simultaneous concentration of four enteroviruses from tap, waste, and natural waters. Applied and Environmental Microbiology, 47, 1311–1315.
Haramoto, E., Katayama, H., Oguma, K., & Ohgaki, S. (2005). Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Applied and Environmental Microbiology, 71, 2403–2411.
Haramoto, E., Katayama, H., & Ohgaki, S. (2004). Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Applied and Environmental Microbiology, 70, 2154–2160.
Haramoto, E., Katayama, H., Utagawa, E., & Ohgaki, S. (2009). Recovery of human norovirus from water by virus concentration methods. Journal of Virological Methods, 160, 206–209.
Hill, W. F., Akin, E. W., & Benton, W. H. (1971). Detection of viruses in water: A review of methods and applications. Water Research, 5, 967–995.
Hill, W. F., Akin, E. W., Benton, W. R., Mayhew, C. J., & Jakubowski, W. (1974). Apparatus for conditioning unlimited quantities of finished waters for enteric virus detection. Applied Microbiology, 27, 1177–1178.
Hill, W. F., Akin, E. W., Benton, W. H., & Metcalf, T. G. (1972). Virus in water: II. Evaluation of membrane cartridge filters for recovering low multiplicities of poliovirus from water. Applied Microbiology, 23, 880–888.
Hill, W. F., Jakubowski, W., Akin, E. W., & Clarke, N. A. (1976). Detection of virus in water: Sensitivity of the tentative standard method for drinking water. Applied and Environmental Microbiology, 31, 254–261.
Hill, V. R., Kahler, A. M., Jothikumar, N., Johnson, T. B., Hahn, D., & Cromeans, T. L. (2007). Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Applied and Environmental Microbiology, 73, 4218–4225.
Hill, V. R., Polaczyk, A. L., Hahn, D., Narayanan, J., Cromeans, T. L., Roberts, J. M., et al. (2005). Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants. Applied and Environmental Microbiology, 71, 6878–6884.
Hill, V. R., Polaczyk, A. L., Kahler, A. M., Cromeans, T. L., Hahn, D., & Amburgey, J. E. (2009). Comparison of hollow-fiber ultrafiltration to the USEPA VIRADEL Technique and USEPA Method 1623. Journal of Environmental Quality, 38, 822–825.
Holowecky, P. M., James, R. R., Lorch, D. P., Straka, S. E., & Lindquist, H. D. A. (2009). Evaluation of ultrafilter cartridges for a water sampling apparatus. Journal of Applied Microbiology, 106, 738–747.
Hsu, B. M., Chen, C. H., Kung, C. M., Wan, M. T., & Shen, S. M. (2007). Evaluation of enterovirus recovery in surface water by different adsorption and elution procedures. Chemosphere, 66, 964–969.
Hurst, C. J., Dahling, D. R., Safferman, R. S., & Goyke, T. (1984). Comparison of commercial beef extracts and similar materials for recovering viruses from environmental samples. Canadian Journal of Microbiology, 30, 1253–1263.
Ikner, L. A., Soto-Beltran, M., & Bright, K. R. (2011). New method using a positively charged microporous filter and ultrafiltration for concentration of viruses from tap water. Applied and Environmental Microbiology, 77, 3500–3506.
Jakubowski, W., Hill, W., & Clarke, N. (1975). Comparative study of four microporous filters for concentrating viruses from drinking water. Applied Microbiology, 30, 58–65.
Jakubowski, W., Hoff, J. C., Anthony, N. C., & Hill, W. F. (1974). Epoxy-fiberglass adsorbent for concentrating viruses from large volumes of potable water. Applied Microbiology, 28, 501–502.
Jansons, J., & Bucens, M. R. (1986). Virus detection in water by ultrafiltration. Water Research, 12, 1603–1606.
Johnson, J. H., Fields, J. E., & Darlington, W. A. (1967). Removing viruses from water by polyelectrolytes. Nature, 213, 665–667.
Jothikumar, N., Khana, P., Paulmurugan, R., Kamatchiammal, S., & Padmanabhan, P. (1995). A simple device of the concentration and detection of enterovirus, hepatitis E virus and rotavirus from water samples by reverse transcription-polymerase chain reaction. Journal of Virological Methods, 55, 401–415.
Karim, M. R., Rhodes, E. R., Brinkman, N., Wymer, L., & Fout, G. S. (2009). New electropositive filter for concentrating enteroviruses and noroviruses from large volumes of water. Applied and Environmental Microbiology, 75, 2393–2399.
Katayama, H., Shimasaki, A., & Ohgaki, S. (2002). Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Applied and Environmental Microbiology, 68, 1033–1039.
Katzenelson, E., Fattal, B., & Hostovesky, T. (1976). Organic flocculation: An efficient second-step concentration method for the detection of viruses in tap water. Applied and Environmental Microbiology, 32, 638–639.
Kelly, S. (1953). Detection and occurrence of Coxsackieviruses in sewage. American Journal of Public Health, 43, 1532.
Kessick, M. A., & Wagner, R. A. (1978). Electrophoretic mobilities of virus adsorbing filter materials. Water Research, 12, 263–268.
Kitajima, M., Haramoto, E., Phanuwan, C., Katayama, H., & Ohgaki, S. (2009). Detection of genogroup IV norovirus in wastewater and river water in Japan. Letters in Applied Microbiology, 49, 655–658.
Kopecka, H., Dubrou, S., Prevot, J., Marechal, J., & Lopez-Pila, J. M. (1993). Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Applied and Environmental Microbiology, 59, 1213–1219.
Lambertini, E., Spencer, S. K., Bertz, P. D., Loge, F. J., Kieke, B. A., & Borchardt, M. D. (2008). Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters. Applied and Environmental Microbiology, 74, 2990–2996.
Lee, H., Kim, M., Lee, J. E., Lim, M., Kim, M., Kim, J. M., et al. (2011a). Investigation of norovirus occurrence in groundwater in metropolitan Seoul, Korea. Science of the Total Environment, 409, 2078–2084.
Lee, H., Kim, M., Paik, S. Y., Lee, C. H., Jheong, W. H., Kim, J., et al. (2011b). Evaluation of electropositive filtration for recovering norovirus in water. Journal of Water and Health, 9, 27–36.
Liu, O. C., Brashear, D. A., Seraichekas, H. R., Barnick, J. A., & Metcalf, T. G. (1971). Virus in water. I. A preliminary study on a flow-through gauze sampler for recovering virus from waters. Applied Microbiology, 21, 405–410.
Logan, K. B., Rees, G. E., Seeley, N. D., & Primrose, S. B. (1980). Rapid concentrations of bacteriophages from large volumes of freshwater: Evaluation of positively charged, microporous filters. Journal of Virological Methods, 1, 87–97.
Logan, K. B., Scott, G. E., Seeley, N. D., & Primrose, S. B. (1981). A portable device for the rapid concentration of viruses from large volumes of natural freshwater. Journal of Virological Methods, 3, 241–249.
Lukasik, J., Scott, T. M., Andryshak, D., & Farrah, S. R. (2000). Influence of salts on virus adsorption to microporous filters. Applied and Environmental Microbiology, 66, 2914–2920.
Ma, J.-F., Naranjo, J., & Gerba, C. P. (1994). Evaluation of MK filters for recovery of enteroviruses from tap water. Applied and Environmental Microbiology, 60, 1974–1977.
McMurry, J. E. (2004). Biomolecules: Amino acids, peptides, and proteins. In Organic chemistry (6th ed., p. 988). Belmont, CA: Brooks/Cole-Thomson Learning.
Melnick, J. L., Emmons, J., Coffey, J. H., & Schoof, H. (1954). Coxsackie viruses from sewage. American Journal of Hygiene, 59, 185–195.
Melnick, J. L., Safferman, R., Rao, V. C., Goyal, S., Berg, G., Dahling, D. R., et al. (1984). Round robin investigation of methods for the recovery of poliovirus from drinking water. Applied and Environmental Microbiology, 47, 144–150.
Menut, C., Beril, C., & Schwartzbrod, L. (1993). Poliovirus recovery from tap water after concentration over glass powder and glass wool. Water Science and Technology, 27, 291–294.
Metcalf, T. G. (1961). Use of membrane filters to facilitate the recovery of virus from aqueous suspensions. Applied Microbiology, 9, 376–379.
Metcalf, T. G., Wallis, C., & Melnick, J. L. (1974). Environmental factors influencing isolation of enteroviruses from polluted surface waters. Applied Microbiology, 27, 920–926.
Morris, R., & Waite, W. M. (1980). Evaluation of procedures for recovery of viruses from water, part 1, concentration systems. Water Research, 14, 791–793.
Myrmel, M., Rimstad, E., Wasteson, Y., & Skjerve, E. (1999). Detection of small round structured viruses in artificially contaminated water using filter adsorption and reverse transcription polymerase chain reaction. International Journal of Food Microbiology, 49, 85–94.
Nordgren, J., Matussek, A., Mattsson, A., Svensson, L., & Lindgren, P.-E. (2009). Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Research, 43, 1117–1125.
Nupen, E. M. (1970). Virus studies on the Windhoek waste-water reclamation plant (South Africa). Water Research, 4, 661–672.
Olszewski, J., Winona, L., & Oshima, K. H. (2005). Comparison of 2 ultrafiltration systems for the concentration of seeded viruses from environmental waters. Canadian Journal of Microbiology, 51, 295–303.
Payment, P., Fortin, S., & Trudel, M. (1984). Ferric chloride flocculation for nonflocculating beef extract preparations. Applied and Environmental Microbiology, 47, 591–592.
Payment, P., Gerba, C. P., Wallis, C., & Melnick, J. L. (1976). Methods for concentrating viruses from large volumes of estuarine water on pleated membranes. Water Research, 10, 893–896.
Payment, P., & Trudel, M. (1979). Efficiency of several micro-fiber glass filters for recovery of poliovirus from tap water. Applied and Environmental Microbiology, 38, 365–368.
Payment, P., & Trudel, M. (1987). Second-step reconcentration of environmental samples by ammonium sulfate flocculation of beef extract. Canadian Journal of Microbiology, 22, 571–572.
Payment, P., & Trudel, M. (1988). Wound fiberglass depth filters as a less expensive approach for the concentration of viruses from water. Canadian Journal of Microbiology, 34, 272.
Polaczyk, A. L., Narayanan, J., Cromeans, T. L., Hahn, D., Roberts, J. M., Amburgey, J. E., et al. (2008). Ultrafiltration-based techniques for rapid and simultaneous concentration of multiple microbe classes from 100-L tap water samples. Journal of Microbiological Methods, 73, 92–99.
Prata, C., Ribeiro, A., Cunha, A., Gomes, N. C. M., & Almeida, A. (2012). Ultracentrifugation as a direct method to concentrate viruses in environmental waters: Virus-like particle enumeration as a new approach to determine the efficiency of recovery. Journal of Environmental Monitoring, 14, 64–70.
Preston, D. R., Vasudevan, T. V., Bitton, G., Farrah, S. R., & Morel, J. L. (1988). Novel approach for modifying microporous filters for virus concentration from water. Applied and Environmental Microbiology, 54, 1325–1329.
Rajal, V. R., McSwain, B. S., Thompson, D. E., Leutenegger, C. M., Kildare, B. J., & Wuertz, S. (2007). Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Research, 41, 1411–1422.
Rao, V. V., Chandorkar, U., Rao, N. U., Kumaran, P., & Lakhe, S. B. (1972). A simple method for concentrating and detecting viruses in wastewater. Water Research, 6, 1565–1576.
Rao, N. U., & Labzoffsky, N. A. (1969). A simple method for the detection of low concentration of viruses in large volumes of water by the membrane filtration technique. Canadian Journal of Microbiology, 15, 399–403.
Rolland, D., & Block, J. C. (1980). Simultaneous concentration of Salmonella and enterovirus from surface water by using micro-fiber glass filters. Applied and Environmental Microbiology, 39, 659–661.
Rose, J. B., Singh, S. N., Gerba, C. P., & Kelley, L. M. (1984). Comparison of microporous filters for concentration of viruses from wastewater. Applied and Environmental Microbiology, 47, 989–992.
Sarrette, B. A., Danglot, C. D., & Vilagines, R. (1977). A new and simple method for the recuperation of viruses from water. Water Research, 11, 355–358.
Schultz, A. C., Perelle, S., Di Pasquale, S., Kovac, K., De Medici, D., Fach, P., et al. (2011). Collaborative validation of a rapid method for efficient virus concentration in bottled water. International Journal of Food Microbiology, 145, S158–S166.
Schwab, K. J., De Leon, R., & Sobsey, M. D. (1993). Development of PCR methods for enteric virus detection in water. Water Science and Technology, 27, 211–218.
Scott, T. M., Lukasik, J., & Farrah, S. R. (2002). Improved method for recovery of bacteriophages from large volumes of water using negatively charged microporous filters. Canadian Journal of Microbiology, 48, 305–310.
Shields, P. A., Berenfeld, S. A., & Farrah, S. R. (1985). Modified membrane-filter procedure for concentration of enteroviruses from tap water. Applied and Environmental Microbiology, 49, 453–455.
Shields, P. A., & Farrah, S. R. (1986). Concentration of viruses in beef extract by flocculation with ammonium sulfate. Applied and Environmental Microbiology, 51, 211–213.
Shuval, H. J., Cymbalista, S., Fatal, B., & Goldblum, N. (1967). Concentration of enteric viruses in water by hydro-extraction and two phase separation. In G. Berg (Ed.), Transmission of viruses by the water route (pp. 45–55). London: Interscience.
Shuval, H. J., Fattal, B., Cymbalista, S., & Goldblum, N. (1969). The phase-separation method for the concentration and detection of viruses in water. Water Research, 3, 225–240.
Singh, S. N., & Gerba, C. P. (1983). Concentration of coliphage from water and sewage with charge-modified filter aid. Applied and Environmental Microbiology, 45, 232–237.
Smith, C. M., & Hill, V. R. (2009). Dead-end hollow-fiber ultrafiltration for diverse recovery of microbes from water. Applied and Environmental Microbiology, 75, 5289–5824.
Sobsey, M. D., & Glass, J. S. (1980). Poliovirus concentration from tap water with electropositive adsorbent filters. Applied and Environmental Microbiology, 40, 201–210.
Sobsey, M. D., & Glass, J. S. (1984). Influence of water quality on enteric virus concentration by microporous filter methods. Applied and Environmental Microbiology, 47, 956–960.
Sobsey, M. D., Glass, J. S., Jacobs, R. R., & Rutala, W. A. (1980). Modifications of the tentative standard method for improved virus recovery efficiency. Journal of the American Water Works Association, -72, 350–355.
Sobsey, M. D., & Hickey, A. R. (1985). Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Applied and Environmental Microbiology, 49, 259–264.
Sobsey, M. D., & Jones, B. L. (1979). Concentration of poliovirus from tap water using positively charged microporous filters. Applied and Environmental Microbiology, 37, 588–595.
Sobsey, M. D., Moore, R. S., & Glass, J. S. (1981). Evaluating adsorbent filter performance for enteric virus concentrations in tap water. Journal of the American Water Works Association, 73, 542–548.
Sobsey, M. D., Wallis, C., Henderson, M., & Melnick, J. L. (1973). Concentration of enteroviruses from large volumes of water. Applied Microbiology, 26, 529–534.
Sorber, C. A., Malina, J. F., & Sagik, B. P. (1972). Virus rejection by reverse osmosis-ultrafiltration processes. Water Research, 6, 1377–1388.
Soule, H., Genoulaz, O., Gratacap-Cavallier, B., Chevallier, P., Liu, J. X., & Seigneurin, J. M. (2000). Ultrafiltration and reverse transcription-polymerase chain reaction: An efficient process for poliovirus, rotavirus, and hepatitis A virus detection in water. Water Research, 34, 1063–1067.
Stevenson, R. (1967). Quantitative recovery of viruses from dilute suspensions. In G. Berg (Ed.), Transmission of viruses by the water route (p. 143). London: Interscience.
Sweet, B. H., McHale, J. S., Hardy, K. J., Morten, F., Smith, J. K., & Klein, E. (1971). Concentration of virus from water by osmotic ultrafiltration—I: Biological aspects. Water Research, 5, 823–829.
Toranzos, G. A., Erdos, G. W., & Farrah, S. R. (1986). Virus adsorption to microporous filters modified by in situ precipitation of metallic salts. Water Science and Technology, 18, 141–148.
U. S. Environmental Protection Agency. (2010). Method 1615. Measurement of enterovirus and norovirus occurrence in water by culture and RT-qPCR. Accessed January 19, 2012, from http://www.epa.gov/oamcinc1/1200011/method1615.pdf.
van Heerden, J., Ehlers, M. M., Heim, A., & Grabow, W. O. K. (2005). Prevalence, quantification and typing of adenoviruses in river and treated drinking water in South Africa. Journal of Applied Microbiology, 99, 234–242.
van Zyl, W. B., Williams, P. J., Grabow, W. O. K., & Taylor, M. B. (2004). Application of a molecular method for the detection of group A rotaviruses in raw and treated water. Water and Science Technology, 50, 223–228.
Victoria, M., Giumaraes, F., Fumian, T., Ferreira, F., Vieira, C., Leite, J. P., et al. (2009). Evaluation of an adsorption-elution method for detection of astrovirus and norovirus in environmental waters. Journal of Virological Methods, 156, 73–76.
Vilagines, P., Sarrette, B., Champsaur, H., Hugues, B., Dubrou, S., Joret, J. C., et al. (1997). Round robin investigation of glass wool method for poliovirus recovery from drinking water and sea water. Water Science and Technology, 35, 445–449.
Vilagines, P., Sarrette, B., Husson, G., & Vilagines, R. (1993). Glass wool for virus concentration at ambient water pH level. Water Science and Technology, 27, 299–306.
Vilagines, P., Sarrette, B., & Vilagines, R. (1982). Preformed magnesium hydroxide precipitate for second-step concentration of enteroviruses from drinking and surface waters. Canadian Journal of Microbiology, 28, 783–787.
Wagner, J. (2001). Membrane filtration handbook; practical tips and hints. Osmonics, Inc. Accessed October 13, 2011, from http://www.ionics.com/content/pdf/1229223-%20Lit-%20Membrane%20Filtration%20Handbook.pdf.
Wallis, C., Henderson, M., & Melnick, J. L. (1972a). Enterovirus concentration on cellulose membranes. Applied Microbiology, 23, 476–480.
Wallis, C., Homma, A., & Melnick, J. L. (1972b). Apparatus for concentrating viruses from large volumes. Journal of the American Water Works Association, 64, 189–195.
Wallis, C., & Melnick, J. L. (1967a). Concentration of enteroviruses on membrane filters. Journal of Virology, 1, 277–472.
Wallis, C., & Melnick, J. L. (1967b). Concentration of viruses on aluminum and calcium salts. American Journal of Epidemiology, 85, 459–468.
Wallis, C., & Melnick, J. (1967c). Concentration of viruses from sewage by adsorption on Millipore membranes. Bulletin of the World Health Organization, 36, 219–225.
Wallis, C., & Melnick, J. (1967d). Concentration of viruses on aluminum phosphate and aluminum hydroxide precipitates. In G. Berg (Ed.), Transmission of viruses by the water route (pp. 120–138). London: Interscience.
Wallis, C., Melnick, J. L., & Fields, J. E. (1970). Detection of viruses in large volumes of natural waters by concentration on insoluble polyelectrolytes. Water Research, 4, 787–796.
Wallis, C., Melnick, J. L., & Gerba, C. P. (1979). Concentration of viruses from water by membrane chromatography. Annual Review of Microbiology, 33, 413–437.
Winona, L. J., Ommani, A. W., Olszewski, J., Nuzzo, J. B., & Oshima, K. H. (2001). Efficient and predictable recovery of viruses from water by small scale ultrafiltration systems. Canadian Journal of Microbiology, 47, 1033–1041.
Zerda, K. S., Gerba, C. P., Hou, K. C., & Goyal, S. M. (1985). Adsorption of viruses to charge-modified silica. Applied and Environmental Microbiology, 49, 91–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikner, L.A., Gerba, C.P. & Bright, K.R. Concentration and Recovery of Viruses from Water: A Comprehensive Review. Food Environ Virol 4, 41–67 (2012). https://doi.org/10.1007/s12560-012-9080-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-012-9080-2