Abstract
A total of 62 samples of commercial horse feed preparations (complementary feeds) containing cereal mixtures (“muesli” or mash, n = 39; pelleted feeds, n = 12), and plain horse feed grains (maize, n = 5; oats, n = 4; barley, n = 2) were purchased from 21 different producers/distributors from the German market. All samples were analysed by competitive enzyme immunoassays (EIA) for six different mycotoxins (mycotoxin groups). Analytes (detection limit, mean recovery) were: deoxynivalenol (DON, 10 µg/kg, 84%), zearalenone (ZEA, 5 µg/kg, 93%), fumonisin B1 (FB1, 2 µg/kg, 113%), T-2 toxin (T-2, 0.1 µg/kg, 71%), sum of T-2 + HT-2 toxin (T-2/HT2, 0.2 µg/kg, 97%), ochratoxin A (OTA, 0.2 µg/kg, 67%), and total ergot alkaloids (Generic Ergot Alkaloids “GEA”, 30 µg/kg, 132%). All samples contained DON (16–4,900 µg/kg, median 220 µg/kg), T-2/HT-2 (0.8–230 µg/kg, median 24 µg/kg), and T-2 (0.3–91 µg/kg, median 7 µg/kg). ZEA was detected in 98% of the samples (7–310 µg/kg, median 61 µg/kg). Most samples (94%) were positive for FB1 (2–2,200 µg/kg, median 27 µg/kg). Ergot alkaloids were detected in 61% of samples (28–1,200 µg/kg, median 97 µg/kg), OTA was found in 42% of samples (0.2–4 µg/kg, median 0.35 µg/kg). The results demonstrate that a co-contamination with several mycotoxins is very common in commercial horse feed from the German market. The toxin concentrations were in most cases well below the levels which are usually considered as critical or even toxic. The highest mycotoxin concentrations were mostly found in single-grain cereal feed: the maximum values for DON and FB1 were found in maize, the highest T-2/HT-2 toxin concentrations were found in oats, and the highest concentration of ergot alkaloids was found in barley. In composed feeds, no correlation between cereal composition and mycotoxin levels could be found.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reports concerning mycotoxins in horse feed are very rare and typically are restricted to fumonisins. To the best of our knowledge, a broader and systematic survey on mycotoxins in commercial horse feed has not been published. This is somehow surprising, since mycotoxins may have various adverse effects in equines. In general, very little is known about the impact of mycotoxins in horse feed.
As a non-ruminant monogastric species, horses may be more sensitive towards adverse effects of mycotoxins, but little is known except a specific sensitivity towards fumonisins. The most severe effect of FB1 in equines is that it causes fatal leucoencephalomalacia (Marasas et al. 1988; Ross et al. 1991). The critical FB1 concentration in horse feed (maize) seems to be at about 10,000 µg/kg (EFSA, 2005b).
Much less is known about other mycotoxins, since very few feeding studies have been performed in horses, and even fewer mycotoxicosis outbreaks have been reported (Morgavi and Riley 2007). Raymond and colleagues (Raymond et al. 2003, 2005) fed naturally contaminated feed containing high levels of DON (11,000–15,000 µg/kg), 15-acetylDON (700–800 µg/kg), and ZEA (800–2,000 µg/kg) to horses and observed reduced feed intake as the predominant (DON-related) effect, but specific effects of ZEA were not reported. In contrast, Johnson et al. (1997) fed barley containing DON at levels up to 44,000 µg/kg to 5 horses and observed no adverse effects, specifically no feed refusal effects. These authors concluded that horses may not be as susceptible as other monogastric species to the toxic effects of DON.
In recent years, the European Food Safety Authority (EFSA) has evaluated several mycotoxins as “undesirable substances in animal feed”, in part with the aim of establishing guidance values for the feed industry.
In its evaluation of DON, EFSA concluded that this toxin exhibits toxic effects in all species, but that horses are more tolerant towards this toxin than pigs (EFSA 2004a). No specific guidance value for DON in horse feed was established.
No information concerning the effects of ZEA on horses was given in the EFSA evaluation of this mycotoxin (EFSA 2004b). We could not find any newer information in literature databases concerning health effects of ZEA in the horse, although preliminary data showed that ZEA (metabolites) can be found in horse blood plasma (Songsermsakul et al. 2006).
Concerning fumonisins, EFSA (2005b) agreed with earlier studies and concluded that the lowest observed adverse effect level is at 0.2 mg/kg body weight. There is also some evidence that the carry-over into milk is low.
In its evaluation concerning OTA in animal feed, EFSA concluded that herbivores such as horses that rely on cecal rather than ruminal fermentation may absorb OTA in the small intestine. These species may be thus more sensitive than ruminants, but quantitative data are lacking (EFSA 2004c).
Horses seem to be relatively sensitive towards ergot alkaloids, in particular towards ergovaline in pasture grasses with endophytic Neotyphodium spp. Ergovaline levels in grass as low as 50–100 µg/kg seem to be critical for mares, resulting in agalactia, delayed parturation, and neurotoxicity. It was further recommended that horse feed should not contain more than 5% rye, a cereal which is thought to be most susceptible for ergot (EFSA 2005a).
About one million horses are kept in Germany, the vast majority as leisure horses (Deutsche Reiterliche Vereinigung 2009). However, a small percentage is slaughtered for meat production, and a very small but growing market exists for horse milk. Therefore, mycotoxin residues may also present a problem if the horse is considered as a food-producing species.
The highest economic importance in relation to horse feed lies with complementary feedingstuffs, mainly cereal-based composed feeds such as “muesli”, mash, and pellets. Concerning single-grain cereal feeds, maize, oats and barley are the most important commodities. The fact that horses typically consume 1–5.5 kg of these feeds per day indicates that horse feed is of considerable economic importance for the German feed industry.
In this study, we present the results of a survey of commercial horse feed performed in 2007–2008 for some mycotoxins of major importance. Samples were purchased in the same form as offered to the regular buyer. All samples were analysed using a set of enzyme immunoassays (EIA) as developed by our research groups in earlier projects.
Materials and methods
Sampling and sample preparation
A total of 62 samples of commercial horse feed preparations (muesli and mash (n = 39), pelleted feedingstuff (n = 12), maize (n = 5), oats (n = 4), barley (n = 2)) were purchased from 21 different producers/distributors, typically in bags containing 20–25 kg each. The sampling included most major horse feed retailers in Germany. The attributed use of these feeds included all relevant feeding purposes, most products fell under the group of “complementary feeds”. The recommended quantity of feeding of these products vary greatly depending on factors such as the exercise condition of the horse and the individual product composition (e.g., oat content). As a rough estimate, typically 0.5–1 kg per 100 kg body weight are fed per day. In addition to barley, maize, oats, and wheat, other ingredients frequently listed in the feed description were alfalfa (Medicago sativa), soy extraction by-products, sugar beet molasses, edible oils (lineseed, sunflower, soy, thistle), minerals, and other nutrient additives. Three samples (nos. 2, 10 and 21) analysed within this study were declared to contain “Mycosorb” as a “mycotoxin binder”.
Several kg (3–5) from each bag were thoroughly mixed in a 10-l plastic bucket, and a subsample of about 1 kg was collected. If necessary (for example, for muesli and mash samples), these subsamples were dried for 16–18 h at 30°C in a laboratory drying oven. In such cases, the loss of weight (typically 5–20%) was recorded, and the analysed mycotoxin content corrected for the original weight. About 1 kg of sample material was ground to a mean particle size of <1 mm in a laboratory mill, and the powdery sample mixed again before each series of analyses.
Sample extraction
T-2 toxin and HT-2 toxin
To 5 g of sample material, 25 ml of extraction solvent (methanol/water, 70/30) were added in a 150-ml beaker and extracted by magnetic stirring (400 rpm) for 30 min. The mixture was filtered through a paper filter. Two ml of the filtrate were transferred into a glass test tube and mixed with 2 ml of distilled water, then 3 ml ethyl acetate were added and the test tube vigorously shaken on a vortex mixer for 1 min. The aqueous and the organic phase were separated by centrifugation (3,000g, 10 min at ambient temperature). The upper organic phase was transferred into a 25-ml evaporation flask, and the solvent was removed in a rotary evaporator at 50°C under reduced pressure. The residue was redissolved with 0.2 ml methanol on a vortex mixer, and then 1.8 ml PBS (pH 7.3) were added. To completely dissolve all residues, the flask was immersed in an ultrasonic bath for 15–20 s. The extract was transferred into a glass test tube, and 1 ml of n-heptane was added. Both solvents were thoroughly mixed on a vortex mixer (15–20 s), then the phases were separated by centrifugation (3,000g, 10 min). The lower aqueous phase was collected with a glass Pasteur pipette and transferred into a glass test tube. This extract was analysed either directly (sample dilution factor: 5), or after dilution with methanol/water (10/90) in case of higher toxin contents. Some sample material absorbed a large portion of the 25 ml extraction solvent, making extraction by magnetic stirring difficult. For such samples, 5 g material were extracted with 50 ml extraction solvent, resulting in a final sample dilution factor of 10.
Deoxynivalenol
To 5 g of sample material, 50 ml of extraction solvent (methanol/PBS (pH 7.3), 10/90) were added in a 150-ml beaker and extracted by magnetic stirring (400 rpm) for 30 min. The mixture was transferred into glass test tubes, centrifuged (1,500g, 15 min, 4°C) and filtered through a paper filter. Two ml of the filtrate and 4 ml of ethyl acetate were thoroughly mixed in a glass test tube on a Vortex for 1 min, then centrifuged (1,500 g, 15 min, 4°C). The upper organic phase was removed, and the aqueous phase extracted again with 4 ml of ethyl acetate. Both ethyl acetate phases were combined in a 50-ml round-bottom evaporation flask, and the solvent was removed in a rotary evaporator at 50°C under reduced pressure. The residue was dissolved with 1 ml of PBS (pH 7.3) by first vortexing (15–20 s) the flask, and then the flask was immersed in an ultrasonic bath for 15–20 s. This extract was analysed either directly (sample dilution factor: 5), or after dilution with PBS (pH 7.3) in the cases of higher toxin contents.
Zearalenone
To 5 g of sample material, 25 ml of extraction solvent (acetonitrile/water, 84/16) were added in a 150-ml beaker and extracted by magnetic stirring (400g) for 30 min. The mixture was filtered through a paper filter. A 100 µl portion of the filtrate was mixed with 15,80 µl PBS (pH 7.3) to give a 5% acetonitrile/PBS for EIA analysis (sample dilution factor: 84). Further dilutions were made with 5% acetonitrile/PBS if necessary.
Fumonisins
To 5 g of sample material, 25 ml of extraction solvent (methanol/water, 75/25) were added in a 150 ml beaker and extracted by magnetic stirring (400 rpm) for 30 min. The mixture was transferred into glass test tubes, centrifuged (3,000g, 15 min) and filtered through a paper filter. A 100-µl portion of the filtrate was mixed with 650 µl PBS (pH 7.3) in order to give a 10% methanol/PBS for EIA analysis (sample dilution factor: 37.5). Further dilutions were made with 10% methanol/PBS if necessary.
Ochratoxin A
To 2 g of sample material, 10 ml of 1 mol/l HCl were added in a 100-ml glass test tube and mixed for 5 min by magnetic stirring (400 rpm). Then 20 ml dichloromethane were added and mixed for another 15 min. The extract was centrifuged (1,500g, 15 min, 4°C). The upper aqueous layer was removed, and the organic phase transferred into an Erlenmeyer flask. Ochratoxin was extracted by liquid-liquid partitioning with 20 ml 0.13 mol/l NaHCO3 solution (pH 8.3) and magnetic stirring (400g) for 15 min. The mixture was transferred into another 100 ml glass test tube and centrifuged (1,500g, 15 min, 4°C). The upper aqueous phase was collected and analysed by EIA (sample dilution factor 10). Further dilutions were made with 0.13 mol/l NaHCO3 solution if necessary.
Ergot alkaloids
To 5 g of sample material, 25 ml of extraction solvent (acetonitrile/PBS (pH 6.0), 60/40) were added in a 150-ml beaker and extracted by magnetic stirring (400 rpm) for 30 min. The solid particles were allowed to settle within 5 min, then 2 ml of this extract were transferred with a Eppendorf pipette into a 2-ml Eppendorf vial. The extract was centrifuged (11,000g, 4 min, 20°C). After a 1:10 dilution with PBS (pH 6.0), this extract was used for EIA analysis (sample dilution factor 10). Further dilutions were made with 5% acetonitrile/PBS (pH 6.0) if necessary.
EIA analysis
Competitive direct enzyme immunoassays were performed as standard microtiter plate EIA using immunoreagents listed in Table 1. Typical standard curves of all six test systems used for mycotoxin quantification are shown in Fig. 1. Four replicate wells were analysed for all standard concentrations and sample dilutions. The original extract plus two serial dilutions (1:3, 1:9) of each sample extract were analysed initially. All dilutions resulting in absorbance values of 20–75% relative absorbance (B/B0) were used to calculate the toxin content. Highly positive extracts were reanalysed in higher dilutions.
Results and discussion
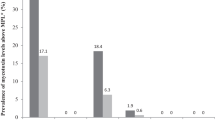
An overview of all results for all six test parameters is shown in Table 2. Since all samples contained high amounts of varying mixtures of cereals, and since relatively low detection limits were achieved in this study, it was no surprise that all samples were positive for DON (EFSA 2004a) and T-2/HT-2 toxin. Nearly all samples were positive for ZEA and FB1, which is also in accordance with most studies on the occurrence of these mycotoxins in cereals (EFSA 2004b, 2005b). It was remarkable, however, that the average level of Fusarium toxins was quite low, and most samples would have met the requirements concerning maximum levels in food (European Union 2006b). The maximum value for FB1 found in one sample (2,200 µg/kg) was well below the guidance value of 5,000 µg/kg for complementary and complete feedingstuffs for horses (European Union 2006a). The maximum level found for DON (4,900 µg/kg) in maize reached the general guidance value of 5,000 µg/kg for complementary and complete feedingstuffs, and exceeded 1,000 µg/kg in two other maize samples. However, all muesli, mash and pellet samples had DON concentrations below 1,000 µg/kg.
Likewise, high ZEA levels were found in maize (290 µg/kg), while the maximum concentration (310 µg/kg) was found in a pellet sample with a relatively high percentage of maize. These values were close to the guidance value of 500 µg/kg for complementary and complete feedingstuffs for cattle. However, in most cases, ZEA was well below 100 µg/kg.
No specific guidance values exist for T-2/HT-2 in feed. However, if a value of about 20 times lower than that for DON is used as a “first and rough” approximate (because of the much higher toxicity of T-2/HT-2), a maximum level of about 250 µg/kg would seem reasonable. Even the highest concentration found in oats for horse feeding (230 µg/kg) was below this level, and the majority of other samples were below 50 µg/kg (T-2 and HT-2). Comparing both the specific T-2 toxin EIA and the group T-2 + HT-2 toxin EIA, it becomes obvious that HT-2 dominates the toxin content in most samples. The overall ratio T-2 toxin:HT-2 toxin was approximately 1:2.2, although this relationship showed a high variability.
When different types of feeds are more specifically analysed, some differences and trends become obvious. The largest group of products, muesli and mash samples, had a high frequency of all toxins, but the levels were very low in nearly all samples (Table 3). In pellet feeds, the frequency of mycotoxins was even higher, but at very low mean and median levels (Table 4). In both groups, there was no clear relationship between the percentage of a certain type of grain and a specific mycotoxin profile.
In those samples which contained only one type of cereal (Table 5), the association between a certain type of grain and specific mycotoxins was more obvious and followed typical profiles. Among the major cereals, maize is most susceptible to co-contamination with DON, ZEA, and FB1, a fact that was also found in maize-based foods (Usleber and Märtlbauer 1998). T-2 and HT-2 toxin are predominantly associated with oats, but there was no quantitative relationship. Obviously the individual toxin concentration of oats raw material is much more important than the percentage of added oats in composed feed.
The levels of OTA were very low in all cases, with a maximum concentration of 4.1 µg/kg in one muesli sample. No relationship between OTA and a particular feed ingredient could be established. In general, OTA was found to be the mycotoxin with the least relevance in aspects of critical concentrations in feed.
An interesting finding was the widespread occurrence of ergot alkaloids in horse feed, while no sample was declared to contain rye. Obviously other cereals, in particular barley, are also subject to contamination with ergot. In fact, the highest value for ergot alkaloids (1,200 µg/kg) was found in barley (Table 5). This particular sample could be considered as critical according to the EFSA evaluation (EFSA 2005a).
In conclusion, the results of this first systematic survey demonstrate that mycotoxins are omnipresent in commercial horse feeds in Germany, exclusively as a multitoxin mélange. However, the toxin concentrations were in most cases relatively low, well below the levels which are usually considered as critical or even toxic. In fact, the vast majority of these materials would have been of “food quality”, since all toxins/toxin groups were present at levels below the respective EU maximum levels for foodstuffs (DON, ZEA, FB1, OTA), or below the de facto accepted levels in foods (T-2/HT-2 toxin, ergot alkaloids). Although these findings are reassuring, it has to be acknowledged that very little is known concerning the adverse effects of these mycotoxins in horses (with the exception of FB1), or concerning their carry-over into edible tissues. Additionally, further studies concerning mycotoxin intake from non-commercial sources of horse feed seem to be advisable.
References
Curtui V, Seidler C, Dietrich R, Märtlbauer E, Schneider E, Usleber E (2003) Bestimmung von Deoxynivalenol in Brot und Bier. Mycotoxin Res 19:144–148
Deutsche Reiterliche Vereinigung (2009). Statistical information concerning horses in Germany. Available at: http://www.pferd-aktuell.de/Wir-ueber-uns/Zahlen-Fakten/-.96/Zahlen-Fakten.htm (Accessed 15 October 2009)
Dietrich R, Schneider E, Usleber E, Märtlbauer E (1995) Use of monoclonal antibodies for the analysis of mycotoxins. Natural Toxins 3:288–293
EFSA, European Food Safety Authority (2004a) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J 73:1–42 Available at: http://www.efsa.europa.eu (Accessed 15 October 2009)
EFSA, European Food Safety Authority (2004b) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to zearalenone as undesirable substance in animal feed. EFSA J 89:1–35 Available at: http://www.efsa.europa.eu (Accessed 15 October 2009)
EFSA, European Food Safety Authority (2004c) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J 101:1–36 Available at: http://www.efsa.europa.eu (Accessed 15 October 2009)
EFSA, European Food Safety Authority (2005a) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ergot as undesirable substance in animal feed. EFSA J 225:1–27 Available at: http://www.efsa.europa.eu. (Accessed 15 October 2009)
EFSA, European Food Safety Authority (2005b) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to fumonisins as undesirable substances in animal feed. EFSA J 235:1–32 Available at: http://www.efsa.europa.eu. (Accessed 15 October 2009)
Esgin S, Märtlbauer E, Terplan G (1989) Entwicklung und Anwendung eines enzymimmunologischen Verfahrens zum Nachweis von T-2 Toxin in Milch. Arch Lebensmittelhyg 40:109–112
European Union (2006a) Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off J Eur Comm L229:7–9 Available at: http://eur-lex.europa.eu/en/index.htm (Accessed 15 October 2009)
European Union (2006b) Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Comm L364:5–24 Available at: http://eur-lex.europa.eu/en/index.htm (Accessed 15 October 2009)
Hack R, Märtlbauer E, Terplan G (1989) A monoclonal antibody-based enzyme immunoassay for the detection of T-2 toxin at picogram levels. Lett Appl Microbiol 9:133–155
Johnson PJ, Casteel SW, Messer NT (1997) Effect of feeding deoxynivalenol (vomitoxin)-contaminated barley to horses. J Vet Diagn Invest 9:219–221
Marasas WF, Kellerman TS, Gelderblom WC, Coetzer JA, Thiel PG, van der Lugt JJ (1988) Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res 55:197–203
Morgavi DP, Riley RT (2007) An historical overview of field disease outbreaks known or suspected to be caused by consumption of feeds contaminated with Fusarium toxins. Anim Feed Sci Technol 137:201–212
Raymond SL, Smith TK, Swamy HVLN (2003) Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry, and hematology of horses, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J Anim Sci 81:2123–2130
Raymond SL, Smith TK, Swamy HVLN (2005) Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, metabolism, and indices of athletic performance of exercised horses. J Anim Sci 83:1267–1273
Ross PF, Rice LG, Reagor JC, Osweiler GD, Wilson TM, Nelson HA, Owens DL, Plattner RD, Harlin KA, Richard JL, Colvin BM, Banton MI (1991) Fumonisin B1 concentrations in feeds from 45 confirmed equine leukoencephalomalacia cases. J Vet Diagn Invest 3:238–241
Schneider E, Usleber E, Dietrich R, Märtlbauer E (2001) Entwicklung eines hochempfindlichen Enzymimmuntests zum Nachweis von Ochratoxin A. Mycotoxin Res 17A:170–173
Seidler C (2007) Nachweis der Fusarientoxine Deoxynivalenol und Zearalenon in Lebensmitteln. Thesis, Giessen. Available at: http://geb.uni-giessen.de/geb/volltexte/2007/4728/pdf/SeidlerCaroline-2007-04-25.pdf. (Accessed 15 October 2009)
Songsermsakul P, Sontag G, Cichna-Markl M, Zentek J, Razzazi-Fazeli E (2006) Determination of zearalenone and its metabolites in urine, plasma and faeces of horses by HPLC-APCI-MS. J Chromatogr B 843:252–261
Usleber E, Märtlbauer E (1998) A limited survey of cereal foods from the German market for Fusarium toxins (deoxynivalenol, zearalenone, fumonisins). Arch Lebensmittelhyg 49:42–45
Usleber E, Märtlbauer E, Dietrich R, Terplan G (1991) Direct enzyme-linked immunosorbent assays for the detection of the 8-ketotrichothecene mycotoxins deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol in buffer solutions. J Agric Food Chem 39:2091–2095
Usleber E, Renz V, Märtlbauer E, Terplan G (1992) Studies on the application of enzyme immunoassays of the Fusarium mycotoxins deoxynivalenol, 3-acetyldeoxynivalenol, and zearalenone. J Vet Med B 39:617–627
Usleber E, Straka M, Terplan G (1994) Enzyme immunoassay for fumonisin B1 applied to corn-based food. J Agric Food Chem 42:1392–1396
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liesener, K., Curtui, V., Dietrich, R. et al. Mycotoxins in horse feed. Mycotox Res 26, 23–30 (2010). https://doi.org/10.1007/s12550-009-0037-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-009-0037-8