Abstract
The Middle to Upper Pennsylvanian Buckhorn Asphalt Quarry (Boggy Formation, Deese Group) of Oklahoma, USA, is well known for its exceptionally preserved fauna of marine invertebrates, including conservation of original skeletal aragonite. Here, we describe for the first time the taxonomy of the Buckhorn bryozoans, recognising nine species, two of which are new: Stenophragmidium buckhornensis sp. nov. and Streblotrypa (Streblotrypa) heltzelae sp. nov. Two further species, Stenoporella sp. and Spinofenestella sp., are described in open nomenclature. The other species show relationships to the Pennsylvanian of the USA and Russia. The genera Shishoviclema and Shulgapora are identified for the first time in North America. Superior preservation of the Buckhorn bryozoans allows some new and poorly known skeletal characters to be described. These include nanoperforations, granule bands, mural spines, spinose hemiphragms and transverse fibrous wall fabric. Nanoperforations, found in the skeletal walls of several Buckhorn bryozoan species, are tiny holes around which laminae are deflected, indicating that they are not post-mortem structures. However, it is unclear whether they are features of the bryozoans or have resulted from the presence of microsymbionts. The primary wall layer of the fenestrate Septopora blanda Moore, 1929 is apparently composed of transverse fibrous crystallites, a skeletal fabric previously known only in post-Palaeozoic cyclostomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The classic fossil Lagerstätten of the Palaeozoic are notable mostly for containing exquisitely preserved arthropod and ‘worm’ faunas, with preservation of non-mineralized cuticle and other soft parts (e.g. Bottjer et al. 2002). Bryozoans are occasionally present in these Lagerstätten (e.g. Hunsrück Slate; Bartels et al. 2009) but apparently without soft part preservation or other features that cannot be found routinely elsewhere. The diverse Pennsylvanian marine biota found in the deposits of the Buckhorn Asphalt Quarry, southern Oklahoma, is unusual among Palaeozoic Lagerstätten. Exceptionally for the Palaeozoic, this biota preserves pristine skeletal microstructures and mineralogy, notably aragonite and high-Mg calcite, which are usually destroyed during diagenesis. Fossils recorded from the Buckhorn Asphalt Quarry include, among others, various molluscs, foraminifera, brachiopods, chaetetid sponges and bryozoans (Seuss et al. 2009), the latter being the subject of the current paper.
Pennsylvanian bryozoans of Oklahoma have been occasionally mentioned in some general faunal descriptions (e.g. Girty 1911, 1915; Morgan 1924; Harlton 1933). However, the bryozoans of the Boggy Formation, which encompasses the rocks found in the Buckhorn Asphalt Quarry, have never been comprehensively described. Although the use of bryozoans in stratigraphy is quite limited, Palaeozoic bryozoans can be important in palaeoecology and palaeobiogeography (e.g. Ross 1981; Bancroft 1987; Buttler et al. 2013). This reflects their taphonomically resistant skeletons of low magnesium calcite and consequent abundance in various marine deposits.
The aims of the current paper are to describe the taxonomy of the bryozoans from the Buckhorn Asphalt Quarry and discuss features evident in their exceptionally preserved skeletons. Many of these features have not been reported before for Palaeozoic bryozoans and have broader significance for understanding the palaeobiology of bryozoans of this age, which mostly belong to the extinct Superorder Palaeostomata.
Geological setting
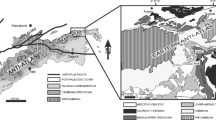
The Buckhorn Asphalt Quarry (Fig. 1) near Sulphur, Oklahoma (GPS NAD84: 34°26′44″N; 96°57′41″W) today is an outcrop approximately 150 m in length, 21 m wide and 6 m high (Seuss et al. 2009, 2012a). The quarry is positioned on the northern edge of the Arbuckle Mountains, near the Texas–Oklahoma state boundary (Seuss et al. 2009, 2012a, b). It contains Middle to Upper Pennsylvanian (Desmoinesian to Virgilian) siliciclastic-carbonate (bioclastic) deposits (Seuss et al. 2009, and unpublished data) that belong to the Boggy Formation (Deese Group) (Fig. 2; Ham 1969; Sadd 1991). The Desmoinesian and Missourian sediments contain a rich (more than 160 species and 125 genera; Seuss et al. 2009) and well- to outstandingly preserved (e.g. colour patterns, aragonite, delicate larval shells and ornament) fauna (Seuss et al. 2009, 2012c), which is dominated by gastropods, foraminifers and bivalves (Seuss et al. 2009, 2012a). The Missourian deposits are capped by the Virgilian Ada conglomerate. Deposition of the most fossiliferous deposits occurred during a transgressive–regressive cycle (Seuss et al. 2009) in only a few metres water depth (Cree 1984), i.e. the shallow euphotic zone II to III (Wisshak et al. 2008), while deeper water deposits are dominated by cephalopod remains (Seuss et al. 2009, 2012b). The exceptional preservation of the fauna is a result of hydrocarbon impregnation during or only very shortly after deposition of the sediments (Seuss et al. 2009).
a Distribution of asphaltic deposits in Oklahoma (Hutchinson 1911, p. 5, modified) and the position of the Buckhorn Asphalt Quarry (marked with a star), b location of the Buckhorn Asphalt Quarry within Middle to Upper Pennsylvanian deposits (modified after Squires 1973 and published by Seuss et al. 2009, fig. 2)
a General view of the quarry (Seuss et al. 2009, fig. 4d), b south-western entrance into the quarry, Missourian deposits in the Buckhorn Asphalt Quarry capped by the Virgilian Ada conglomerate, c idealized lithological section of the sediments in the Buckhorn Asphalt Quarry (after Seuss et al. 2009, fig. 6) showing occurrence of bryozoan species
Material and methods
Bryozoans were studied both in thin sections and using scanning electron microscopy (SEM). SEM investigation was made possible because the samples from the Buckhorn Asphalt Quarry were disaggregated by using a Soxhlet apparatus and the organic solvent methylene chloride to remove asphalt and to set free the fossils (Seuss et al. 2009). For thin sections, individual colonies of bryozoans were first embedded in epoxy resin (SpeciFix 40; Fa. Struers), then cut to prepare a total of 36 thin sections which were investigated using a binocular microscope in transmitted light.
SEM at the Natural History Museum of the United Kingdom (NHMUK) employed two instruments: a LEO 1455-VP and a FEI Quanta 650 FEG for higher resolution study. Both microscopes were operated under low vacuum, using back-scattered electrons to image uncoated specimens.
Morphological character terminology is partly adopted from Anstey and Perry (1970) for trepostomes, from Hageman (1991) for fenestrates, and Hageman (1993) for cryptostomes. The spacing of structures is measured as the distance between their centres (Ernst et al. 2015). Statistics were summarised using arithmetic mean, sample standard deviation, coefficient of variation, and minimum and maximum values. The studied material is deposited at the Bayerische Staatssammlung für Paläontologie und Geologie, Munich (SNSB-BSPG).
Systematic palaeontology
Phylum Bryozoa Ehrenberg, 1831
Class Stenolaemata Borg, 1926
Superorder Palaeostomata Ma, Buttler and Taylor, 2014
Order Trepostomata Ulrich, 1882
Suborder Amplexoporina Astrova, 1965
Family Stenoporidae Waagen and Wentzel, 1886
Genus Stenophragmidium Bassler, 1952
Type species: Stenophragma lobatum Munro, 1912. Lower Carboniferous, Mississippian, Viséan; England.
Diagnosis: Colonies encrusting, rarely ramose. Autozooecia with rounded-polygonal and oval apertures. Hemiphragms short, often curved proximally, positioned on one side of autozooecia. Exilazooecia rare. Acanthostyles both large and small, rarely one size. Exozonal walls laminated, merged, without distinct autozooecial boundaries, with moniliform thickenings.
Remarks: Stenophragmidium Bassler, 1952 is similar to Tabulipora Young, 1883 in having moniliform walls and acanthostyles of two sizes, but differs from it by having hemiphragms instead of ring septa. Stenophragmidium differs from Stenopora Lonsdale, 1844 by having both hemiphragms and diaphragms, whereas Stenopora lacks any kind of diaphragms.
Occurrence: Lower Carboniferous (Mississippian) to Lower Permian; Europe, North America, China, Mongolia, Russia.
Stenophragmidium buckhornensis sp. nov.
a–e Stenophragmidium buckhornensis sp. nov.: a, b fragment of colony with autozooecial apertures, macro- and microacanthostyles, holotype SNSB-BSPG 2011 X 34, c autozooecial chamber with hemiphragm (arrow), holotype SNSB-BSPG 2011 X 34, d fragment of colony with macro- and microacanthostyles, paratype SNSB-BSPG 2011 X 35, e tangential thin section showing hemiphragms in autozooecial chambers, SNSB-BSPG 2011 X 39, f, g Tabulipora hispida (Coryell in Morgan, 1924), fragment of colony with autozooecial apertures, macro- and microacanthostyles and ring septa, SNSB-BSPG 2011 X 42, h, i Stenoporella sp., fragment of colony with autozooecial apertures, macro- and microacanthostyles and mural spines in autozooecial chambers (i, arrow), SNSB-BSPG 2011 X 45
Holotype: SNSB-BSPG 2011 X 34.
Paratypes: SNSB-BSPG 2011 X 33, SNSB-BSPG 2011 X 35-SNSB-BSPG 2011 X 41, SNSB-BSPG 2011 X 196-SNSB-BSPG 2011 X 197.
Type locality: Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Type stratum: Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation.
Etymology: The species named after the type locality, the Buckhorn Asphalt Quarry.
Diagnosis: Colony encrusting; exilazooecia rare; macroacanthostyles prominent, 1–4 surrounding each autozooecial aperture; microacanthostyles arranged in one row between macroacanthostyles; hemiphragms closely spaced.
Description: Encrusting colony, 0.68–0.94 mm in thickness. Exilazooecia rare. Acanthostyles of two sizes: large macroacanthostyles and small microacanthostyles. Macroacanthostyles prominent, 1–4 surrounding each autozooecial aperture. Microacanthostyles arranged in a single row between macroacanthostyles. Hemiphragms closely spaced, occupying more than half of the autozooecial chamber space. Autozooecial walls in exozone laminated, moniliform, 0.04–0.08 mm in thickness.
Remarks: Stenophragmidium buckhornensis sp. nov. is similar to S. granulosum Dunaeva, 1964 from the Mississippian (Viséan) of Ukraine in having large macroacanthostyles and a series of microacanthostyles between them, but the new species has slightly smaller autozooecia (aperture width 0.10–0.18 vs. 0.18–0.19 mm in S. granulosum). Stenophragmidium buckhornensis differs from S. megistum Perry and Gutschick, 1959 from the Amsden Formation (Mississippian, Serpukhovian) of Montana, by its smaller autozooecia (aperture width 0.10–0.18 vs. 0.15–0.25 mm in S. megistum).
Genus Tabulipora Young, 1883
Type species: Cellepora urii Fleming, 1828. Lower Carboniferous (Mississippian); Scotland.
Diagnosis: Colonies ramose, encrusting, cylindrical or massive. Autozooecia with basal diaphragms and ring septa. Autozooecial walls irregularly thickening with development of monilae. Exilazooecia rare. Acanthostyles of two sizes: large macroacanthostyles and small microacanthostyles.
Remarks: Tabulipora Young, 1883 differs from Stenopora Lonsdale, 1844 and Stenodiscus Crockford, 1945 by the development of ring septa.
Occurrence: Carboniferous–Permian; worldwide.
Tabulipora hispida (Coryell in Morgan, 1924)
1924 Stenopora hispida Coryell in Morgan, p. 181, pl. 41, figs. 1, 2.
Material: SNSB-BSPG 2011 X 42-SNSB-BSPG 2011 X 44.
Description: Colony encrusting, thickness unknown. Exilazooecia rare to common. Acanthostyles of two sizes: large macroacanthostyles and small microacanthostyles. Macroacanthostyles prominent, 4–6 surrounding each autozooecial aperture. Microacanthostyles arranged in a single row between macroacanthostyles. Ring septa common, with semicircular openings occupying about a half of the autozooecial chamber space and showing preferred orientation in the colony. Autozooecial walls in exozone laminated, monilae-shaped, 0.030–0.055 mm in thickness.
Remarks: Tabulipora hispida (Coryell in Morgan, 1924) is similar to T. demisa Trizna, 1961 from the Moscovian of Urals, but differs in the presence of both micro-/macroacanthostyles, whereas T. demisa possesses only macroacanthostyles. Tabulipora hispida differs from T. micropora (Coryell in Morgan, 1924) from the Wapanucka Limestone of Oklahoma by its less abundant exilazooecia.
Occurrence: Carboniferous, Pennsylvanian, Wapanucka Limestone; Oklahoma, USA. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Genus Stenoporella Bassler, 1936
Type species: Stenoporella romingeri Bassler, 1936. Chesterian (= Serpukhovian); Arkansas (USA).
Diagnosis: Colonies massive or encrusting laminar. Endozone indistinctly developed. Autozooecia prismatic, budding from thin basal epitheque, having polygonal apertures. Complete or perforated diaphragms may be present. Thick, short spines in zooecial walls present, projecting into the zooecial cavity. Walls thin, locally crenulated in the endozone; laminations reversed U-shaped, moniliform thickenings sometimes present in the exozone. Exilazooecia polygonal in cross section. Large acanthostyles at zooecial junctions, originating in the endozone. Microacanthostyles may or may not be present along zooecial borders.
Remarks: The genus Stenoporella Bassler, 1936 differs from other stenoporid genera by the presence of spines in the zooecial walls.
Occurrence: Chesterian (Late Mississippian) of Arkansas, Alabama, and Utah, USA.
Stenoporella sp.
(Figs. 3h–l, 4a, b, 9b–d; Table 3)
a, b Stenoporella sp., fragment of colony with autozooecial apertures, macro- and microacanthostyles and mural spines in autozooecial chambers, SNSB-BSPG 2011 X 46, c–i Shishoviclema carbonaria (Coryell in Morgan, 1924), c fragment of colony with autozooecial apertures, acanthostyles and paurostyles (arrow metazooecium), SNSB-BSPG 2011 X 47, d, e, h tangential section showing autozooecial apertures, acanthostyles and paurostyles (arrow metazooecium), SNSB-BSPG 2011 X 124, f, g longitudinal section showing autozooecial chambers and aktinotostyles, SNSB-BSPG 2011 X 125, i branch transverse section, SNSB-BSPG 2011 X 198
Material: SNSB-BSPG 2011 X 45-SNSB-BSPG 2011 X 46.
Description: Colony encrusting and erect branched; thickness of encrusted colony unknown; erect colony with branches 0.83 mm in diameter. Autozooecia short and wide; autozooecial diaphragms, hemiphragms and ring septa absent. Autozooecial apertures rounded-polygonal. Short mural spines present in zooecial walls, projecting into the zooecial cavity, 6.9–8.5 μm in diameter. Exilazooecia rare to abundant. Acanthostyles of two sizes: large macroacanthostyles and smaller microacanthostyles. Macroacanthostyles positioned at junctions between autozooecial apertures, 0.033–0.038 mm in diameter. Microacanthostyles arranged in a single row between macroacanthostyles, 0.021–0.025 mm in diameter. Autozooecial walls in exozone 0.025–0.058 mm in thickness.
Remarks: The Buckhorn species shows similarities to the genus Stenoporella Bassler, 1936, which is known from three species from the Mississippian of USA. It shares the following characters with Stenoporella (in part): thin walls with mural spines; acanthostyles of two sizes; and encrusting colonies. However, the mural spines in the present material are smaller than those in the three known species of Stenoporella (McKinney 1971), spine diameter being 6.9–8.5 versus 30 μm in Stenoporella. The Buckhorn species differs from Stenoporella sparsispinifera McKinney, 1971 from the Chesterian of Alabama, in its smaller autozooecia (average autozooecial diameter 0.20 vs. 0.24 mm in S. sparsispinifera), and smaller macroacanthostyles (0.033–0.038 vs. 0.03–0.10 mm in S. sparsispinifera).
Occurrence: Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Order Cryptostomata Vine, 1884
Suborder Rhabdomesina Astrova and Morozova, 1956
Family Rhomboporidae Simpson, 1895
Genus Shishoviclema Gorjunova, 1985
Type species: Shishoviclema ninae Gorjunova, 1985, by original designation. Carboniferous, Pennsylvanian (Gzhelian); Russia.
Diagnosis: Colonies erect with cylindrical branches. Autozooecia relatively short, teardrop shaped in the transverse section of exozone, growing from a distinct median axis, then bending sharply in exozone. Hemisepta absent; basal diaphragms occasionally developed. Aktinotostyles abundant, regularly arranged between autozooecial apertures. Single large acanthostyle between successive autozooecial apertures, with narrow hyaline core and wide laminated sheaths. Metazooecia usually absent, but a few occasionally present. Autozooecial walls laminated, without distinct boundaries in exozones.
Remarks: Shishoviclema Gorjunova, 1985 differs from Rhombopora Meek, 1872 by the absence of paurostyles and the presence of aktinotostyles, and from Saffordotaxis Bassler, 1952 by the presence of acanthostyles.
Occurrence: Devonian to Permian; worldwide.
Shishoviclema carbonaria (Coryell in Morgan, 1924)
1924 Acanthoclema carbonarium Coryell in Morgan, p. 176, pl. 36, figs. 1, 2.
Material: SNSB-BSPG 2011 X 47- SNSB-BSPG 2011 X 49, SNSB-BSPG 2011 X 120-SNSB-BSPG 2011 X 135, SNSB-BSPG 2011 X 198.
Description: Colonies erect, branches 0.61–1.10 mm in diameter, with 0.17–0.32 mm wide exozones and 0.34–0.52 mm wide endozones. Autozooecia short, growing in spiral pattern from a distinct median axis at angles of 30–38° in endozone, abruptly bending in exozones and intersecting colony surface at angles of 75–80°; triangular to rhombic, teardrop shaped in transverse sections of endozone. Autozooecial apertures oval, arranged in quincunx on colony surface. Aktinotostyles abundant, arranged in a single row between autozooecial apertures forming relatively regular hexagons. Single large acanthostyle between successive autozooecial apertures, with narrow hyaline core and wide laminated sheaths. Metazooecia rare to absent. Autozooecial walls laminated, without distinct boundaries in exozone. Autozooecial walls hyaline, 0.010–0.015 mm thick in endozone; laminated in exozone.
Remarks: Shishoviclema carbonaria (Coryell in Morgan, 1924) differs from S. ninae Gorjunova, 1985 from the Gzhelian of Russia in its thinner branches (branch diameter 0.61–1.10 vs. 1.08–1.53 mm in S. ninae) and in the smaller acanthostyles (acanthostyle diameter 0.04–0.05 vs. 0.07–0.10 mm in S. ninae).
Occurrence: Carboniferous, Pennsylvanian, Wapanucka Limestone; Oklahoma, USA. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Family Hyphasmoporidae Vine, 1885
Genus Streblotrypa Vine, 1885
Subgenus Streblotrypa (Streblotrypa) Vine, 1885
Type species: Streblotrypa nicklesi Vine, 1885. Lower Carboniferous; England.
Diagnosis: Colonies erect, branched. Indistinct bundle of about 10 or fewer axial zooecia in the endozone. Autozooecia budding from axial bundle, having long inflated proximal parts, rounded-polygonal in transverse section in the endozone, bending abruptly at the transition between endo- and exozone. Autozooecial apertures rounded to oval. Diaphragms rare. Hemisepta usually present. Metazooecia usually restricted to rows between the autozooecial apertures; styles usually lacking but poorly developed acanthostyles sometimes occurring. Autozooecial walls laminated, without distinct autozooecial boundaries.
Remarks: Streblotrypa (Streblotrypa) Vine, 1885 differs from S. (Streblascopora) Bassler, 1929 by an indistinctly defined axial bundle of axial zooecia and well-developed hemisepta.
Occurrence: Carboniferous to Permian; worldwide.
Streblotrypa (Streblotrypa) heltzelae sp. nov.
(Figs. 5a–i, 6a, 8b, c, e–g; Table 5)
a–i Streblotrypa (Streblotrypa) heltzelae sp. nov.: a fragment of colony showing autozooecial apertures, metazooecia, acanthostyles and microstyles, holotype SNSB-BSPG 2011 X 136, b, c fragment of colony showing autozooecial apertures, metazooecia, macroacanthostyles and microstyles (c, black arrow macroacanthostyles, white arrow microstyles), holotype SNSB-BSPG 2011 X 136, d fragment of colony split in the middle showing autozooecial chambers with hemisepta (arrows) and metazooecia, paratype SNSB-BSPG 2011 X 139, e fragment of colony with secondary branch, paratype SNSB-BSPG 2011 X 141, f broken part of the secondary branch showing bundle of axial zooecia (arrow), paratype SNSB-BSPG 2011 X 182, g tangential thin section showing autozooecial apertures, metazooecia, and acanthostyles, paratype SNSB-BSPG 2011 X 180, h longitudinal section showing autozooecial chambers, paratype SNSB-BSPG 2011 X 179, i longitudinal section showing inferior hemiseptum (arrow), paratype SNSB-BSPG 2011 X 178
a Streblotrypa (Streblotrypa) heltzelae sp. nov., branch transverse section, paratype SNSB-BSPG 2011 X 175, b–f Rhombocladia delicata Rogers, 1900: b, c fragment of colony showing autozooecial apertures, macro- and microacanthostyles, SNSB-BSPG 2011 X 183, d–f tangential section showing autozooecial chambers (f, hemiseptum, arrow), SNSB-BSPG 2011 X 187, g, h Spinofenestella sp., fragment of colony showing autozooecial apertures and nodes on median keel, SNSB-BSPG 2011 X 189
Holotype: SNSB-BSPG 2011 X 136.
Paratypes: SNSB-BSPG 2011 X 137-SNSB-BSPG 2011 X 182.
Type locality: Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Type stratum: Carboniferous, Pennsylvanian, Early-Mid Desmoinesian (Moscovian), Missourian (Kasimovian), Deese Group, Boggy Formation.
Etymology: Named in honour of Mary Lou Heltzel, the owner of the Buckhorn Asphalt Quarry, who kindly provided access to the quarry.
Diagnosis: Colonies erect, ramose, branching at angles of 80–90°; axial bundle formed by 2–5 axial zooecia; superior hemisepta absent; inferior hemisepta well developed; 4–8 metazooecia arranged in 2–3 rows between apertures; up to 3 acanthostyles arranged around distal end of autozooecial aperture; microstyles arranged between acanthostyles or on ridges around metazooecia and autozooecial apertures, positioned very shallowly in the laminated skeleton.
Description: Colonies erect, ramose, branched, branches 0.55–0.84 mm in diameter, with 0.20–0.36 mm wide endozones and 0.14–0.24 mm wide exozones. Branch transverse sections rounded to oval. Secondary branches arising at angles of 80–90°. Autozooecia budding from the poorly developed axial bundle in a regular spiral pattern at angles of 27–33°, bending in exozones and intersecting colony surface at angles of 75–90°, having long inflated proximal parts, rounded-polygonal in transverse section in the endozone. Axial bundle formed by 2–5 axial zooecia, 0.10–0.15 mm in diameter. Autozooecial apertures oval, opening around the branches in regular diagonal rows. Autozooecial boundaries marked by sharp ridges on the colony surface, which form a roughly hexagonal pattern. Superior hemisepta absent; inferior hemisepta well developed, thin, placed approximately in the middle of the chamber, curved proximally. Autozooecial diaphragms rarely occurring. Metazooecia oval to rounded, usually 4–8 arranged in 2–3 rows between apertures, but often clustered in parts of branches lacking apertures. Acanthostyles scattered on the colony surface; usually up to 3 acanthostyles arranged around distal end of each autozooecial aperture. Microstyles arranged between acanthostyles or on ridges around metazooecia and autozooecial apertures, positioned very shallowly in the laminated skeleton, 0.005–0.008 mm in diameter. Autozooecial walls hyaline, 0.005–0.008 mm thick in endozone; laminated in exozone.
Remarks: This new species is similar to Streblotrypa (Streblotrypa) merceri Morningstar, 1922 from the Lower Mercer Limestone of Ohio. However, the latter species has uniformly four metazooecia between autozooecial apertures (rarely 3–6 metazooecia) and lacks acanthostyles. The interior morphology of S. (S.) merceri is unknown. The Buckhorn species differs from S. (S.) multipora Warthin, 1930 from the Wewoka Formation of Oklahoma by having smaller autozooecial apertures (aperture width 0.08–0.11 vs. 0.12–0.14 mm in S. (S.) multipora). The interior morphology of S. (S.) multipora is unknown. The new species differs from S. (S.) mongolica Popeko, 1967 from the Pennsylvanian of Mongolia by its smaller branches (diameter 0.55–0.84 vs. 1.0–2.0 mm in S. (S.) mongolica), presence of hemisepta, and smaller autozooecial apertures (aperture width 0.08–0.11 vs. 0.09–0.13 mm in S. (S.) mongolica).
Order Fenestrata Elias and Condra, 1957
Suborder Phylloporinina Lavrentjeva, 1979
Family Chainodictyonidae Nickles and Bassler, 1900
Genus Rhombocladia Rogers, 1900
Type species: Rhombocladia delicata Rogers, 1900. Pennsylvanian (Upper Coal Measures); Kansas, USA.
Diagnosis: Colonies ramose, dendroid. Flattened branches bearing 4–12 zooecial rows. Vestibule weakly developed. Diaphragms rare. Superior hemisepta usually developed. Oval apertures arranged in a diagonal pattern. Autozooecial chambers rhombic in mid-tangential section. Macroacanthostyles often occurring at distal ends of autozooecial apertures. Microacanthostyles present in zooecial walls, sometimes forming star-like accumulations. Leptozooecia rarely present on the frontal surface or on lateral parts of branches. Dorsal wall very thin.
Remarks: The genus Rhombocladia differs from Chainodictyon Foerste, 1887 by having a dendroid instead of a reticulate colony-form and by the development of hemisepta. From Kallodictyon Morozova, 1981, it differs by colony-form, the thin dorsal wall and the absence of leptozooecia on the dorsal surface of the colony.
Rhombocladia delicata Rogers, 1900
(Figs. 6b–f, 8a, d, 9a; Table 6)
1900 Rhombocladia delicata Rogers, p. 12, pl. 1, figs. 1–1d.
pars 1906 Rhombocladia delicata Rogers; Johnsen, p. 58, pl. 11, fig. 30a [? non 30b].
pars 1929 Rhombocladia delicata Rogers; Moore, p. 149, pl. 17, figs. 26–28, 30, 31 [non 29, 32].
1930 Rhombocladia delicata Rogers; Warthin, p. 42, pl. 3, fig. 17.
1963 Rhombocladia delicata Rogers form A; Ceretti, p. 327, pl. 7, fig. 10.
1964 Rhombocladia delicata Rogers; Ceretti, pp. 184, 185, pl. 33, fig. 2a, b, 4a, b.
2003 Rhombocladia delicata Rogers; Ernst, pp. 63, 64, pl. 5, figs. 4–7; text-fig. 3.
Material: SNSB-BSPG 2011 X 183-SNSB-BSPG 2011 X 188.
Description: Colonies erect, ramose, dendroid; branches 0.76–1.12 mm wide, 0.48 mm deep, flattened, bearing 5–7 zooecial rows; dorsal wall rugose. Diaphragms not observed. Superior hemisepta present. Oval apertures arranged in a diagonal pattern. Autozooecial chambers rhombic in mid-tangential section. Single macroacanthostyle at the distal end of each autozooecial aperture present. Microacanthostyles present in the zooecial walls of exozone. Leptozooecia rare, small, occurring near lateral parts of branches, adjacent to the thick calcification forming the dorsal wall. Autozooecial walls in endozone 0.01–0.02 mm thick, hyaline; finely laminated in exozone.
Remarks: Rhombocladia delicata Rogers, 1900 differs from R. coronata Schulga-Nesterenko, 1955 from the Moscovian of Russian Platform in having macroacanthostyles. It differs from R. carnica Ceretti, 1964 from the Pennsylvanian (lower Gzhelian) of Italy by having wider branches (0.76–1.12 vs. 0.63–0.96 mm).
The surface texture of the dorsal surface in Buckhorn material of R. delicata has the appearance of calcified exterior wall. Unlike the smooth textured, style-covered skeletal walls on the frontal side, it is rugose and lacks styles.
Occurrence: Carboniferous, Pennsylvanian; Kansas, USA. Carboniferous, Middle Pennsylvanian (Wewoka Formation); Oklahoma, USA. Carboniferous, Pennsylvanian, Corona Formation (lower Gzhelian); Kron Alpe (Monte Corona), Carnic Alps (Austria). Carboniferous, Pennsylvanian, Auernig Formation (lower Gzhelian); Auernig, Carnic Alps (Udine, Italy). Strata probably attributable to the Las Llacerias Formation, Pennsylvanian (Kasimovian); Cantabrian Mountains, Asturias, NW Spain. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Suborder Fenestellina Astrova and Morozova, 1956
Family Fenestellidae King, 1849
Genus Spinofenestella Termier and Termier, 1971
Type species: Fenestella spinulosa Condra, 1902 by original designation. Upper Carboniferous, Pennsylvanian, Coal Measures; USA.
Diagnosis: Colony fan-shaped or conical. Branches straight, connected by thin dissepiments. Two rows of autozooecia per branch, overlapped basally. Autozooecial chambers triangular in deep tangential section, oval immediately below the distal tube. Chambers circular in cross-section. Proximal hemisepta absent or poorly developed. Low keel a single row of nodes on the observe surface.
Remarks: Alternifenestella Termier and Termier, 1971 is a synonym of Spinofenestella due to the close morphological similarities of their type species, according to Hageman and McKinney (2010), although Gorjunova and Weiss (2012) retained Alternifenestella as a valid genus.
Occurrence: Silurian to Upper Permian; worldwide.
Spinofenestella sp.
a Spinofenestella sp., broken part of branch showing autozooecial chambers with superior hemiseptum, SNSB-BSPG 2011 X 189, b–d Septopora blanda Moore, 1929. b fragment of colony showing autozooecial chambers, nodes and cyclozooecia, SNSB-BSPG 2011 X 191, c, d fragment of colony showing autozooecial chambers, nodes and cyclozooecia (c, arrows), SNSB-BSPG 2011 X 192, e–h Shulgapora helenae (Schulga-Nesterenko, 1951): e fragment of colony showing autozooecial chambers and cyclozooecia, SNSB-BSPG 2011 X 193, f fragment of colony showing autozooecial chambers with apertural nodes and cyclozooecia, SNSB-BSPG 2011 X 194, g, h fragment of colony showing autozooecial chambers with apertural nodes and cyclozooecia (arrow), SNSB-BSPG 2011 X 195
Material: SNSB-BSPG 2011 X 189-SNSB-BSPG 2011 X 190.
Exterior description: Colony reticulate, formed by straight branches joined by narrow dissepiments. Fenestrules oval to rectangular, long, narrow. Autozooecia arranged in two rows on branches. Autozooecial apertures circular, with low smooth peristome; two apertures per fenestrule length. Small elliptical nodes on the low keel, closely spaced.
Interior description: Autozooecia short, triangular to trapezoidal in mid-tangential section; vestibules short. Axial wall between autozooecial rows strongly zigzag; aperture positioned at distal end of chamber. Short superior hemisepta present.
Remarks: The Buckhorn species is similar to Fenestella sasakwaensis Warthin, 1930 from the Holdenville Formation (Pennsylvanian, Moscovian) of Oklahoma, but differs in the smaller distance between branch centres (on average 0.32 vs. 0.58 mm in Fenestella sasakwaensis), and smaller distances between dissepiment centres (0.36 vs. 0.66 mm in F. sasakwaensis), as well as in the closer spacing of keel nodes (on average 0.17 vs. 0.28 mm in F. sasakwaensis). The internal morphology of Fenestella sasakwaensis is unknown. The Buckhorn species is also similar to Fenestella moorei Sayre, 1930 from the Pennsylvanian (Kasimovian) of Kansas, USA, but differs from in its smaller fenestrules (average fenestrule width 0.13 vs. 0.20 mm in F. moorei; average fenestrule length 0.25 vs. 0.36 mm in F. moorei). The internal morphology of F. moorei is also unknown.
Occurrence: Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Family Septoporidae Morozova, 1962
Genus Septopora Prout, 1859
Type species: Septopora cesteriensis Prout, 1859. Lower Carboniferous; North America.
Diagnosis: Colonies reticulate, consisting of moderately thick branches and broad, often curved dissepiments. New branches originate by bifurcation or from dissepiments. Fenestrules irregularly shaped. Autozooecia arranged in two rows on branches and dissepiments, rectangular to pentagonal in mid-tangential section. Superior hemisepta present; inferior hemisepta absent. Keel broad, low, carrying a single row of nodes. Cyclozooecia spaced irregularly through the colony (modified after Morozova 2001).
Remarks: Septopora Prout, 1859 differs from the similar genus Synocladiella Lisitsyn in Lisitsyn and Ernst, 2004 in having 2 rows of autozooecia on branches instead of 6–8 in Synocladiella.
Occurrence: Mississippian (Lower Carboniferous) to Upper Permian; North America, Europe, Asia.
Septopora blanda Moore, 1929
1929 Septopora blanda Moore, p. 130, pl. 16, figs. 6, 12, pl. 17, fig. 2.
1930 Septopora blanda Moore, 1929 – Warthin, p. 39, pl. 3, figs. 8a, b.
Material: SNSB-BSPG 2011 X 191-SNSB-BSPG 2011 X 192.
Description: Colonies reticulate, formed by undulating branches 0.41–0.42 mm wide joined by 0.31- to 0.42-mm-wide dissepiments. Dissepiments straight to reversed V-shaped, carrying autozooecia. Fenestrules oval to reversed V-shaped, 0.29–0.33 mm and 0.58–0.68 mm long. Autozooecia arranged in two on the branches. Dissepiments bearing two to four autozooecia. Four apertures spaced per fenestrule length. Autozooecial apertures circular to oval. Elliptical nodes regularly arranged in a single row on the low keel between apertures, 0.11–0.13 mm wide and spaced 0.42–0.55 mm from centre to centre. Cyclozooecia common, spaced irregularly on obverse surface, 0.036–0.048 mm in diameter.
Remarks: Septopora blanda Moore, 1929 differs from S. ivanovi Shishova, 1952 from the Moscovian of the Russian Platform by its smaller fenestrules (fenestrule width 0.29–0.33 vs. 0.40–0.60 mm in S. ivanovi; fenestrule length 0.58–0.68 vs. 0.60–0.90 mm in S. ivanovi).
Occurrence: Upper Carboniferous, Pennsylvanian, Upper Graham Formation; Texas, USA. Carboniferous, Middle Pennsylvanian (Wewoka Formation); Oklahoma, USA. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Genus Shulgapora Termier and Termier, 1971
Type species: Polypora abundans Schulga-Nesterenko, 1951, by subsequent designation of Morozova (2001). Carboniferous, Pennsylvanian, Kasimovian; Russia.
Diagnosis: Reticulate colonies of different shape built by straight or slightly undulating, bifurcating branches, joined at regular intervals by straight dissepiments without autozooecia. Autozooecia arranged in 4–5 alternating rows on branches. Autozooecial chambers tubular, elongated, regularly hexagonal to rhombic in mid tangential section, with short vestibules. Hemisepta absent. Autozooecial apertures rounded. Keels between longitudinal rows of autozooecia weakly developed or absent. Cyclozooecia regularly present on obverse side, sometimes occurring on reverse side. Microacanthostyles and nodes usually present on obverse surface (modified after Morozova 2001).
Remarks: Termier and Termier (1971, p. 33) did not designate a type species from among the three assigned species within the Polypora helenae –Polypora kolvae group. In this group Schulga-Nesterenko (1951, p. 131) named P. helenae, P. abundans, and by inference P. kolvae Stuckenberg, 1895 (P. N. Wyse Jackson, personal communication). Additionally, Termier and Termier (1971) did not give a formal diagnosis of Shulgapora. Morozova and Krutchinina (1986, p. 111) presented a formal diagnosis of Shulgapora and designated Polypora helenae Schulga-Nesterenko, 1951 as type species of the genus. Schastlitseva (1992) followed this diagnosis. Finally, Morozova (2001, p. 72) proposed Polypora abundans Schulga-Nesterenko, 1951 as type species of the genus. This designation is followed in the present paper.
Shulgapora is similar to Thamniscus King, 1849 (sensu Ernst in Lisitsyn and Ernst 2004) but differs in having a reticulate colony instead of a freely branching one without lateral connections. Shulgapora differs from Synocladiella Lisitsyn in Lisitsyn and Ernst, 2004 by branches being connected by dissepiments rather than by branch fusion.
Occurrence: Carboniferous (Pennsylvanian)–Permian (Guadalupian); Russia, Europe, Australia, North America.
Shulgapora helenae (Schulga-Nesterenko, 1951)
1951 Polypora helenae Schulga-Nesterenko, p. 31, pl. 29, fig. 3, text-fig. 50.
Material: SNSB-BSPG 2011 X 193-SNSB-BSPG 2011 X 195.
Description: Colony reticulate, formed by straight branches joined by narrow dissepiments. Complete fenestrules not observed in the Buckhorn material. Fenestrule length c. 1.35 mm. Autozooecia arranged in 3–5 rows on branches. Autozooecial apertures circular, with low peristome; five apertures spaced per fenestrule length. Nodes and keels absent; longitudinal striation between apertures developed. Cyclozooecia common, spaced regularly on obverse surface, positioned preferably on distal ends of apertures, 0.037–0.075 mm in diameter. Internal morphology unknown.
Remarks: The present material fits perfectly with the species Polypora helenae Schulga-Nesterenko, 1951, originally described from the Moscovian of the Russian Platform. It differs from Shulgapora hungarica Zágoršek, 1993 from the Moscovian of Hungary, in its narrower branches (branch width 0.39–0.59 vs. 0.53–0.70 mm in S. hungarica) and in its shorter fenestrules (fenestrule length c. 1.35 vs. 2.0–2.6 mm in S. hungarica), as well as in its smaller apertures (aperture width 0.07–0.11 vs. c. 0.22 mm in S. hungarica). Shulgapora helenae differs from S. abundans (Schulga-Nesterenko, 1951) from the Kasimovian of Russian Platform in its narrower branches (branch width 0.39–0.59 vs. 0.60–0.70 mm in S. abundans) and shorter fenestrules (fenestrule length c. 1.35 vs. 1.50–2.05 mm in S. abundans), as well as in the absence of nodes.
Occurrence: Carboniferous, Pennsylvanian, Moscovian; Russian Platform. Carboniferous, Pennsylvanian, Missourian (Kasimovian), Deese Group, Boggy Formation; Buckhorn Asphalt Quarry near Sulphur, Oklahoma, USA.
Preservation
One of the outstanding characteristics of the Buckhorn Asphalt Quarry is the occurrence of pristine skeletons composed of aragonite and high-Mg calcite. Reliable reports of aragonitic bryozoans are lacking in the Palaeozoic and only a few early taxa can be inferred to have possessed skeletons of high-Mg calcite (see Taylor et al. 2014). Although the mineralogies of the Buckhorn bryozoans have not been analysed, all taxa present are represented elsewhere in the Palaeozoic, including in deposits where diagenesis has resulted in aragonite dissolution, and are, therefore likely to have secreted low-Mg calcite skeletons. Therefore, the main preservational interest in the Buckhorn bryozoans does not concern their mineralogy but rather the fine preservation of skeletal morphology.
In contrast to the Buckhorn bryozoans, the preservation of most bryozoans throughout the fossil record is compromised by diagenetic changes. Diagenesis of bryozoans typically results in: (1) epitaxial cement growth on skeletal walls; (2) binding of sediment to skeletal surfaces; and (3) fusion of the original crystallites of the skeleton. The first two processes obscure micromorphological details on the colonies surfaces while the third makes it harder to determine the original fabric of the skeletal walls. All three problems are either lacking or are ameliorated in the Buckhorn bryozoans.
The mild diagenesis of the Buckhorn bryozoans has allowed several skeletal features to be observed for the first time and others to be seen more clearly than before in Palaeozoic bryozoans. Several of the Buckhorn species show the node-like surface expressions of styles that are normally only apparent in thin sections of Palaeozoic bryozoans. Styles are solid rods, typically with an internal ‘cone-in-cone’ laminated structure, that grow parallel with distal wall extension and become embedded in the growing walls. The Buckhorn bryozoans reveal the growing surfaces of styles varying in size, from the small 5-μm-wide stylets of the fenestrate bryozoans to the large styles of the trepostome Stenophragmidium buckhornensis reaching over 50 μm in diameter. In Rhombocladia delicata and Streblotrypa (Streblotrypa) heltzelae both micro- and mega-styles are evident on pristine colony surfaces. In all the Buckhorn bryozoans, the styles project by an amount approximately equivalent to their diameters; none form long, or sharp, spines extending significantly above the colony surface. This suggests that their function is less likely to have been predator defence (cf. the long spines of some modern bryozoans; Harvell 1984, 1992; Yoshioka 1982) than skeletal strengthening or soft tissue attachment/positioning.
Styles are notably lacking from the dorsal sides of branches in the fenestrate Rhombocladia delicata and the surface texture is smooth but with growth wrinkles (Fig. 8a; also observed in R. dichotoma M’Coy, 1844 from the Viséan of Ireland in Wyse Jackson 1996, fig. 53), suggesting that this wall is formed by exterior calcification (e.g. Taylor et al. 2014), i.e. that the mineralised layer directly underlay the outer organic cuticle without an intervening coelom (or pseudocoel). If this interpretation is correct, then Rhombocladia differs from other fenestrates in which the dorsal calcification comprises the interior wall secreted beneath a coelomic cover (e.g. Tavener-Smith 1969). A question mark must therefore be placed over the classification of Rhombocladia, and indeed the family Chainodictyonidae, within the order Fenestrata. Exterior-walled calcification on branch surfaces above the basal lamina is also lacking in the other orders of Palaeostomata making Rhombocladia unique among Palaeozoic bryozoans. However, branches with exterior-walled dorsal sides are present in many post-Palaeozoic cyclostome stenolaemates (e.g. Ostrovsky and Taylor 1996). An interesting comparison can be made between Rhombocladia and the un-named Silurian trepostome depicted by Taylor (1984, pl. 2, fig. 2) from the Silurian Waldron Formation of Tennessee. The latter has ribbon-like encrusting colonies with autozooids in a biserial or triserial arrangement and lateral borders of the branches apparently formed by the upturned basal lamina (which is an exterior wall), rather like the edges of the erect branches of Rhombocladia when viewed from the frontal side (Fig. 6c). A detailed study of chainodictyonid morphology, beyond the scope of the present paper, is needed to re-evaluate the ordinal attribution of this unusual family.
a Rhombocladia delicata Rogers, 1900, autozooecial wall structure, SNSB-BSPG 2011 X 184, b Streblotrypa (Streblotrypa) heltzelae sp. nov. nanoperforations in the autozooecial wall, holotype SNSB-BSPG 2011 X 136, c Streblotrypa (Streblotrypa) heltzelae sp. nov., microborings in the autozooecial wall, paratype SNSB-BSPG 2011 X 142, d Rhombocladia delicata Rogers, 1900, autozooecial wall ultrastructure, SNSB-BSPG 2011 X 184, e, f, Stenophragmidium buckhornensis sp. nov., microborings and nanoperforations in the autozooecial wall, paratype SNSB-BSPG 2011 X 33, g, h Streblotrypa (Streblotrypa) heltzelae sp. nov., band of granules, wall structure, paratype SNSB-BSPG 2011 X 139
Some additional features observed in the Buckhorn bryozoans warrant individual description.
Nanoperforations
Very tiny holes, here termed nanoperforations, are visible in the skeletons of three Buckhorn bryozoan species when observed at high magnifications. These were initially found in Streblotrypa (Streblotrypa) heltzelae sp. nov. (Fig. 8b) and subsequently observed in Stenophragmidium buckhornensis sp. nov. (Fig. 8f) and Rhombocladia delicata (Fig. 8d), i.e. taxa traditionally assigned to three different suborders, Cryptostomata, Trepostomata and Fenestrata, respectively. In all three species, the nanoperforations are visible on the surfaces of well-preserved endozonal walls. They are distributed semi-regularly, have a diameter of about 0.5 μm, and are countersunk (bevelled) into the laminated skeletal walls.
We have been unable to find similar structures described in other bryozoans, living or fossil, and the genesis of these nanoperforations is unclear. Interzooidal pores in bryozoans are an order of magnitude larger in size and more distantly spaced. Important questions to be considered are whether the nanoperforations were produced by the bryozoans themselves or by microendoliths, and if the latter is correct, whether they were formed syn-vivo or post-mortem. Their presence in three distantly related species favours the idea that the nanoperforations were made by endoliths. However, they are very small in size and do not match any of the microborings previously described from the Buckhorn Asphalt Quarry (Wisshak et al. 2008). Unequivocal microborings of a larger size and oriented subparallel to wall surfaces occur in the Buckhorn bryozoans (Fig. 8c, e) and these do resemble the linear superficial borings depicted by Wisshak et al. (2008, fig. 2) from Buckhorn gastropods, bivalves and ostracods. If the nanoperforations in the Buckhorn bryozoans are microendoliths, their countersunk openings suggest that they existed during the life of the bryozoans, as post-mortem microborings would cut through the walls with sharply truncated edges. Confinement of the nanoperforations to endozones is also consistent with formation syn-vivo.
Further research would benefit from resin casting of the nanoperforations to reveal their three-dimensional structure. Until this has been undertaken, the most appealing hypothesis is that the nanoperforations are due to very small-sized symbionts (?bacteria) that lived embedded in the endozonal walls of the zooids of the Buckhorn bryozoans.
Granule bands
Branches of the cryptostome Streblotrypa (Streblotrypa) heltzelae sp. nov., when fractured longitudinally, reveal lines of closely spaced ‘granules’ on the walls of the autozooids just proximally of the endozone/exozone transition and oriented approximately at right angles to the axis of the zooids (Fig. 5d). An elemental analysis could not be performed at this stage of the research but will be implemented in future studies of the material. The granules are partly enveloped in a layer of organic residue, making it difficult to resolve details of their structure. They appear bright when imaged using back-scattered electrons (Fig. 8g, h), suggesting that they consist of material with a high molecular weight, possibly pyrite. About 6 μm in maximum diameter, individual granules have ‘arms’ and an almost tetrahedral morphology, although their exact shape is unclear. This morphology is not one that is normally associated with pyrite.
The origin of the granule bands is problematical. Comparable structures have not previously been recorded in cryptostome or any other bryozoans. Their uniform position within the zooids implies that they are not artefacts, but if they are truly pyritic then their composition must have been altered from its original organic state. The location of the granule bands invites comparison with that of the diverse attachment organs described by Boardman (1998) in living stenolaemate bryozoans. Attachment organs anchor the vestibular wall and membranous sac to the skeleton, sometimes via ligaments. However, none appear to have a morphology matching the enigmatic granule bands of Streblotrypa (Streblotrypa) heltzelae sp. nov.
Mural spines
In contrast to styles, which are oriented parallel to the direction of wall growth and are visible at the distal edges of walls, mural spines are oriented roughly at right angles to wall growth direction and can be seen projecting into zooidal chambers from the sides of the walls. Mural spines are common and varied in Recent cyclostome bryozoans (e.g. Farmer 1979) but have been infrequently recorded in Palaeozoic stenolaemates (e.g. Corneliussen and Perry 1970; Blake 1973), where they have been called zooecial spines or axial spines, probably because pristine wall surfaces are seldom exposed and these spines may be difficult to observe in thin section.
Mural spines in the Buckhorn bryozoans have been observed in the fenestrate Rhombocladia delicata and the trepostome Stenoporella sp. (Figs. 3h, 4a, b). The mural spines of R. delicata are sparse and cone-shaped (Fig. 9a), tubercle-like with a rounded top and about 23 μm high by 28 μm wide at the base. Those of Stenoporella sp. are denser and seem to have a more varied morphology, although some of the variability may be preservational. They are about 7–7.5 μm high and either flat-topped (Fig. 9b) and anvil-shaped, or thorn-like (Fig. 9c). Bifid mural spines (Fig. 9d) are also present in Stenoporella sp. and it seems likely that the flat-topped spines were originally bifid but have had their ends broken off.
a Rhombocladia delicata Rogers, 1900, cone-shaped mural spine, SNSB-BSPG 2011 X 185, b Stenoporella sp., mural spines, SNSB-BSPG 2011 X 45, c, d Stenoporella sp., mural spines, SNSB-BSPG 2011 X 46, e Stenophragmidium buckhornensis sp. nov., spines on the underside of a hemiphragm, paratype SNSB-BSPG 2011 X 33, f Stenophragmidium buckhornensis sp. nov., spines on the underside of a hemiphragm, paratype SNSB-BSPG 2011 X 197, g, h wall ultrastructure of Septopora blanda Moore, 1929, showing degraded transverse fibres oriented at right angles to wall growth direction (g), and overlying laminae (h), SNSB-BSPG 2011 X 191
The function of mural spines in bryozoans as a whole is enigmatic. Farmer (1979) argued that they serve to support and/or anchor soft tissue. This is the most credible hypothesis for short spines such as those of the Buckhorn species, although some of the longer spines found in Recent bryozoans may have had an anti-predator function.
Spinose hemiphragms
Hemiphragms are transverse walls found in some trepostomes that extend only part of the way across the zooecial chamber. In Stenophragmidium buckhornensis sp. nov., the hemiphragms are slight, forming crescent-like partial partitions. They are notable, however, for being spinose. The spines are located on the inner edge (Fig. 9e) and underside of the hemiphragms (Fig. 9f). Usually densely packed, most of the spines point proximally but some are oriented oblique and chaotically (Fig. 9f). Their length varies from about 3 to 30 μm.
As far as we are aware, similar structures have not previously been reported in trepostomes with hemiphragms. While in some ways resembling acicular cement crystals, the smoothly rounded surfaces of the spines point to a biological origin. However, their function is unclear.
Transverse fibrous fabric
Walls fractured obliquely to the surface in a specimen of the fenestrate Septopora blanda reveal the individual crystallites making up the skeleton. Outer layers of an exfoliated wall show broad sheets, about 0.5 mm thick, consisting of probably tabular crystallites seemingly oriented parallel to wall growth direction (Fig. 9h), although it cannot be determined whether the crystallites grew in a distal or proximal direction. At deeper levels within the walls, the fabric appears to be different, comprising narrow crystallites oriented at right angles to wall growth direction (Fig. 9g). The crystallites are degraded but most appear to be only 1–2 μm in width. By comparison with cyclostome bryozoans, these crystallites may constitute a transverse fibrous fabric. This fabric type is widespread among living cyclostomes (Taylor and Weedon 2000) and has also been described in some exceptionally preserved Jurassic and Cretaceous cyclostomes (Taylor and Weedon 1996). In modern cyclostomes, the fibres range from 0.5 μm to more than 15 μm in width, although most are 1–5 μm wide. The fibres in modern cyclostomes originate by splitting of larger crystallites in divergent zones with a fountain-like appearance. Transverse fibres produced from different zones meet at convergences where fibres grow in opposing directions.
Transverse fibrous fabric has not previously been reported in palaeostomates. Its location in the studied specimen of the fenestrate Septopora blanda corresponds with the ‘primary granular layer’ recognised by Tavener-Smith (1969). This layer, which was earlier referred to as ‘germinal plate’ or ‘colonial plexus’, was described by Tavener-Smith (1969, p. 285) as “apparently clear and structureless” and “granular or rubbly” in appearance under SEM. Importantly, the clear appearance of the primary granular layer in thin sections of fenestrates can resemble that of the transverse fibrous layer in thin sections of cyclostomes such as Cinctipora elegans (Boardman et al. 1992, fig. 4).
Discussion
The studied bryozoan fauna from the Boggy Formation (Deese Group, Pennsylvanian) of the Buckhorn Asphalt Quarry, Oklahoma, USA contains nine species belonging to nine genera. This assemblage includes two new species: Stenophragmidium buckhornensis sp. nov. and Streblotrypa (Streblotrypa) heltzelae sp. nov. Two species had to be described in open nomenclature: Stenoporella sp. and Spinofenestella sp. Three genera belong to Trepostomata (Stenophragmidium, Tabulipora, and Stenoporella), two genera to the suborder Rhabdomesina of the Order Cryptostomata (Shishoviclema and Streblotrypa), and four genera (Rhombocladia, Septopora, Spinofenestella, and Shulgapora) to the Order Fenestrata. The assignment of Rhombocladia to the fenestrates is questioned here, however, due to the apparent presence of an exterior dorsal wall. Cystoporate bryozoans were not found in the Buckhorn Asphalt Quarry. The studied bryozoan assemblage is mainly associated with the Missourian (Kasimovian), and only one species, Streblotrypa (Streblotrypa) heltzelae sp. nov., also occurs in the Desmoinesian (Moscovian-Kasimovian). This is the most abundant in the assemblage, followed by another rhabdomesine (Shishoviclema carbonaria), which is the second ranked species in abundance. Other species are less abundant: Stenophragmidium buckhornensis appears to be the most common trepostome species, and Rhombocladia delicata is the most common species among the rest of the assemblage.
Palaeobiogeography
Five identified species show various palaeobiogeographical relationships. Tabulipora hispida (Coryell in Morgan, 1924) and Shishoviclema carbonaria (Coryell in Morgan, 1924) are known from the Wapanucka Limestone (Pennsylvanian, Morrowan) of Oklahoma. Rhombocladia delicata Rogers, 1900 was originally described from the Pennsylvanian of Kansas. This species has been recorded from the Wewoka Formation (Pennsylvanian, Desmoinesian) of Oklahoma, the lower Gzhelian of Austria and Italy, and from the Kasimovian of Cantabrian Mountains, NW Spain. Septopora blanda Moore, 1929 is known from the Upper Graham Formation (Pennsylvanian, Virgilian) of Texas and from the Wewoka Formation (Pennsylvanian, Desmoinesian) of Oklahoma.
Shulgapora helenae (Schulga-Nesterenko, 1951) was originally described from the Moscovian of the Russian Platform. The genus Stenoporella is restricted in its distribution to the Chesterian (Late Mississippian) of Arkansas, Alabama, and Utah.
Broader implications of exceptional bryozoan preservation
Several microskeletal features are recorded here for the first time in Palaeozoic bryozoans. Understanding their broader significance for Palaeozoic bryozoan palaeobiology first requires an evaluation of whether these features are peculiar to the bryozoans from the Buckhorn Asphalt Quarry, and might be due to the unusual depositional and/or diagenetic environment, or are more widespread among Palaeozoic bryozoans but have not been seen before because of the poorer preservation.
The small holes with countersunk edges, termed nanoperforations, observed in the skeletons of three distantly related bryozoans (Streblotrypa (Streblotrypa) heltzelae sp. nov., Stenophragmidium buckhornensis sp. nov. and Rhombocladia delicata), seem most likely to be features specific to the Buckhorn Asphalt Quarry bryozoans, especially if their interpretation as microsymbiont traces is correct. On the other hand, the granule bands found in Streblotrypa (Streblotrypa) heltzelae, and the transverse fibrous fabric of the primary walls of Septopora blanda, are considered more likely to be features that are widely distributed in Palaeozoic bryozoans but not normally preserved. The transverse fibres are particularly important in linking the primary layer of fenestrates, which is usually described in the literature as clear or granular, with a fabric found in the early layers of the walls of many post-Palaeozoic cyclostomes.
References
Anstey, R. L., & Perry, T. G. (1970). Biometric procedures in taxonomic studies of Paleozoic bryozoans. Journal of Paleontology, 44(3), 383–398.
Astrova, G. G. (1965). Morphology, history of development, and systematics of Ordovician and Silurian Bryozoa. Trudy Paleontologischeskogo Instituta Akademii Nauk SSSR, 106, 1–432 (in Russian).
Astrova, G. G., & Morozova, I. P. (1956). On systematics of the order Cryptostomata. Doklady Akademii Nauk SSSR, 110(4), 661–664 (in Russian).
Bancroft, A. J. (1987). Biostratigraphical potential of Carboniferous Bryozoa. Courier Forschungsinstitut Senckenberg, 98, 193–197.
Bartels, C., Briggs, D. E. G., & Brassel, G. (2009). The fossils of the Hunsrück Slate. Cambridge: Cambridge University Press. 324 pp.
Bassler, R. S. (1929). The Permian Bryozoa of Timor. Paläontologie von Timor, 16, 37–90.
Bassler, R. S. (1936). Nomenclatorial notes on fossil and Recent Bryozoa. Journal of the Washington Academy of Science, 26, 156–162.
Bassler, R. S. (1952). Taxonomic notes on genera of fossil and Recent Bryozoa. Journal of the Washington Academy of Sciences, 42, 381–385.
Blake, D. B. (1973). Acanthopore morphology and function in the bryozoan family Rhabdomesidae. Journal of Paleontology, 47, 412–435.
Boardman, R. S. (1998). Reflections on the morphology, anatomy, evolution, and classification of the Class Stenolaemata (Bryozoa). Smithsonian Contributions to Paleobiology, 86, 1–59.
Boardman, R. S., McKinney, F. K., & Taylor, P. D. (1992). Morphology, anatomy, and taxonomy of the Cinctiporidae, new family (Bryozoa: Stenolaemata). Smithsonian Contributions in Paleobiology, 70, 1–81.
Borg, F. (1926). Studies on Recent cyclostomatous Bryozoa. Zoologiska Bidrag från Uppsala, 10, 181–507.
Bottjer, D. J., Etter, W., Hagadorn, J. W., & Tang, C. M. (Eds.). (2002). Exceptional fossil preservation. New York: Columbia University Press. 403 pp.
Buttler, C. J., Wyse Jackson, P. N., Ernst, A., & McKinney, F. K. (2013). A review of the early Palaeozoic biogeography of bryozoans. In: D. Harper & T. Servais (Eds.), Early Palaeozoic Palaeobiogeography and Palaeogeography. Geological Society, London, Memoirs, 38, 145–155.
Ceretti, E. (1963). Briozoi carboniferi della Carnia. Giornale di Geologia. Annali del Museo Geologico di Bologna (series 2), 30, 254–360.
Ceretti, E. (1964). Su alcuni Briozoi criptostomi delle Alpi Carniche. Giornale di Geologia, Annali del Museo Geologico di Bologna, 32(2), 175–199.
Condra, G. E. (1902). New Bryozoa from the Coal Measures of Nebraska. The American Geologist, 30, 337–358.
Corneliussen, E. F., & Perry, T. G. (1970). The ectoproct Batostoma? cornula (Cumings & Galloway) and its enigmatic intrazooecial spines, Fort Atkinson Limestone (Cincinnatian), Wilmington, Illinois. Journal of Paleontology, 44, 997–1008.
Cree, S. B. (1984). A biostratigraphic study of the asphalt-bearing limestones of Pennsylvanian age in the Arbuckle Mountains, Oklahoma. Unpublished Master Thesis, Graduate School, University of Texas, Arlington. 82 pages.
Crockford, J. (1945). Stenoporids from the Permian of New South Wales and Tasmania. Proceedings of the New South Wales Linnean Society, 70, 9–24.
Dunaeva, N. N. (1964). New bryozoans of the order Trepostomata from the Lower Carboniferous of Donetz Basin. Paleontologicheskij Zhurnal, 1964(2), 39–44 (in Russian).
Ehrenberg, C. G. (1831). In Symbolae Physicae, seu Icones et descptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegiptum, Nubiam, Dongalaam, Syriam, Arabiam et Habessiniam, studia annis 1820–25, redirent. Pars Zoologica, 4, Animalia Evertebrata exclusis Insectis. Berolini, 10 pls.
Elias, M. K., & Condra, G. E. (1957). Fenestella from the Permian of west Texas. Geological Society of America Memoir, 70, 1–158.
Ernst, A. (2003). Upper Palaeozoic bryozoans from the Carnic Alps (Austria). Freiberger Forschungshefte, C499, 55–77.
Ernst, A., Wyse Jackson, P. N., & Aretz, M. (2015). Bryozoan fauna from the Mississippian (Viséan) of Roque Redonde (Montagne Noire, southern France). Geodiversitas, 37(2), 151–213.
Farmer, J. D. (1979). Morphology and function of zooecial spines in cyclostome Bryozoa: implications for paleobiology. In G. P. Larwood & M. B. Abbott (Eds.), Advances in Bryozoology (pp. 219–246). London: Academic Press.
Foerste, A. F. (1887). The Clinton Group of Ohio (Bryozoa). Bulletin of Scientific Laboratory, Denison University, 2(1/2), 71–88. 149–176.
Girty, G. H. (1911). On some new genera and species of Pennsylvanian fossils from the Wewoka Formation of Oklahoma. Annals of the New York Academy of Sciences, 21, 119–156.
Girty, G. H. (1915). Fauna of the Wewoka Formation of Oklahoma. U.S. Geological Survey Bulletin, 544, 1–353.
Gorjunova, R. V. (1985). Morphology, system und phylogeny of Bryozoa (Order Rhabdomesida). Trudy Paleontologicheskogo instituta Akademii Nauk SSSR, 208, 1–152 (in Russian).
Gorjunova, R. V., & Weis, O. B. (2012). A new genus Acupipora gen. nov. from the Upper Carboniferous of the East European platform and problem of classification of bryozoans of the Order Fenestellida. Paleontological Journal, 46(1), 16–28.
Hageman, S. J. (1991). Approaches to systematic and evolutionary studies of perplexing groups: an example using fenestrate Bryozoa. Journal of Paleontology, 65, 630–647.
Hageman, S. J. (1993). Effects of nonnormality on studies of the morphological variation of a rhabdomesine bryozoan, Streblotrypa (Streblascopora) prisca (Gabb and Horn). The University of Kansas Paleontological Contributions, 4, 1–13.
Hageman, S., & McKinney, F. K. (2010). Discrimination of fenestrate bryozoan genera in morphospace. Palaeontologia Electronica, 13.2.7A:43 pp, 7 MB; http://palaeo-electronica.org/2010_2/206/index.html.
Ham, W. E. (1969). Regional geology of the Arbuckle Mountains Oklahoma Part 1. Regional Geology. In Geology of the Arbuckle Mountains. Oklahoma Geol. Surv. Guide Book XVII, pp 5–50.
Harlton, B. H. (1933). Micropalaeontology of the Pennsylvanian Johns Valley Shale of the Ouachita Mountains, Oklahoma, and its relationship to the Mississippian Caney Shale. Journal of Paleontology, 7, 3–29.
Harvell, C. D. (1984). Predator-induced defense in a marine bryozoan. Science, 224, 1357–1359.
Harvell, C. D. (1992). Inducible defenses and allocation shifts in a marine bryozoan. Ecology, 73, 1567–1576.
Hutchinson, L. L. (1911). Preliminary report on the rock asphalt, asphaltite, petroleum and natural gas in Oklahoma. Oklahoma Geological Survey, Bulletin, 2, 1–256.
Johnsen, A. (1906). Bryozoen aus dem karnischen Fusulinenkalk. Neues Jahrbuch für Geologie und Paläontologie, 1906(2), 135–166.
King, W. (1849). On some families and genera of corals. Annals and Magazine of the Natural History, 2, 388–390.
Lavrentjeva, V. D. (1979). Phylloporinina – new Suborder of Palaeozoic Bryozoa. Paleontologicheskij Zhurnal, 1979(1), 59–68 (in Russian).
Lisitsyn, V. P., & Ernst, A. (2004). Revision of the Paleozoic bryozoan genera Synocladia and Thamniscus. Paleontologicheskij Zhurnal, 2004(3), 53–59 (in Russian).
Lonsdale, W. (1844). Geological observations on the Volcanic Islands visited during the voyage of H. M. S. „Beagle“. Smith. In C. Darwin (Ed.), Description of six species of corals from the Palaeozoic formation of Van Diemen's Land (pp. 161–169). London: Elder & Co.
Ma, J.-Y., Buttler C. J., & Taylor, P. D. (2014). Cladistic analysis of the 'trepostome' Suborder Esthonioporina and the systematics of Palaeozoic bryozoans. In A. Rosso, P. N. Wyse Jackson, & J. S. Porter (Eds), Bryozoan Studies 2013. Studi Trentini di Scienze Naturali, 94, 153–161
McKinney, F. K. (1971). Stenoporella, a Late Mississippian trepostomatous ectoproct (bryozoan). Journal of Paleontology, 45, 713–723.
M‘Coy, F. (1844). A synopsis of the characters of the Carboniferous Limestone fossils of Ireland (p. 207). Dublin: Dublin University Press.
Meek, F. B. (1872). Report on the paleontology of eastern Nebraska. In F. V. Hayden (Ed.), Final report on the United States Geological Survey of Nebraska and Portions of adjacent Territories: 81–239. Washington: U.S. Government Printing Office.
Moore, R. C. (1929). A bryozoan faunule from the Upper Graham Formation, Pennsylvanian, of north central Texas. Journal of Paleontology, 3(1–27), 121–156.
Morgan, G. D. (1924). Geology of the Stonewall Quadrangle, Oklahoma. Bulletin of Bureau of Geology, Norman, Oklahoma, 2, 179–183.
Morningstar, H. (1922). Pottsville fauna of Ohio. Bulletin (Geological Survey of Ohio), 25, 1–352.
Morozova, I. P. (1962). On the systematics and phylogeny of Fenestelloidea. Paleontologicheskii Zhurnal, 1962(4), 101–115 (in Russian).
Morozova, I. P. (1981). Late Paleozoic bryozoans of the nord–west of the USSR. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 188, 1–119 (in Russian).
Morozova, I. P. (2001). Bryozoans of the Order Fenestellida (morphology, system, historical development). Trudy Paleontologicheskogo Instituta Rossiiskoi Akademii Nauk, 277, 1–177 (in Russian).
Morozova, I. P. & Kruchinina, O. N. (1986). Permian bryozoans of the Arctic region (Western Sector). 143 pp., Nauka, Moscow (in Russian).
Munro, M. (1912). Description of some new forms of trepostomatous Bryozoa from the Lower Carboniferous rocks of the north–western province. Quarterly Journal of the Geological Society, 68, 574–579.
Nickles, J. M., & Bassler, R. S. (1900). A synopsis of American fossil Bryozoa, including bibliography and synonymy. United States Geological Survey Bulletin, 173, 1–663.
Ostrovsky, A. N., & Taylor, P. D. (1996). Systematics of some Antarctic Idmidronea and Exidmonea (Bryozoa: Cyclostomata). Journal of Natural History, 30, 1549–1575.
Perry, T. G., & Gutschick, R. S. (1959). Bryozoa from the Amsden Formation, south-west Montana. Journal of Paleontology, 33(2), 313–322.
Popeko, L. I. (1967). Phylum Bryozoa. In G. V. Kotlyar, & L. I. Popeko (Eds.), Biostratigraphy, bryozoans, and brachiopods of the Upper Paleozoic in Transbaikalia. Zapiski Zabaikalskogo filiala Geografichesko SSSR, 5, 1–85 (in Russian).
Prout, H. A. (1859). Third series of descriptions of Bryozoa from the Paleozoic rocks of western states and territories. Transactions of St. Louis Academy of Sciences, 1, 443–452 (series 3).
Ross, J. R. P. (1981). Biogeography of Carboniferous ectoproct Bryozoa. Palaeontolgy, 24(2), 313–341.
Rogers, A. F. (1900). A new genus and species of Bryozoa from the Coal Measures of Kansas and Missouri. The Kansas University Quarterly, A9, 1–12.
Sadd, J. L. (1991). Tectonic influences on carbonate deposition and diagenesis, Buckhorn Asphalt, Deese Group (Desmoinesian), Arbuckle Mountains, Oklahoma. Journal of Sedimentary Petrology, 61, 28–42.
Sayre, A. N. (1930). The fauna of the Drum Limestone of Kansas and western Missouri. Bulletin of the Kansas Geological Survey, 31(17), 75–203.
Schastlitseva, N. P. (1992). New species of the genus Shulgapora (Bryozoa). Paleontologicheskii Zhurnal, 1992(3), 9–14.
Schulga-Nesterenko, M. I. (1951). Carboniferous Fenestellida of the Russian Platform. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 32, 1–157 (in Russian).
Seuss, B., Nützel, A., Mapes, R. H., & Yancey, T. E. (2009). Facies and fauna of the Pennsylvanian Buckhorn Asphalt Quarry deposit: a review and new data on an important Palaeozoic fossil Lagerstätte with aragonite preservation. FACIES, 55, 609–645.
Seuss, B., Titschak, J., Seifert, S., Neubauer, J., & Nützel, A. (2012a). Oxygen and stable carbon isotopes from a nautiloid from the middle Pennsylvanian (Late Carboniferous) impregnation Lagerstätte ‘Buckhorn Asphalt Quarry’ – Primary paleoenvironmental signals versus diagenesis. Palaeogeography, Palaeoclimatology, Palaeoecology, 319–320, 1–15.
Seuss, B., Mapes, R. H., Klug, C., & Nützel, A. (2012b). Exceptional cameral deposits in a sublethally injured Carboniferous orthoconic nautiloid from the Buckhorn Asphalt Quarry Lagerstätte in Oklahoma, USA. Acta Palaeontologica Polonica, 57, 375–390.
Seuss, B., Nützel, A., Scholz, H., & Frýda, J. (2012c). The Paleozoic evolution of the gastropod larval shell: larval armor and tight coiling as results of predation-driven heterochronic character displacement. Evolution & Development, 14, 212–222.
Shishova, N. A. (1952). Moscovian and Don-Medvedetzki Carboniferous bryozoans of the genus Septopora. Trudy Paleontologičkogo Instituta Akademiya Nauk SSSR, 49, 159–174 (in Russian).
Simpson, G. B. (1895). A discussion of the different genera of the Fenestellidae. Thirteenth Annual Report of the State Geologist of New York for the year 1894, 687–727.
Stuckenberg, A. A. (1895). Corals and bryozoans from the Carboniferous sediments in the Urals and Timan. Trudy Geologicheskogo Komiteta, 10, 1–244 (in Russian).
Tavener-Smith, R. (1969). Skeletal structure and growth in the Fenestellidae (Bryozoa). Palaeontology, 12, 281–309.
Taylor, P. D. (1984). Adaptations for spatial competition and utilization in Silurian encrusting bryozoans. Special Papers in Palaeontology, 32, 197–210.
Taylor, P. D., & Weedon, M. J. (1996). Skeletal ultrastructure and affinities of eleid (melicerititid) cyclostomate bryozoans. In D. P. Gordon, A. M. Smith, & J. A. Grant-Mackie (Eds.), Bryozoans in Space and Time (pp. 341–350). Wellington: NIWA. 442 pp.
Taylor, P. D., & Weedon, M. J. (2000). Skeletal ultrastructure and phylogeny of cyclostome bryozoans. Zoological Journal of the Linnean Society, 128, 337–399.
Taylor, P. D., Lombardi, C., & Cocito, S. (2014). Biomineralization in bryozoans: present, past and future. Biological Reviews. doi:10.1111/brv.12148.
Termier, H., & Termier, G. (1971). Bryozoaires du Paleozoique superieur de Làfganistan. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon, 47, 1–52.
Trizna, V. B. (1961). Bryozoans of the Middle and Upper Carboniferous of some regions of west slope of the Urals. Trudy VNIGRI, 179, 27–136 (in Russian).
Ulrich, E. O. (1882). American Palaeozoic Bryozoa. The Journal of the Cincinnati Society of Natural History, 5(121–175), 233–257.
Vine, G. R. (1884). Fourth report of the Committee consisting of Dr. H. R. Sorby and Mr. G. R. Vine, appointed for the purpose of reporting on fossil Polyzoa. Reports of the 53rd Meeting of the British Association for the Advancement in Sciences, 161–209.
Vine, G. R. (1885). Further notes on new species and other Yorkshire Carboniferous fossil Polyzoa described by Prof. John Phillips. Proceedings of the Yorkshire Geological and Polytechnic Society, 8, 377–393. new series.
Waagen, W., & Wentzel, J. (1886). Salt–Range Fossils. Productus–Limestone Fossils: Coelenterata. Memoirs of the Geological Survey of India, Paleontologica Indica, Series, 13(1), 835–924.
Warthin, A. S., Jr. (1930). Micropalaeontology of the Wewoka, and Holdenville Formations. Bryozoa. Oklahoma Geological Survey, Bulletin, 53, 35–43.
Wisshak, M., Seuss, B., & Nützel, A. (2008). Evolutionary implications of an exceptionally preserved Carboniferous microboring assemblage in the Buckhorn Asphalt lagerstätte (Oklahoma, USA). In M. Wisshak & L. Tapanila (Eds.), Current developments in Bioerosion (pp. 21–54). Berlin Heidelberg New York: Springer.
Wyse Jackson, P. N. (1996). Bryozoa from the Lower Carboniferous (Viséan) of County Fermanagh, Ireland. Bulletin of the Natural History Museum (Geology), 52, 119–171.
Yoshioka, P. (1982). Predator-induced polymorphism in the bryozoan Membranipora membranacea (L.). Journal of Experimental Marine Biology and Ecology, 61, 233–242.
Young, J. (1883). On Ure’s “Millepore”. Tabulipora (Cellepora) Urii, Flem. Annals and Magazine of Natural History, 12(5), 154–158.
Zágoršek, K. (1993). New Carboniferous Bryozoa from Nagyvisnyó (Bükk Mts., Hungary). Foldtani Kozlony, 123(4), 417–440.
Acknowledgements
Thanks go to Mrs Heltzel to allow us to sample from deposits on her private property. We are grateful to Catherine Reid, Canterbury, and Patrick Wyse Jackson, Dublin, for their helpful and constructive reviews. We also thank the DFG for funding the research within the projects NU 96/10-1, 2 and ER 278/6.1.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is registered in Zoobank under urn:lsid:zoobank.org:pub:36E948A4-2509-45CB-879A-56A6973BA49D
Rights and permissions
About this article
Cite this article
Ernst, A., Seuss, B., Taylor, P.D. et al. Bryozoan fauna of the Boggy Formation (Deese Group, Pennsylvanian) of the Buckhorn Asphalt Quarry, Oklahoma, USA. Palaeobio Palaeoenv 96, 517–540 (2016). https://doi.org/10.1007/s12549-016-0231-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12549-016-0231-6